Abstract
Little is known about the potential of non-human mammal vocalizations to signal information on the hormonal status of the caller. In the current study, we used endocrine data and acoustic analyses to determine whether male giant panda bleats provide reliable information about the caller's current androgen levels. Our results revealed significant relationships between acoustic features of male giant panda bleats and the caller's faecal androgen metabolite concentrations. To our knowledge, this constitutes the first demonstration that the acoustic structure of a non-human mammal call has the potential to yield information about the caller's current androgen levels. We go on to discuss the anatomical basis for our findings and the potential functional relevance of signalling information on male androgen levels in giant panda sexual communication.
Keywords: vocal communication, giant panda, cues to androgen levels
1. Introduction
Giant panda (Ailuropoda melanoleuca) vocal activity increases significantly during the breeding season [1]. In particular, males ‘bleat’ at high rates during encounters with oestrous females [2], indicating a key role for these vocalizations in reproductive contexts. Recent work has shown that male giant panda bleats encode information on the caller's identity and body size [3,4]; however, male bleats may also contain information about a caller's hormonal quality that is highly relevant in reproductive contexts. For example, given that testosterone is an important component of male competitive ability and sperm quality in mammals [5,6], vocal cues to male androgen levels may be important in this species' sexual communication for intrasexual assessment or mate choice, respectively. Indeed, intra-male competition figures prominently in giant panda mating strategies [1] and both male and female giant pandas use chemical signals to assess male competitive status [7].
Mammal vocal signals are generated by the conversion of airflow from the lungs to acoustic energy by the larynx, which is subsequently filtered by the vocal tract. The rate at which the vocal folds open and close in the larynx determines the fundamental frequency (F0) of the vocalization—perceived as the pitch—and the supra-laryngeal vocal tract acts as a spectral filter, selectively transmitting certain frequencies termed ‘formants’ [8]. Because acoustic features generated by the larynx and vocal tract are produced separately, they have the potential to yield information about different aspects of the caller's phenotype. For example, formants are reliable acoustic cues to a caller's body size in several non-human mammals because of a close relationship between formant spacing, vocal tract length and overall body size [9]. In addition, because male vocal folds are susceptible to changes in testosterone levels [10], source-related acoustic features could contain information about male hormonal quality [11].
Here, we use a combination of hormone measurements and acoustic analyses to determine whether male giant panda bleats have the potential to directly signal the caller's current androgen levels. In line with previous human studies, we predicted that the mean F0 of male bleats would be negatively correlated with the caller's androgen levels [11], and jitter (a measure of F0 perturbation) would increase with circulating androgen levels [12]. We had no a priori directional predictions for our measures of F0 modulation. In addition, since male giant panda testosterone levels fluctuate during the breeding season [13] and the formants of giant panda bleats appear to be static components of these calls [4], we did not expect to find a relationship between male androgen levels and formant spacing.
2. Material and methods
The study was conducted during the 2009 breeding season (February–April). Recordings were captured using a Marantz PMD660 recorder (44.1 kHz sampling rate, 16 bits amplitude resolution) and a Sennheiser MKH70 P48 directional microphone at the China Research and Conservation Centre for the Giant Panda (CRCCGP), China, and San Diego Zoo in the United States. A total of 181 bleats from seven adult male giant pandas (aged 6–19 years) were analysed using Praat v. 5.0.29 (www.praat.org). Acoustic values were averaged across each recording session (see table 1 for the composition of the sample by subject).
Table 1.
