Abstract
Trans-generational antibody transfer constitutes an important mechanism by which mothers may enhance offspring resistance to pathogens. Thus, differential antibody deposition may potentially allow a female to differentiate offspring performance. Here, we examined whether maternal immunization with sheep red blood cells (SRBC) prior to egg laying affects sex-specific yolk antibody transfer and sex-specific offspring performance in zebra finches (Taeniopygia guttata). We showed that immunized mothers deposit anti-SRBC antibodies into the eggs depending on embryo sex and laying order, and that maternal exposure to SRBC positively affects the body size of female, but not male offspring. This is the first study reporting sex-specific consequences of maternal immunization on offspring performance, and suggests that antibody transfer may constitute an adaptive mechanism of maternal favouritism.
Keywords: antibodies, growth, maternal effects, offspring sex, sheep red blood cells
1. Introduction
Maternal antibodies are important factors affecting offspring resistance to pathogens, as they provide passive protection against specific pathogens before a newborn offspring is able to cope with infections itself. Maternal antibodies may also affect the development of the offspring's own immunity, their growth and survival (e.g. [1,2]). Therefore, maternal antibody transfer is considered to be an adaptive mechanism allowing mothers to enhance the fitness of their offspring, especially when they share the same disease environment (e.g. [1]).
In birds, differential yolk antibody transfer to individual eggs has been reported with respect to offspring sex and egg-laying sequence, and was suggested to be a strategy by which a mother may enhance the performance of the more vulnerable offspring [3–5]. However, sex-specific yolk antibody transfer is poorly documented and, more importantly, evidence on the consequences of maternal antibody transfer for the performance of male and female offspring is lacking.
Here, we present results of two independent experiments conducted on captive zebra finches (Taeniopygia guttata). In the first experiment, we immunized females with non-pathogenic sheep red blood cells (SRBC) and studied the relationship between maternal antibody deposition of specific antibodies in egg yolk and embryo sex. In the second experiment, we examined the potential consequences of mothers' immunization for the performance of sons and daughters. In zebra finches, female offspring have repeatedly been shown to be the more vulnerable sex in the face of adverse environmental conditions (e.g. [6,7]). Thus, mothers might be expected to produce daughters in the eggs containing more antibodies, which should result in the enhanced performance of this sex.
2. Material and methods
Zebra finches used in our studies originated from the same laboratory population, and experiments were conducted in 2005 and 2008 on different individuals. Birds were kept in a climatized chamber at 20 ± 2°C, under a 13 L : 11 D photoperiod, lights on at 07.00 h. They were fed ad libitum with a standard mixture of seeds (Megan, Poland) and chopped hard-boiled eggs and received a cuttlebone and grit.
In the first experiment, 36 females were immunized with 100 µl of 40 per cent suspension of SRBC in phosphate-buffered saline (PBS) one month before and again 4 days before the day of pairing with males. The average time between secondary immunization and laying of the first egg was 9.5 days. One female did not show a detectable immune response, so her clutch was removed from the dataset. Finally, 138 eggs from 35 females were analysed. Six days after the second immunization, blood was taken and anti-SRBC antibody titre was assessed using a haemagglutination assay [8] (details in the electronic supplementary material). Eggs were collected on the day of laying, artificially incubated for 72 h and then frozen at −25°C. The frozen egg was broken, the yolk separated from the albumen and the embryo tissue isolated. Maternal antibodies were extracted from the whole yolk and were quantified using a haemagglutination assay [9] (details in the electronic supplementary material). DNA was extracted from embryo tissue with the Kit Blood Mini (A&A Biotechnology, Poland) and the CHD-W and CHD-Z genes from the sex chromosomes were amplified using PCR (protocol in [10]).
In the second experiment, 15 females were immunized with 100 µl of 40 per cent suspension of SRBC in PBS and another 15 females were injected with 100 µl of PBS two weeks before mating. Primary immunization with SRBC two weeks before egg laying has been shown to be efficient for transmitting anti-SRBC antibodies to the eggs [9]; however, to facilitate antibody production, all females received the second injection (SRBC or PBS, respectively) on the day of laying the first egg. All immunized females responded to SRBC and control females have no positive anti-SRBC antibody titres. Eggs were numbered on the day of laying and hatching order was assessed by frequent nests inspection around the expected hatching day. To control for potential post-hatching effects of maternal immunization, two nestlings were cross-fostered on the day of hatching in pairs of nests of immunized and control females with similar clutch size (±1 egg). The first nestling always originated from eggs 1 and 2 and the second one from eggs 3–6. Nestlings were swapped according to the position of the egg in the laying sequence. Body mass of freshly hatched nestlings were determined. Next, 12 days after hatching, nestlings were weighed and tarsus length measured. Offspring sex was determined at maturity by plumage or using PCR techniques (as above) in case of earlier death.
