Abstract
Young men who have sex with men (YMSM) of color are at particularly increased risk for HIV infection compared to white MSM. National data highlight the need to link YMSM of color to care to improve their overall health and stem further infections, yet, there is limited data on interventions and clinical outcomes focused on engaging and retaining youth, specifically HIV-infected YMSM of color in care. To address the medical care needs of this underserved population, in 2005, the Health Research and Services Administration (HRSA) created the YMSM of Color Initiative. Utilizing a social marketing campaign targeting youth and members of their sexual and social networks, testing and outreach on college campuses and within the broader community, and a tightly linked medical–social support network, we created STYLE (Strength Through Youth Livin’ Empowered), a novel intervention that sought to diagnose, engage, and retain HIV-positive black and Latino YMSM in HIV primary care services. Over a 3-year period, 81 men were either newly diagnosed or reengaged in care. Overall, 63% of the cohort was retained in clinical care; defined as attending at least one medical visit every 4 months. Compared to the 3 years prior to STYLE, the odds ratio for whether or not someone attended a clinic visit was 2.58 (95% confidence interval [CI] 1.34–4.98) if enrolled in STYLE. We conclude that compared to a pre-STYLE cohort, STYLE was an effective intervention that increased HIV diagnoses, provided efficient and timely engagement in care for both those newly diagnosed and those who had fallen out of care and improved overall retention.
Introduction
Young men who have sex with men (YMSM) of color are at particularly increased risk for HIV infection compared to white MSM. From 2001 to 2006, a 12.4% increase in the number of HIV/AIDS diagnoses among all black MSM was observed, with an increase of 93.1% observed among black MSM aged 13–24 years.1 In a 7-city study of young MSM age 15–22, HIV prevalence was greatest among young MSM of color: 14% among non-Hispanic blacks, 12% among mixed race, 7% among Hispanic/Latinos compared to only 3% among whites.2
In addition to elevated rates of HIV acquisition and transmission and engagement in high-risk sexual behaviors, youth aged 15–24 have the lowest utilization of medical office visits of any age group and this rate has actually decreased over the period from 1995 to 2005.3 Among those aged 20–29, men have lower rates of utilization of ambulatory and preventive care compared to women. Moreover, for both males and females, black and Hispanic individuals have lower utilization rates than whites.4 We are thus missing crucial opportunities to counsel youth on prevention strategies and to provide HIV testing and linkage to care for those at risk for or infected with HIV.
Youth diagnosed with HIV face a wide variety of problems, many of which existed prior to and are exacerbated by HIV infection, including financial and housing instability, substance abuse, mental health concerns, stigma and isolation, as well as the impact of the disease itself on their overall health and wellness.5–11 Engagement and retention in care has been linked to improved health outcomes, better medication adherence and increased overall survival.12,13 One study of HIV-infected and at-risk youth found the probability of being retained in primary care beyond an initial visit for males was 64% and only 24% beyond 1 year.5 There are limited data on interventions and clinical outcomes focused on engaging and retaining youth, specifically HIV-infected YMSM of color in care.12,14–16
To address the medical care needs of this underserved population, in 2005, the Health Research and Services Administration (HRSA) created the YMSM of Color Initiative. The goal of the initiative was to design and test novel interventions to engage and retain young (ages 17–24) HIV-positive MSM of color in HIV care. Although the southeastern United States is experiencing disproportionate HIV infection rates, has higher numbers of AIDS cases, has higher proportions of blacks, and is experiencing the most rapid growth rate of Latinos in the country,17,18 there have been limited HIV interventions in this part of the country.19 A previously unrecognized outbreak of HIV infection among black YMSM college students in North Carolina was discovered,20 and through December 2006, 191 HIV-infected college students of whom 84% were black and 92% MSM or men who have sex with men and women were identified.20,21 To address these findings, the University of North Carolina School of Medicine, Division of Infectious Diseases (UNC-ID) developed STYLE (Strength Through Youth Livin’ Empowered). Utilizing a social marketing campaign targeting youth and members of their sexual and social networks targeted outreach to venues where YMSM congregate, and tailored HIV support services, we sought to diagnose, engage, and retain HIV-positive black and Latino YMSM in HIV primary care services. The primary goal of the study was to improve retention in HIV care for YMSM, with the hypothesis that YMSM of color will demonstrate improved retention compared to historical controls if HIV services are specifically targeted to this community. Moreover, we hypothesized that newly diagnosed men would have better retention in care compared to those who had previously been in care but had been out for 6 months or more.
