Abstract
Lungfishes are the closest living relatives of the tetrapods, and the ear of recent lungfishes resembles the tetrapod ear more than the ear of ray-finned fishes and is therefore of interest for understanding the evolution of hearing in the early tetrapods. The water-to-land transition resulted in major changes in the tetrapod ear associated with the detection of air-borne sound pressure, as evidenced by the late and independent origins of tympanic ears in all of the major tetrapod groups. To investigate lungfish pressure and vibration detection, we measured the sensitivity and frequency responses of five West African lungfish (Protopterus annectens) using brainstem potentials evoked by calibrated sound and vibration stimuli in air and water. We find that the lungfish ear has good low-frequency vibration sensitivity, like recent amphibians, but poor sensitivity to air-borne sound. The skull shows measurable vibrations above 100 Hz when stimulated by air-borne sound, but the ear is apparently insensitive at these frequencies, suggesting that the lungfish ear is neither adapted nor pre-adapted for aerial hearing. Thus, if the lungfish ear is a model of the ear of early tetrapods, their auditory sensitivity was limited to very low frequencies on land, mostly mediated by substrate-borne vibrations.
Keywords: lungfish, hearing, vibration, tetrapod, sound, evolution
1. Introduction
The evolutionary transition of tetrapods from water to land involved a series of adaptations, including the evolution of the tympanic ear to facilitate aerial pressure hearing. However, recent important insights from palaeontology show that the early tetrapods were atympanate [1]; the tetrapodomorph ancestors may have had a middle ear bone (homologous to the human stapes) that was almost in a suitable position for tympanic stimulation, but the middle ear bone was not connected to a tympanum. Tympanic ears are first thought to have evolved in the Triassic, more than 150 Myr after the origin of tetrapods and probably 100 Myr after the truly terrestrial amniotes emerged, and the tympanum originated four or five times independently among the tetrapods [2].
A major question in the evolution of terrestrial hearing is therefore what hearing in the terrestrial tetrapods was like before the origin of the tympanic ear [3]. In water, hair cells in the inner-ear sensory maculae in combination with otoliths make the unaided auditory systems of fishes act as accelerometers that respond to the particle motion component of a sound field [4]. The otolith organs in fishes are the saccule, lagena and utricle. Beyond the resonance frequency of the otolith-hair cell complex, fishes have very poor hearing sensitivity; however, many species have a mechanical coupling of an air volume to the inner ear as a secondary specialization. Such a coupling acts as a pressure to particle motion transducer. Because a pressure sensitive ear will be much more sensitive than a particle motion-sensitive ear at higher frequencies, auditory specializations among aquatic vertebrates usually entail a mechanical coupling of the inner ear to air-filled structures [5,6].
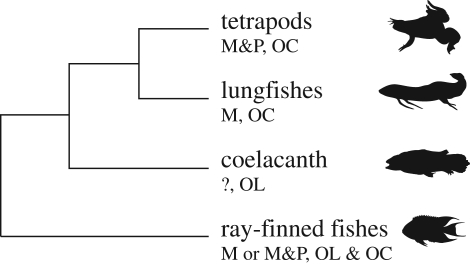
In ray-finned fishes, the swim bladder often serves the dual purpose of maintaining neutral buoyancy and improving hearing abilities of the fish. Similarly, the lung of lungfishes may also act as a pressure to particle motion transducer, providing the basis for high-frequency (above 200 Hz) pressure hearing. Like other non-teleosts (including tetrapods), recent lungfishes have otoconia in the inner-ear maculae instead of the solid otoliths, and the inner ear shows many unusual anatomical features, such as a fused sacculo-lagenar macula [7]. Since lungfishes are to be considered the closest living relatives of the tetrapods ([2,8]; figure 1), the physiology of hearing in lungfishes may thus provide important insights into the hearing in the early tetrapods,
Figure 1.
Phylogeny of the tetrapods and their closest living vertebrate sister groups (after Zardoya & Meyer [8]). p is the pressure and M is the motion-sensitive hearing (unknown in the coelacanth). OL designates inner-ear organs with a solid otolith (in teleosts, the major subgroup of the ray-finned fishes, and the coelacanth), OC inner-ear organs with an otoconial mass (most non-teleosts).
Here we studied the pressure and vibration sensitivity of the West African lungfish (Protopterus annectens), to test the hypothesis that they are sensitive to pressure by using their lung as a pressure to particle motion transducer, implying that tetrapodomorph ancestors may have had pressure hearing before the water to land transition.