Composition of the sample by subject.
| subject | recording session | number of recordings | faecal androgen metabolite concentrations (ng g−1) |
|---|---|---|---|
| Ling Ling | 1 | 4 | 83 |
| Ling Ling | 2 | 3 | 74 |
| Ling Ling | 3 | 6 | 79 |
| Ling Ling | 4 | 9 | 73 |
| Lu Lu | 1 | 4 | 141 |
| Lu Lu | 2 | 5 | 77 |
| Lu Lu | 3 | 6 | 61 |
| Lu Lu | 4 | 10 | 377 |
| Wu Gang | 1 | 3 | 175 |
| Wu Gang | 2 | 10 | 253 |
| Wu Gang | 3 | 12 | 138 |
| Wu Gang | 4 | 7 | 196 |
| Gao Gao | 1 | 11 | 146 |
| Gao Gao | 2 | 12 | 91 |
| Gao Gao | 3 | 9 | 157 |
| Gao Gao | 4 | 7 | 137 |
| Yuan Yuan | 1 | 5 | 244 |
| Yuan Yuan | 2 | 5 | 362 |
| Yuan Yuan | 3 | 7 | 320 |
| Yuan Yuan | 4 | 4 | 526 |
| Mei Sheng | 1 | 7 | 237 |
| Mei Sheng | 2 | 4 | 127 |
| Mei Sheng | 3 | 8 | 120 |
| Xi Meng | 1 | 7 | 88 |
| Xi Meng | 2 | 6 | 134 |
| Xi Meng | 3 | 10 | 88 |
| total | 26 | 181 | mean = 173 |
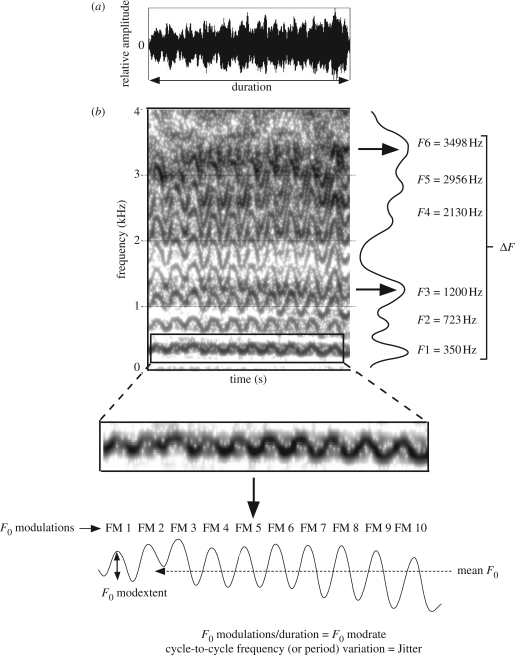
F0 was measured using the To Pitch (cc) command in Praat (time step: 0.01 s). A five point average smoothing filter was used to remove any rapid variations in the F0 contour caused by analysis imprecision, and to limit the possibility of ‘octave jumps’, the minimum and maximum values for F0 were set according to the F0 contour as observed on the spectrogram. Time-varying numerical representations of the F0 contour were then compared with the F0 contour as visualized on a spectrogram before the mean F0 was extracted (figure 1). In addition, to quantify the characteristic F0 modulation of giant panda bleats, we measured the number of complete cycles of F0 modulation per second (F0 modrate) and the average peak-to-peak variation of each F0 modulation (F0 modextent) (figure 1). We also quantified the cycle-to-cycle frequency variation across the call using a measure of F0 perturbation termed jitter [8]. To increase the validity of our jitter measurements, we averaged three measurement values (local, relative average perturbation and five point period perturbation quotient). Finally, we used linear predictive coding to measure the frequency values of the first six formants of giant panda bleats, allowing us to calculate the average formant spacing (sensu [4]) (for more details on the acoustic analysis see electronic supplementary material).
Figure 1.
(a) Waveform and (b) spectrogram to illustrate the acoustic features of giant panda bleats we measured. Spectrogram settings: window length, 0.03 s; time steps, 250; frequency steps, 1000; Gaussian window; dynamic range, 50 dB.