Methods
Program description
The main elements of STYLE included: (1) a social marketing campaign developed with the input of a youth advisory board and focus groups; (2) intensified outreach to black and Latino MSM youth-serving venues and increased provision of HIV testing services on college campuses, and within the broader community utilizing both venue-based and social and sexual network testing approaches;22 and (3) a tightly linked medical–social support network for HIV-infected youth newly diagnosed or reengaging in care that included an infectious disease board-certified physician who oversaw the provision of care to all patients; the majority being seen at one of two clinical sites (one tied to a local academic medical center and the other located within a local health department). Prior to the initiation of STYLE, there were no services specifically provided for young HIV-positive MSM of color in the local area. STYLE was designed to address the previously unmet needs of this population by providing an array of services above and beyond the standard of care. These services included, a peer outreach worker, a case manager and as well as members of the research staff that formed a medical–social support network for the youth, the creation of weekly support group meetings, and availability of members of the research staff by text and/or phone to assist with appointment scheduling or to answer medical questions. HIV-positive YMSM of color identified at STYLE sponsored HIV testing events or through HIV testing conducted through partner agencies and local health departments received an appointment with the physician within 72 h, creating a near-immediate connection to medical care. Partner agencies and health departments had a strong incentive to refer YMSM to our program because it was the only program with services specifically targeting YMSM of color in the region. While both black and Latino HIV-infected YMSM were recruited into the study, printed outreach materials bearing the STYLE logo were targeted specifically for black MSM; a similar campaign was not designed for Latino MSM. However, other HIV/STD related information distributed at STYLE events and at STYLE clinical sites were made available in Spanish. In addition to HIV medical care services, STYLE provided clients with ancillary social support services, including case management and support groups, through a partnership with a local AIDS Service Organization (ASO). STYLE was able to leverage the resources of an academic medical center, an ASO and a local historically black University (HBU) as part of an overall strategy to identify, test, and link HIV-positive YMSM of color into medical care.
Data collection
For the overall HRSA/SPNS project, eight study sites and one evaluation and support center was funded to assist local evaluations and conduct a multisite evaluation and these data are presented elsewhere.23 Each of the eight sites operated independently, using different strategies to conduct outreach, HIV testing and linkage to care for HIV-positive YMSM of color. The eight sites used a common data collection tool and common eligibility requirements to allow for cross-site comparisons. Data collected from the sites were entered into a secure Web-based data entry portal maintained by the evaluation center. STYLE-specific data collected between the start of our site's client enrollment in June 2006 and the end of the grant cycle in August 2009 were analyzed for this paper. Eligible participants were (1) biologically born male, (2) HIV positive, (3) diagnosed HIV positive within the past 6 months or reengaged in care after being out of care for at least 6 months, (4) a male who had sex with males, (5) self-identified as non-white, (6) between 17 and 24 years at the time of the first interview, and (8) able to provide written informed consent. Eligible participants were administered a standardized face-to-face interview by experienced interviewers at baseline and every 3 months thereafter. For newly diagnosed HIV-positive YMSM, baseline interviews were administered within 30 days of the client's initial visit with a physician. For clients reengaging in care, baseline interviews were administered within 30 days of their reengaging visit. Baseline interviews lasted approximately 1 hour and also included a qualitative interview component that was not conducted during the follow-up interviews, which averaged 30 min in duration. Potential participants were referred to the STYLE study through being identified as HIV positive through STYLE-sponsored outreach HIV testing events, the NC Screening and Tracing Active Transmission Acute HIV testing program (STAT)24 and through referrals from HIV testing conducted at local health departments and ASOs. Potential participants were also identified and referred to STYLE by the NC Disease Intervention Specialists (DIS). DIS conduct voluntary postdiagnosis interviews with all individuals with reported cases of HIV and syphilis. During the interview, they conduct a risk assessment, provide risk reduction information, and make referrals for medical care and case management. Although the DIS were not a formal part of the STYLE intervention, the strong ties between the university and the NC HIV/STD Prevention and Care Branch of the North Carolina Department of Health helped to make our project highly visible to the DIS, which facilitated the referral process. Over the course of the 3 years of participant recruitment, only two individuals who were approached about participating in the STYLE cohort study refused to participate. These two individuals stated that their discomfort with discussing issues related to HIV as the primary reason for refusing to participate in the study. Interviews were generally administered immediately after scheduled HIV clinic appointments. However, if a participant was unable to stay beyond the time allotted for his clinical appointment, study staff would make a separate appointment to meet with the participant to complete the interview, within a 2-week time period. Interviews were conducted in either English or Spanish based on participant preference. Participants were compensated $50.00 for completion of the baseline interview and $25.00 for each follow-up.