2. Material and methods
We used five specimens (40–60 cm) of the African lungfish (P. annectens). The fish were lightly anaesthetized by brief immersion prior to the experiments in MS-222. To investigate the sensitivity and modality (pressure versus particle motion) of hearing in the African lungfish, we measured the auditory brainstem response (ABR; [9]) to sound in air and water and vibrations only. The ABR is an evoked potential response measured by two differential electrodes inserted subdermally, one above the ear and the other above the brain stem with reference to a ground electrode placed dorsally behind the head. The electrode signal was recorded using a low-impedance headstage (Tucker-Davis Technologies, RA4LI), amplified 20× (TDT, RA4PA) and processed using a digital signal processor (Tucker-Davis Technologies, RM2). Robust ABRs were measured by averaging the neural response 400 times. The vibration and sound click stimuli were broad-band, hence exciting hair cells tuned to a wide range of frequencies. By masking the click response by a tone and subtracting the masked from the unmasked ABR click response, we obtained the response to the tone and could estimate thresholds and hence produce audiograms [10]. The masked ABR enabled us to measure auditory responses at very low frequencies, where it is difficult to get a good response using tone burst ABR measurements.
The underwater hearing sensitivity was measured in a 1 by 1 m PVC tank with 70 cm depth of water. The lungfish were suspended in a ceiling mounted sling at a depth of 20 cm, 50 cm above an UW30 transducer. We calibrated the sound field at the position of the fish head along all three orthogonal axes (and ±5 cm in all directions) using two calibrated B&K 8103 hydrophones connected to two B&K 2635 charge amplifiers. The hydrophones were placed in a rigid holder spaced by exactly 2 cm. The holder was rotated to measure the particle motion in all three dimensions. In all cases, the particle motion in the vertical direction was the most prominent. Particle acceleration was computed from the pressure gradient over the two hydrophones [11], and the ratio of pressure to particle acceleration was used to scale the pressure-derived ABR thresholds to the corresponding vertical particle acceleration at the head of the lungfish. Sound pressure and particle motion were relatively constant (±2 dB) in the horizontal plane in the tank, but the particle motion varied by 6 dB/5 cm in the vertical plane.
For the measurement of sensitivity to air-borne sound, the fish were transferred to a small, wet sandbox placed in the centre of an anechoic room. They were stimulated by sound emitted from a laterally placed loudspeaker (JBL 1G calibrated with a B&K ½ inch microphone) 1 m from the animal. Vibration sensitivity measurements were also performed in the anechoic room, with the head of the animal placed on a calibrated vibration exciter (B&K 4809).
3. Results
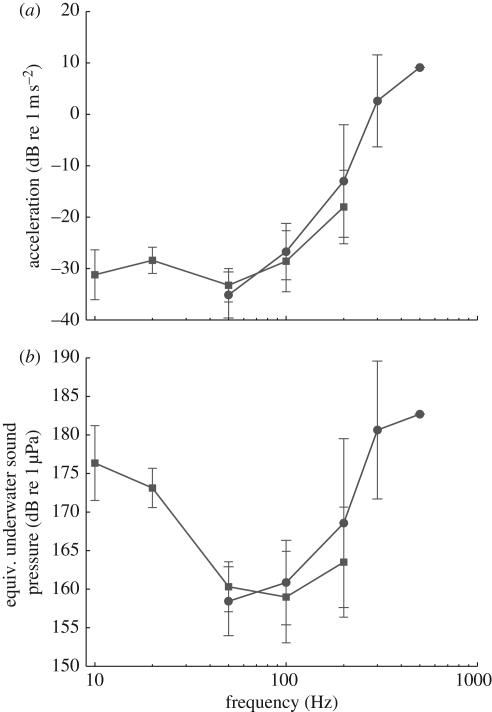
For the air-borne sound field, the stimulation range was limited by the equipment to frequencies above 100 Hz. No response could be measured for any of the frequencies of up to 1 kHz of sound pressures up to approximately 110 dB re 20 µPa (r.m.s.). However, ABR responses to head vibrations (10–200 Hz) and underwater sound (50–500 Hz) were collected from five animals (figure 2a). The lowest thresholds of approximately 2 cm s−2 were found at frequencies around 50 Hz. The sensitivities to head vibration and underwater sound stimulation when measured as acceleration are very similar. There is thus no evidence for special sensitivity to the pressure component of the sound field and no increased pressure sensitivity at the resonance frequency of the lungs (approx. 300 Hz, judged by their volume). In the audiogram shown in figure 2b, the vibration thresholds are recalculated as equivalent far field underwater sound pressure levels. The resulting audiogram is V-shaped with the best frequency at approximately 50 Hz. Thresholds increase above 100 Hz and is approximately 10 dB higher at 200 Hz than at the frequency of best hearing. The sensitivity in units of particle acceleration would translate to sound pressure levels from 150 to 190 dB re 1 µPa in an acoustic free field.