Because peak faecal glucocorticoid concentrations are detected approximately 12 h after peak serum levels [14], fresh faecal samples were collected at 08.00 h the day after each recording session. The samples were frozen at −20°C until faecal androgen metabolite concentrations could be measured at the Centre for Research on Endangered Species endocrinology laboratory, San Diego, CA, USA (see electronic supplementary material). We used log transformations to normalize the data distribution and linear mixed-effect models (with subject identity entered as a random factor) to examine relationships between acoustic features of male giant panda bleats averaged across each of 26 recording sessions and the caller's current androgen levels. Within-subject centring was used to decompose the within- and between-subject effect of male androgen levels on bleat acoustic structure (see electronic supplementary material). To control for interindividual differences in arousal level and give an appropriate match between hormonal and acoustic changes, we noted whether males were involved in a vocal interaction with a female in an adjacent enclosure during each recording session and the time delays between each recording session and faecal sampling, and entered these as covariates in the analysis. In addition, minimum body weight recorded over the previous year was entered as a covariate to control for the possible affects of male body size on bleat acoustic structure [3].
3. Results
A positive within-subject relationship between male faecal androgen values and bleat duration was revealed (table 2). By contrast, no between-subject effect was detected (table 2). We also found a positive between-subject effect of male faecal androgen values on F0 modrate but no within-subject effect (table 2). No significant relationships between male faecal androgen values and any other acoustic features were detected (table 2).
Table 2.
Results of the linear mixed-effects models to show the relationships between acoustic features of giant panda bleats and log male faecal androgen metabolite concentrations. (Significant p-values are in bold.)
| acoustic measures | within-subject effect |
between-subject effect |
||||||
|---|---|---|---|---|---|---|---|---|
| estimates ± s.e. | d.f. | F | p | estimates ± s.e. | d.f. | F | p | |
| log duration | 0.294 ± 0.137 | 1,16 | 7.62 | 0.01 | −0.506 ± 0.258 | 1,4 | 1.56 | 0.28 |
| log mean F0 | 0.000 ± 0.038 | 1,16 | 0.28 | 0.61 | 0.071 ± 0.138 | 1,4 | 0.45 | 0.54 |
| log F0 modrate | 0.029 ± 0.064 | 1,16 | 0.58 | 0.46 | 0.199 ± 0.006 | 1,4 | 12.14 | 0.02 |
| F0 modextent | −0.023 ± 44.797 | 1,16 | 0.00 | 0.96 | −33.377 ± 68.147 | 1,4 | 0.07 | 0.81 |
| log jitter | 0.004 ± 0.098 | 1,16 | 0.06 | 0.80 | 0.042 ± 0.095 | 1,4 | 0.26 | 0.64 |
| formant spacing | −10.880 ± 9.873 | 1,16 | 0.75 | 0.40 | 4.839 ± 15.877 | 1,4 | 0.37 | 0.55 |
4. Discussion
Our results show that giant panda bleats encode reliable information on male androgen levels. Although vocal cues to testosterone have been identified in birds [15] and humans [11], to our knowledge, this study constitutes the first demonstration that the acoustic structure of a non-human mammal call contains this type of information. In particular, male bleats from callers with higher faecal androgen values were characterized by longer duration and higher rates of F0 modulation. Work on other mammals has shown that call duration increases with arousal state [16]. Accordingly, the longer duration of bleats produced by males with high androgen levels might be a simple consequence of their greater arousal. Indeed, the within-subject variation and lack of between-subject effect support the contention that duration varies according to the subject's own androgen-mediated arousal level, as opposed to being a robust cue to androgen levels across subjects.