To compare differences in yolk antibody titres between the sexes, we used a linear mixed model (PROC MIXED in SAS) that included laying order as a covariate and female identity as a random factor. We also used linear mixed models to analyse variation in body mass and tarsus length in relation to maternal treatment and sex defined as fixed effects, laying order as a covariate and female and foster female identities as random factors.
3. Results
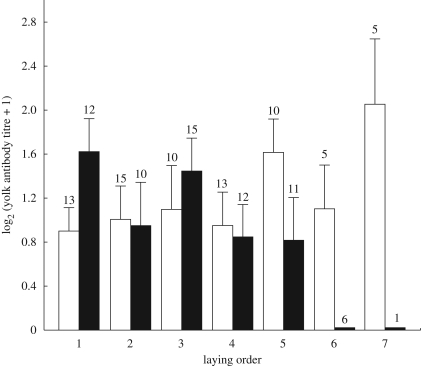
Mean antibody level in the egg yolk positively correlated with anti-SRBC antibody titre in maternal plasma (r = 0.48, p = 0.003, n = 35). Anti-SRBC antibody level in the egg yolk was shaped by the significant interaction between embryo sex and laying order (table 1). This interaction resulted from the fact that the level of maternal anti-SRBC antibodies significantly decreased with laying order among eggs bearing sons (F1,44.2 = 14.75, p = 0.0004; figure 1), but it did not change with laying order among eggs bearing daughters (F1,54.4 = 1.22, p = 0.27; figure 1).
Table 1.
Results of the linear mixed model analysis in which anti-SRBC antibody level in egg yolk was examined in relation to embryo sex and laying order.
| sources of variation | d.f. | F | p-value |
|---|---|---|---|
| sex | 1,115 | 6.03 | 0.016 |
| laying order | 1,112 | 3.79 | 0.054 |
| sex × laying order | 1,113 | 12.85 | 0.0005 |
Figure 1.
Anti-SRBC antibody titre (means ± s.e.) in egg yolks in relation to embryo sex and laying order. Sample sizes are given above bars. White bars, daughters; black bars, sons.
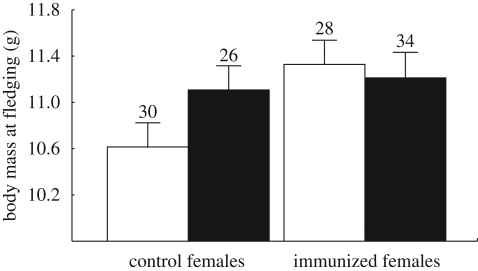
Further, we found that maternal immunization did not affect hatchling body mass but it significantly influenced body mass and tarsus length of nestlings measured 12 days after hatching (table 2). Specifically, there was a significant interaction between maternal treatment and offspring sex (table 2). In the separate analyses performed within sexes, we found that daughters originating from immunized mothers were heavier and had longer tarsi 12 days after hatching compared with daughters originating from control ones (body mass: F1,49.6 = 12.83, p = 0.0008, figure 2: tarsus length: F1,20.2 = 6.62, p = 0.018). In contrast, sons were not affected by maternal immunization (body mass: F1,27.6 = 0.53, p = 0.47, figure 2; tarsus length: F1,27.8 = 3.13, p = 0.09).
Table 2.
Results of the linear mixed model analyses in which body mass and tarsus length were examined in relation to maternal treatment, laying order and offspring sex.
| sources of variation | d.f. | F | p-value |
|---|---|---|---|
| body mass at hatching | |||
| maternal immunization | 1,28.7 | 2.23 | 0.146 |
| laying order | 1,107 | 22.16 | <0.0001 |
| sex | 1,108 | 1.80 | 0.182 |
| body mass at fledging | |||
| maternal immunization | 1,16.6 | 1.99 | 0.176 |
| laying order | 1,91.6 | 1.84 | 0.179 |
| sex | 1,99 | 2.03 | 0.156 |
| maternal immunization × sex | 1,94.6 | 4.05 | 0.047 |
| tarsus length at fledging | |||
| maternal immunization | 1,98.9 | 0.37 | 0.542 |
| laying order | 1,102 | 0.33 | 0.567 |
| sex | 1,107 | 1.66 | 0.200 |
| maternal immunization × sex | 1,108 | 13.55 | 0.0004 |
Figure 2.