As the majority of eligible YMSM of color at the UNC-ID clinic during the study period consented to enroll in STYLE, and thus there was no comparison group, data were abstracted from a clinical cohort preceding the implementation of STYLE to serve as a control group. Abstracted data were restricted to the 30 black or Latino YMSM (age 17–24) who had their first visit in the UNC-ID HIV clinic between January 1, 2003 and December 31, 2005, as they were most similar to STYLE participants based on available demographic data. Because these patients were not participants in STYLE they did not complete any questionnaires. The data available for these patients are restricted to their demographic information (age, race, gender, and sexual identity), which was used to select them from the other patients receiving HIV care at UNC-ID clinic.
Clinical recommendations about attending regularly scheduled visits were similar during this time period as during the implementation of STYLE.
All participants provided written informed consent to participate in the study. The University of North Carolina Institutional Review Board (IRB), and the George Washington University IRB approved all instruments and protocols.
Study variables
A participant was considered newly diagnosed if they had no prior diagnosis of HIV infection before entering STYLE, and was considered reengaged in care if they entered STYLE after having been out of prior HIV clinical care for at least 6 months. All participants were analyzed in six periods of 4 months each (periods 1–6) from their entry into clinical care for up to a total of 2 years. Participants who entered STYLE later and consequently were not enrolled for at least a 2-year period were analyzed with five or fewer 4-month periods corresponding to the amount of time they were enrolled. A visit was defined as having had a medical care visit attended by participants in which a follow-up survey was completed or for which clinical data was abstracted (e.g., CD4 count, viral load). If both clinical data and follow-up survey data was absent for any given 4-month period that participant was considered to have missed their scheduled visit. Patients were considered retained in regular care if they had at least one visit per 4-month period. Conversely, patients were considered to not to be retained in regular care if they missed one of their scheduled visits. Our retention measure was broad enough to capture a wide range of care utilization patterns and reflects the HIV Department of Health and Human Services (DHHS) guideline recommendations for patients to have CD4 and viral loads drawn every 3–4 months.25
The questionnaire used was adapted from standardized tools, including the Young Men's Survey, the Youth Risk Behavior Survey, National HIV Behavioral Surveillance tools, the HIV Cost and Service Utilization Study, and previous SPNS and Adolescent Trials Network instruments. Client related factors measured included age, race/ethnicity, income, educational level, and sexual identity. The Center for Epidemiologic Studies Depression Scale (CES-D) was used to measure depressive feelings at baseline and behaviors and has been used extensively in medical, and nonpatient populations, including those at risk for or infected with HIV.26,27 Distance to care was estimated as the driving distance from each patient's home address zip code reported at the time of enrollment to his respective clinic site. Clinic addresses and zip codes were geo-coded using ArcGIS Version 9.3 (Environmental Systems Research Institute, Inc., Redlands, CA) with point-locations assigned to the centroid of zip codes. Driving distance was then calculated between points using the StreetMap Find Route Tool. Clinical health outcomes were obtained by a chart review of the participants' CD4 count, viral load, antiretroviral (ART) medication usage, and baseline ART resistance testing evaluations (genotypes). While the follow-up survey included most of the same measures as the baseline survey, it did not include many of the lengthy scales included in the baseline survey, most notably the CES-D scale used to measure depression, which precludes our ability to make longitudinal assessments of depression in our study.
Statistical analysis
Univariate and bivariate analyses were used to describe participant characteristics. We also compared retention in care for participants enrolled in STYLE to a cohort of similar age black and Latino male patients being seen in the same academic HIV clinic over the 3-year period prior to STYLE's creation. Visits across the 4-month periods were modeled longitudinally as a function of whether or not the participant was enrolled in STYLE. To model our binary outcome (if a visit was made or not) over the 4-month periods, we utilized the hierarchical generalized linear model (HGLM). Normal hierarchical linear models take into account the nested structure of data, but are inappropriate to use when the dependent variable is not continuous. Similarly, logistic regression should be used for binary dependent variables, but is inappropriate to use when there is a nested structure in the data (e.g. repeated measures in individuals over time). HGLM, however, can be used in the analysis of multilevel categorical dependent variables.28 HGLM has been used to model condom use and HIV status disclosure in longitudinal data, and has also been used in a similar manner to our analysis to model program retention.29,30 Analyses were conducted using SAS software, version 9.2 (SAS Institute Inc., Cary, NC) and HLM software, version 6.08 (Scientific Software International Inc., Lincolnwood, IL).