An explanation for the higher F0 modulation rate of males with high androgen levels may be found if we consider that testosterone is known to increase the collagen/elastin ratio of tissue [17], and that the levels of collagen and elastin could contribute differentially to vocal fold elasticity. In humans, significantly higher levels of collagen are found in the male vocal fold (both the cover and ligament) and the male vocal fold ligament is three to five times stiffer than females [18]. If testosterone-mediated increases in collagen levels lead to greater stiffness of the vocal fold ligament in male giant pandas, this would be likely to increase the elastic recoil of the vocal folds. In turn, this could facilitate increased levels of F0 modulation by allowing the vocal folds to be restored to their original length more quickly after deformation. Since F0 modulation rate did not vary consistently according to circulating androgen levels within individual males, but males with high overall androgen levels produced bleats with higher F0 modulation rates (indicated by the between-individual effect), we suggest that the F0 modulation rate of giant panda bleats is likely to constitute a robust cue to male quality (in this case overall androgen levels), allowing female giant pandas to choose between different males in mate choice contexts and for male giant pandas to assess the relative androgen levels and competitive ability of rivals.
As expected, no relationship between the formant spacing of male giant panda bleats and the caller's androgen levels was found. Indeed, the results of human studies investigating the relationship between male testosterone levels and formant spacing are somewhat equivocal [11,19]. Moreover, male giant panda androgen levels fluctuate during the breeding season [13] and, because formant spacing is explicitly related to the caller's vocal tract length, there is no reason to expect this acoustic feature to vary according to fluctuating androgen levels unless the caller actively changes its vocal tract length. In addition, and contrary to our predictions, males with high faecal androgen concentrations did not have significantly lower mean F0 or higher jitter. A possible explanation is that these acoustic features have more potential to reveal moment-to-moment changes in the caller's motivational state [16] and, therefore, are more subject to interindividual variation. It must also be noted that human studies, in which high testosterone speakers have been shown to have lower mean F0 [11], typically use paradigms that provide more control over the vocalizer's motivation levels than was possible here.
In summary, our findings show that giant panda bleats contain reliable information on male androgen levels. In several mammal species, males with relatively higher levels of testosterone are typically more aggressive and also preferred as mating partners [20]. Consequently, acoustic features that signal male androgen levels may have use in giant panda sexual communication, allowing female giant pandas to choose high-quality mates and male giant pandas to better avoid more competitive/dangerous rivals. Future playback studies to test these predictions are now required.
Acknowledgements
The procedures used in the research did not affect the housing, diet or management of the animals and comply with the law of the People's Republic of China. The director of the CRCCGP and Zoo Atlanta's Institute for Animal Care and Use Committee approved the research.
We thank the keepers and staff at the CRCCGP and San Diego Zoo. This material is based upon work supported in part by the STC Programme of the National Science Foundation under Agreement No. IBN-9876754.
References
- 1.Schaller G. B., Hu J., Pan W., Zhu J. 1985. The giant pandas of Wolong. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Kleiman D. G., Peters G. 1990. Auditory communication in the giant panda: motivation and function. In Second International Symposium on the giant panda (eds Asakura S., Nakagawa S.), pp. 107–122 Tokyo, Japan: Tokyo Zoological Park Society [Google Scholar]