Figure 2.
ABR-derived audiogram of the African lungfish (n = 5). The two curves are measurements of mean masked ABR thresholds to head vibration (squares) and to underwater sound (circles). (a) Thresholds measured as particle acceleration. (b) The thresholds plotted are equivalent free-field sound pressures in dB RMS re 1 µPa, i.e. the free-field sound pressure corresponding to the vibration thresholds as derived with ABR. Note that these evoked potential thresholds are probably at least 10–30 dB above psychophysical thresholds.
4. Discussion
The overlap in acceleration thresholds in air and water strongly suggests that lungfish hearing is based on the detection of the particle motion component of sound. In contrast, the pressure component is apparently not transduced, and there is very little sensitivity to air-borne sound. However, sound-induced vibrations of the substrate or the skull may stimulate the ear in both air and water. We propose that the lack of pressure sensitivity is due to poor coupling between the lungs and the auditory system, and a lack of specialized sensory regions in the inner ear responding to frequencies above some 100 Hz. Thus, the lungfish seem to hear like the non-specialized ray-finned fishes, such as dab (Limanda limanda; [12]), where the ear operates as an accelerometer with little or no coupling to gas-filled structures that can act as a pressure to particle motion transducer.
The audiogram of the dab, as measured by heart-rate conditioning, has generally the same shape as found here for the lungfish, but the thresholds are considerably higher for the lungfish. The main explanation for this discrepancy is probably that ABR measurements depend on synchronous responses in large groups of nerve cells. In a variety of animals, ABR thresholds are at least 10–30 dB above the thresholds of the most sensitive neurons [13]. The vibration sensitivity (best ABR thresholds of 2 cm s−2) for lungfish is similar to that of two frog species (Rana temporaria and Bombina orientalis, ABR thresholds around 1 cm s−2) measured using the same methodology in a pilot study, suggesting that the pronounced vibration sensitivity of the frog ear [14] may be an ancestral trait.
The finding of an ear that is unresponsive to higher frequencies in the closest relative to tetrapods leads to two scenarios for the evolution of the tetrapod inner ear. Both are hypothetical, since the structure of the early tetrapod inner ear is presently unknown [3,15].
(1) The early lungfish had a more diversified inner ear, more similar to the ears of recent tetrapods with patches of hair cells uncovered by otoconia and possibly sensitive to higher frequencies. The structure of the ear of recent lungfish therefore reflects independent reduction (e.g. by paedomorphosis). (2) The ear of early tetrapods resembled the ear of recent lungfish, with essentially no biologically relevant hearing sensitivity above a few hundred hertz and a reasonably well-developed vibration sensitivity resembling the sensitivity of modern-day tetrapods and non-specialized actinopterygian bony fishes. It has been suggested that the coelacanth ear has some similarities with the tetrapod ear. For example, Fritzsch [16] has proposed that the basilar papilla in frogs should be homologous to an area in the inner ear of the coelacanth. This hypothesis would agree with scenario 1, but the evidence for homology is weak [7].
In scenario 2, we propose that the evolution of high-frequency sensitivity could have proceeded by initial diversification of the fused sacculo-lagenar inner-ear organ, creating a new patch of sensory cells. This patch could be derived from sensory regions such as the extrastriolar region between the saccule and lagena reported by Platt et al. [7], and it is interesting that this extrastriolar region receives separate innervation [7]. Following the emergence of high-frequency sensitivity, the ear could increase its sensitivity to skull vibrations, for example, by using the middle ear bone as an inertial element [17], which would require a movable link between middle ear bone and otic capsule. The final step would be the connection of the middle ear bone to the skin covering the spiracle, creating a tympanic ear. During the roughly 100 Myr when tetrapods were terrestrial, but atympanate, the main mechanisms of hearing would probably be by sound-induced vibrations of the skull, i.e. similar to bone conduction in humans or the extratympanic hearing in frogs [3].
Acknowledgements
The experiments were approved by the Danish Animal Experimentation Committee.
Professor Tobias Wang kindly provided the lungfish. The research was supported by Widex (C.B.), Oticon (M.Wi.) and grants from the Danish Natural Science Council to J.C.D., M.Wa. and P.T.M.