Nestling body mass (least-square means ± s.e.) at 12 days after hatching in relation to maternal immunization and offspring sex. Sample sizes are given above bars. White bars, daughters; black bars, sons.
4. Discussion
We showed that immunized mothers transmit anti-SRBC antibodies to the egg yolk, and more importantly, eggs bearing male and female embryos differ in the antibody level in relation to laying order. In zebra finches, mothers were previously shown to vary their deposition of resources in the eggs to counteract sex-specific differences in offspring sensitivity and negative consequences of hatching asynchrony (e.g. [7,10,11]). The pattern of antibody transfer observed in the current study may be another form of maternal favouritism, which can differentiate the performance of male and female offspring hatched from initial and last laid eggs. The observed pattern of antibody transfer may serve to enhance protection of newly hatched chicks against infections, especially if offspring from early and late laid eggs differ in sensitivity to pathogens, as reported in some other species (e.g. [12]). These possibilities require more detailed studies, as mechanisms involved in differential antibody deposition remain unknown. Maternal antibody transfer to the eggs is thought to be a passive process (e.g. [1]), but if oocytes resulting in male or female embryos differ in the duration of growth, they could accumulate unequal amounts of antibodies. Such sex-specific differences in oocyte growth, resulting in differential deposition of maternal agents, were shown in the house finch (Carpodacus mexicanus) [13]. The non-exclusive mechanism is that offspring sex determination is under the influence of yolk content and the order of oocyte sequestration [14].
In our second experiment, we found that daughters of immunized mothers grew larger compared with daughters of control mothers, while such differences were not observed among sons. Given that the nestlings were partially cross-fostered, the observed differences must be attributed to maternal substances contained in the eggs but not differences in provisioning. Variation in the growth of daughters may have important fitness consequences, as in zebra finches female body mass at fledging is a significant predictor of survival and fecundity [15,16]. The observed sex-specific differences in response to maternal immunization could be possibly caused by increased transfer of maternal antibodies to female eggs, as maternal antibodies have already been shown to enhance nestling growth (e.g. [1]). However, this result is not entirely consistent with the pattern of antibody transfer shown in the first experiment. In fact, one should expect the observed sex-specific effects to be related to laying order. In a statistical sense, an interaction of maternal treatment × offspring sex × laying order should appear significant, which was not the case. This may suggest that the deposition of some other maternal micro- or macronutrients in the eggs, such as proteins, lipids, hormones or carotenoids, might be affected by maternal immunization and cause the observed differences in growth (e.g. [17]). Thus, we are not entirely confident on the detailed mechanism of the observed sex-specific differences in growth in response to maternal immunization.
To our knowledge, our study is the first to report sex-specific effects of maternal immunization on offspring performance. We also found differences in antibody levels in eggs bearing daughters and sons in relation to laying order. However, it is not known whether enhanced growth of female offspring is directly related to increased maternal antibody transfer. The observed effects may constitute an adaptive strategy of maternal favouritism by which the female enhances the performance of the more sensitive sex, but direct fitness advantages of this strategy must be further explored.
Acknowledgements
We thank E. B. Śliwińska and A. Arct for help in sexing of embryos, and anonymous referees for comments. Financial support was provided by the Polish Ministry of Science and Higher Education in years 2006–2010 (grants N30405331/2019, N30400931/0397), partly by DS/WBiNoZ/INoŚ/757/07-09 and by The Foundation for Polish Science (J.R.).