- 3.Charlton B. D., Zhihe Z., Snyder R. J. 2009. The information content of giant panda, Ailuropoda melanoleuca, bleats: acoustic cues to sex, age and size. Anim. Behav. 78, 893–898 10.1016/j.anbehav.2009.06.029 (doi:10.1016/j.anbehav.2009.06.029) [DOI] [Google Scholar]
- 4.Charlton B. D., Zhihe Z., Snyder R. J. 2009. Vocal cues to identity and relatedness in giant pandas (Ailuropoda melanoleuca). J. Acoust. Soc. Am. 126, 2721–2732 10.1121/1.3224720 (doi:10.1121/1.3224720) [DOI] [PubMed] [Google Scholar]
- 5.Minter L. J., DeLiberto T. J. 2008. Seasonal variation in serum testosterone, testicular volume, and semen characteristics in the coyote (Canis latrans). Theriogenology 69, 946–952 10.1016/j.theriogenology.2008.01.010 (doi:10.1016/j.theriogenology.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 6.Zielinski W. J., Vandenbergh J. G. 1993. Testosterone and competitive ability in male house mice, Musmusculus: laboratory and field studies. Anim. Behav. 45, 873–891 10.1006/anbe.1993.1108 (doi:10.1006/anbe.1993.1108) [DOI] [Google Scholar]
- 7.White A. M., Swaisgood R. R., Zhang H. 2002. The highs and lows of chemical communication in giant pandas (Ailuropoda melanoleuca): effect of scent deposition height on signal discrimination. Behav. Ecol. Sociobiol. 51, 519–529 10.1007/s00265-002-0473-3 (doi:10.1007/s00265-002-0473-3) [DOI] [Google Scholar]
- 8.Titze I. R. 1994. Principles of voice production. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- 9.Fitch W. T., Hauser M. D. 2002. Unpacking ‘Honesty’: generating and extracting information from acoustic signals. In Animal communication (eds Megala-Simmons A., Popper A.), pp. 65–137 Berlin, Germany: Springer [Google Scholar]
- 10.Beckford N. S., Schain D., Roor S. R., Schanbacher B. 1985. Androgen stimulation and laryngeal development. Ann. Otol. Rhinol. Laryngol. 94, 634–640 [DOI] [PubMed] [Google Scholar]
- 11.Evans S., Neave N., Wakelin D., Hamilton C. 2008. The relationship between testosterone and vocal frequencies in human males. Physiol. Behav. 93, 783–788 10.1016/j.physbeh.2007.11.033 (doi:10.1016/j.physbeh.2007.11.033) [DOI] [PubMed] [Google Scholar]
- 12.King A., Ashby J., Nelson C. 2001. Effects of testosterone replacement on a male professional singer. J. Voice 15, 553–557 10.1016/S0892-1997(01)00055-8 (doi:10.1016/S0892-1997(01)00055-8) [DOI] [PubMed] [Google Scholar]
- 13.MacDonald E., Czekala N. M., Wang P., Gual-Sil F., Nakao T. 2006. Urinary testosterone and cortisol metabolites in male giant pandas Ailuropoda melanoleuca in relation to breeding, housing, and season. Acta Zool. Sin. 52, 242–249 [Google Scholar]
- 14.Kersey D. C. 2009. Reproductive and adrenal endocrinology of the giant panda (Ailuropoda melanoleuca). Environmental science and public policy. PhD thesis, George Mason University, Fairfax, VA [Google Scholar]
- 15.Galeotti P., Saino N., Sacchi R. 1997. Song correlates with social context, testosterone and body condition in male barn swallows. Anim. Behav. 53, 687–700 10.1006/anbe.1996.0304 (doi:10.1006/anbe.1996.0304) [DOI] [Google Scholar]
- 16.Rendall D. 2003. Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J. Acoust. Soc. Am. 113, 3390–3402 10.1121/1.1568942 (doi:10.1121/1.1568942) [DOI] [PubMed] [Google Scholar]
- 17.Fisher G. M., Swain M. L. 1980. Influence of contraceptive and other sex steroids on aortic collagen and elastin. Exp. Mol. Path. 33, 15–24 10.1016/0014-4800(80)90003-9 (doi:10.1016/0014-4800(80)90003-9) [DOI] [PubMed] [Google Scholar]
- 18.Chan R. W., Fu M., Young L., Tirunagari N. 2007. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann. Biomed. Eng. 35, 1471–1483 10.1007/s10439-007-9314-x (doi:10.1007/s10439-007-9314-x) [DOI] [PubMed] [Google Scholar]
- 19.Bruckert L., Lienard J. S., Lacroix A., Kreutzer M., Leboucher G. 2006. Women use voice parameters to assess men's characteristics. Proc. R. Soc. B 273, 83–89 10.1098/rspb.2005.3265 (doi:10.1098/rspb.2005.3265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clutton-Brock T., McAuliffe K. 2009. Female mate choice in mammals. Q. Rev. Biol. 84, 3–27 10.1086/596461 (doi:10.1086/596461) [DOI] [PubMed] [Google Scholar]