Results
Demographic characteristics
Eighty-one HIV-infected YMSM of color were enrolled in STYLE. The mean age of the sample was 21 years; 83% identified as black and 11% as Latino. Sixty-two percent described themselves as gay, 22% as bisexual, 1% as heterosexual, and 15% as other. Two thirds of the men reported a history of vaginal sex with a woman over the course of their lifetime. Almost half of the sample was enrolled in school at study entry. Participants lived a mean of 47 miles from the HIV clinic where they are receiving care.
Two thirds of the cohort was newly diagnosed. The mean time from diagnosis to enrollment for those newly diagnosed was 56 days; the mean time from last clinic visit to enrollment for those reengaging in care was 509 days (or approximately 17 months). The majority (75%) of newly diagnosed persons had been diagnosed less than 3 months prior to enrollment. One third of the STYLE cohort was diagnosed during the acute stage of HIV infection, defined as having a negative HIV antibody test in the presence of positive HIV nucleic acid testing.24,31 Twelve percent of the sample had transmitted drug resistance, defined as having a baseline genotype that demonstrated the presence of at least one mutation in the 2009 World Health Organization revised listing of surveillance drug resistance mutations.32 As shown in Table 1, there were no differences in ethnicity or education when comparing newly diagnosed to those reengaged in care. However, compared to those participants who were reengaged in care, newly diagnosed persons were on average younger, had less depressive symptomatology and reported lower levels of financial distress. Health status data indicate that compared to those newly diagnosed, those reengaging in care had similar CD4 counts but slightly lower viral loads at baseline.
Table 1.
Characteristics of Newly Diagnosed and Recently Reengaged Persons in STYLE Cohort
| Newly diagnosed n = 52 | Reengaged in care n = 29 | Test statistica | p Value | |
|---|---|---|---|---|
| Age | 20.7 | 21.9 | −2.81 | 0.006 |
| Race | 0.63 | 0.73 | ||
| Black, % | 80.8 | 86.2 | ||
| Latino, % | 11.5 | 10.3 | ||
| Multiracial/other, % | 7.7 | 3.5 | ||
| Sexual Identity | ||||
| Homosexual/gay | 63.4 | 58.6 | 0.18 | 0.67 |
| Bisexual | 26.9 | 13.8 | 1.86 | 0.17 |
| Heterosexual | 1.9 | 0 | 0.56 | 0.45 |
| Otherb | 7.7 | 27.6 | 0.02 | |
| Comfort with sexual identity | 1.26 | 0.53 | ||
| Very comfortable | 58.8 | 46.4 | ||
| Comfortable | 37.3 | 46.4 | ||
| Uncomfortable | 3.9 | 7.2 | ||
| Education (%) | 4.71 | 0.09 | ||
| <High school | 19.2 | 24.1 | ||
| High school or GED | 26.9 | 6.9 | ||
| >High school | 53.9 | 69.0 | ||
| Distance to clinic, miles (mean) | 51.3 | 38.7 | 0.95 | 0.35 |
| Had health insurance, % | 59.6 | 44.8 | 1.64 | 0.20 |
| Baseline CD4 count (mean) | 520.1 | 568.1 | −0.66 | 0.51 |
| Baseline CD4, % | 3.24 | 0.36 | ||
| <200 | 11.8 | 10.7 | ||
| 200–350 | 15.7 | 21.4 | ||
| 351–500 | 27.4 | 10.7 | ||
| >500 | 45.1 | 57.1 | ||
| Baseline viral load (log10) | 4.4 | 3.7 | 2.43 | 0.02 |
| Substance use in last 30 days | ||||
| Alcohol | 55.8 | 55.6 | <0.01 | 0.99 |
| Marijuana | 46.2 | 35.7 | 0.81 | 0.37 |
| Any other recreational drug use | 19.2 | 14.3 | 0.31 | 0.58 |
| CES-D (mean) | 15.4 | 20.4 | −2.01 | 0.05 |
| Suicide, % | ||||
| Ever made a plan | 13.5 | 31.0 | 3.63 | 0.06 |
| Ever attempted | 7.7 | 27.6 | 5.84 | 0.02 |
| Ran out of money (last 3 months), % | 71.2 | 82.8 | 11.74 | 0.02 |
| Study enrollment, days (mean) | 357.5 | 389.4 | −0.39 | 0.70 |
For continuous variables a t-statistic was used, for categorical variables a χ2 was used.