References
- 1.Grindstaff J. L., Brodie E. D., Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasselquist D., Nilsson J. A. 2009. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Phil. Trans. R. Soc. B 364, 51–60 10.1098/rstb.2008.0137 (doi:10.1098/rstb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargitai R., Prechl J., Török J. 2006. Maternal immunoglobulin concentration in collared flycatcher (Ficedula albicollis) eggs in relation to parental quality and laying order. Funct. Ecol. 20, 829–838 10.1111/j.1365-2435.2006.01171.x (doi:10.1111/j.1365-2435.2006.01171.x) [DOI] [Google Scholar]
- 4.Müller W., Groothuis T. G. G., Dijksra C., Siitari H., Alatalo R. V. 2004. Maternal antibody transmission and breeding densities in the black-headed gull Larus ridibundus. Funct. Ecol. 18, 719–724 10.1111/j.0269-8463.2004.00902.x (doi:10.1111/j.0269-8463.2004.00902.x) [DOI] [Google Scholar]
- 5.Saino N., Romano M., Ferrari R. P., Martinelli R., Moller A. P. 2003. Maternal antibodies but not carotenoids in barn swallow eggs covary with embryo sex. J. Evol. Biol. 16, 516–522 10.1046/j.1420-9101.2003.00534.x (doi:10.1046/j.1420-9101.2003.00534.x) [DOI] [PubMed] [Google Scholar]
- 6.Birkhead T. R., Fletcher F., Pellatt E. J. 1999. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc. R. Soc. Lond. B 266, 385–390 10.1098/rspb.1999.0649 (doi:10.1098/rspb.1999.0649) [DOI] [Google Scholar]
- 7.Kilner R. 1998. Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 56, 155–164 10.1006/anbe.1998.0775 (doi:10.1006/anbe.1998.0775) [DOI] [PubMed] [Google Scholar]
- 8.Hudson L., Hay F. C. 1989. Practical immunology. Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 9.Grindstaff J. L., Demas G. E., Ketterson E. D. 2005. Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. J. Anim. Ecol. 74, 1051–1058 10.1111/j.1365-2656.2005.01002.x (doi:10.1111/j.1365-2656.2005.01002.x) [DOI] [Google Scholar]
- 10.Rutkowska J., Cichoń M. 2005. Egg size, offspring sex and hatching asynchrony in zebra finches. J. Avian Biol. 36, 12–17 10.1111/j.0908-8857.2005.03469.x (doi:10.1111/j.0908-8857.2005.03469.x) [DOI] [Google Scholar]
- 11.Gilbert L., Rutstein A. N., Hazon N., Graves J. A. 2005. Sex-biased investment in yolk androgens depends on female quality and laying order in zebra finches (Taeniopygia guttata). Naturwissenschaften 92, 178–181 10.1007/s00114-004-0603-z (doi:10.1007/s00114-004-0603-z) [DOI] [PubMed] [Google Scholar]
- 12.Roulin A., Brinkhof M. W. G., Bize P., Richner H., Jungi T. W., Bavoux C., Boileau N., Burneleau G. 2003. Which chick is tasty to parasites? The importance of host immunology versus parasite life history. J. Anim. Ecol. 72, 75–81 10.1046/j.1365-2656.2003.00677.x (doi:10.1046/j.1365-2656.2003.00677.x) [DOI] [Google Scholar]
- 13.Badyaev A. V., Young R. Y., Hill G. E., Duckworth R. A. 2008. Evolution of sex-biased maternal effects in birds. IV. Intra-ovarian growth dynamics can link sex determination and sex-specific acquisition of resources. J. Evol. Biol. 21, 449–460 10.1111/j.1420-9101.2007.01498.x (doi:10.1111/j.1420-9101.2007.01498.x) [DOI] [PubMed] [Google Scholar]
- 14.Rutkowska J., Badyaev A. V. 2008. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil. Trans. R. Soc. B 363, 1675–1686 10.1098/rstb.2007.0006 (doi:10.1098/rstb.2007.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kogel C. H. 1997. Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J. Anim. Ecol. 66, 167–178 [Google Scholar]
- 16.Haywood S., Perrins C. M. 1992. Is clutch size in birds affected by environmental conditions during growth? Proc. R. Soc. Lond. B 249, 195–197 10.1098/rspb.1992.0103 (doi:10.1098/rspb.1992.0103) [DOI] [PubMed] [Google Scholar]
- 17.Gil D., Marzal A., de Lope F., Puerta M., Moller A. P. 2006. Female house martins (Delichon urbica) reduce egg androgen deposition in response to a challenge of their immune system. Behav. Ecol. Sociobiol. 60, 96–100 10.1007/s00265-005-0145-1 (doi:10.1007/s00265-005-0145-1) [DOI] [Google Scholar]