References
- 1.Clack J. A. 1989. Discovery of the earliest-known tetrapod stapes. Nature 342, 425–427 10.1038/342425a0 (doi:10.1038/342425a0) [DOI] [PubMed] [Google Scholar]
- 2.Clack J. A. 1997. The evolution of tetrapod ears and the fossil record. Brain, Behav. Evol. 50, 198–212 10.1159/000113334 (doi:10.1159/000113334) [DOI] [PubMed] [Google Scholar]
- 3.Christensen-Dalsgaard J., Carr C. E. 2008. Evolution of a sensory novelty: tympanic ears and the associated neural processing. Brain Res. Bull. 75, 365–370 10.1016/j.brainresbull.2007.10.044 (doi:10.1016/j.brainresbull.2007.10.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalmijn J. 1989. Functional evolution of lateral line and inner ear sensory systems. In The mechano sensory lateral line (eds Coombs S., Görner P., Münz H.), pp. 187–215 New York, NY: Springer [Google Scholar]
- 5.Popper A. N., Fay R. R. In press. Rethinking sound detection by fishes. Hear. Res. (doi:10.1016/j.heares.2009.12.023) [DOI] [PubMed] [Google Scholar]
- 6.Popper A. N., Fay R. R., Platt C., Sand O. 2003. Sound detection mechanisms and capabilities of teleost fishes. In Sensory processing in aquatic environments (eds Collin S. P., Marshall N. J.), pp. 3–38 New York, NY: Springer [Google Scholar]
- 7.Platt C., Jørgensen J. M., Popper A. 2004. The inner ear of the lungfish Protopterus. J. Comp. Neurol. 3, 277–288 [DOI] [PubMed] [Google Scholar]
- 8.Zardoya R., Meyer A. 1997. Molecular phylogenetic information on the identity of the closest living relative(s) of land vertebrates. Naturwissenschaften 84, 389–397 10.1007/s001140050415 (doi:10.1007/s001140050415) [DOI] [PubMed] [Google Scholar]
- 9.Corwin J. T., Bullock T. H., Schweitzer J. 1982. The auditory brain stem response in five vertebrate classes. Electroencephalogr. Clin. Neurophysiol. 54, 629–641 10.1016/0013-4694(82)90117-1 (doi:10.1016/0013-4694(82)90117-1) [DOI] [PubMed] [Google Scholar]
- 10.Brandt C., Andersen T., Christensen-Dalsgaard J. 2008. In Auditory signal processing in hearing-impaired listeners. Int. Symp. on Auditory and Audiological Research (eds Dau T., Buchholz J. M., Harte J. M., Christiansen T. U.), pp. 241–274 Copenhagen, Denmark: Centertryk [Google Scholar]
- 11.Christensen-Dalsgaard J., Breithaupt T., Elepfandt A. 1990. Underwater hearing in the clawed frog Xenopus laevis. Tympanic motion studied with laser vibrometry. Naturwissenschaften 77, 135–137 10.1007/BF01134478 (doi:10.1007/BF01134478) [DOI] [PubMed] [Google Scholar]
- 12.Chapman C. J., Sand O. 1974. Field studies of hearing in two species of flatfish, Pleuronectes platessa and Limanda limanda. Comp. Biochem. Physiol. 47, 371–385 [DOI] [PubMed] [Google Scholar]
- 13.Brittan-Powell E. F., Christensen-Dalsgaard J., Tang Y., Carr C., Dooling R. J. 2010. The auditory brainstem response in two lizard species. J. Acoust. Soc. Am. 128, 787–794 10.1121/1.3458813 (doi:10.1121/1.3458813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen-Dalsgaard J., Jørgensen M. B. 1988. The response characteristics of vibration-sensitive saccular fibers in the grassfrog, Rana temporaria. J. Comp. Physiol. A 162, 633–638 10.1007/BF01342638 (doi:10.1007/BF01342638) [DOI] [PubMed] [Google Scholar]
- 15.Clack J. A., Allin E. 2004. The evolution of single- and multiple-ossicle ears in fishes and tetrapods. In Evolution of the vertebrate auditory system (eds Manley G. A., Popper A. N., Fay R. R.), pp. 128–163 New York, NY: Springer [Google Scholar]
- 16.Fritzsch B. 1999. Hearing in two worlds. Theoretical and actual adaptive changes of the aquatic and terrestrial ear. In Comparative hearing: fish and amphibians (eds Fay R. R., Popper A. N.), pp. 15–42 New York, NY: Springer [Google Scholar]
- 17.Lombard R. E., Bolt J. 1979. Evolution of the tetrapod ear: an analysis and reinterpretation. Biol. J. Linn. Soc. 11, 19–76 10.1111/j.1095-8312.1979.tb00027.x (doi:10.1111/j.1095-8312.1979.tb00027.x) [DOI] [Google Scholar]