Other includes: DL, confused/deciding (2), me (4), a man with a diverse sexual preference, I do what I do, I don't label/identify myself (2), open-minded.
CES = D, Center for Epidemiologic Studies Depression Scale.
Outcomes after enrollment
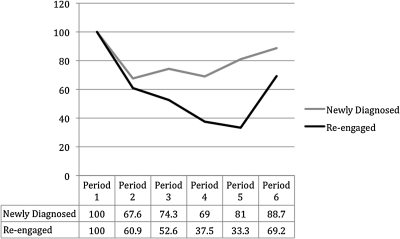
Overall, 63% of the STYLE cohort was retained in clinical care (67% in the newly diagnosed group and 55% in the re-engaging group made all of their scheduled visits). Figure 1 shows the percent attending visits in each four-month period. Among the newly diagnosed group, 84% of all scheduled visits were made, compared to 73% of scheduled visits made in the reengaged group (t statistic = 1.96; p = 0.05). Importantly, among those who missed at least one 4-month visit, and were therefore considered not retained in regular clinical care, among the newly diagnosed, participants still made 73% of their visits, and the reengaged still made 67% of their visits. The two most common reasons cited for missing appointments included forgetting and having issues with transportation.
FIG. 1.
Retention over 2 years of newly diagnosed or recently reengaged young men who have sex with men (MSM) of color in STYLE cohort.
Sixty-two percent of subjects (n = 50) initiated ART during the course of their enrollment in STYLE. Most participants, 68% (n = 34) were started on a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen consisting of the only currently available single tablet regimen, 30% (n = 15) were placed on a boosted protease inhibitor (PI) regimen and one subject was started on an integrase inhibitor-based regimen. Thirty-four of the 50 subjects on ART were enrolled prior to August 31, 2008 and thus had the ability of having at least 1 year of follow-up data. Seventy-nine percent (n = 29), 75% (n = 24), and 76% (n = 21) of subjects were suppressed (viral loads <200 copies), at 3, 6, and 12 months, respectively. The mean change in CD4 count for all persons over the course of the study was an increase in 100 cells/mm3 (n = 79). Notably, the percentage of persons with CD4 counts 350 or more increased from 71% at baseline to 85% at study end. While not statistically significant there was a trend towards improved clinical outcomes in the newly diagnosed subjects compared to those recently reengaged in care (Table 2). The mean study enrollment time in days for those on ART was 385.6, and was not significantly different from 352.2 for those not on ART.
Table 2.
Clinical Outcomes of Newly Diagnosed and Recently Reengaged Persons in STYLE Cohort
| Variable | Newly diagnosed N = 52 | Reengaged N = 29 | Total N = 81 | Test-statistic | p Value |
|---|---|---|---|---|---|
| Started ART | 32 (61.5%) | 18 (62.1%) | 50 (61.7%) | 0.002 | 0.96 |
| Suppresseda at: | |||||
| 3 monthsb | 82.4% (n = 17) | 75% (n = 12) | 79.3% (n = 29) | <0.01 | >0.99 |
| 6 monthsb | 78.6% (n = 14) | 70% (n = 10) | 75% (n = 24) | <0.01 | >0.99 |
| 12 monthsb | 91.7% (n = 12) | 55.6% (n = 9) | 76.2% (n = 21) | 1.67 | 0.19 |
| Change in CD4 countc | N = 51 | N = 28 | N = 79 | 0.41 | 0.68 |
| Mean [SD] | 109.5 [244.9] | 88.1 [174.4] | 101.9 [221.5] | 0.41 | 0.68 |
| Median | 59 | 15.5 | 41 | 0.41 | 0.68 |
Viral load <200.
Only includes those clients who had at least one year of follow-up.
Includes all clients who at least one CD4 count measured. For those with only one value, a change of 0 was used.
ART, antire troviral; SD, standard deviation.
Comparison to prior clinic data
Thirty black or Latino MSM (age 17–24) had their first visit in the HIV clinic between January 1, 2003 and December 31, 2005. The pre-STYLE cohort attended 67% of their visits, compared to 80% of attended visits by those enrolled in STYLE (t statistic = 2.16; p = 0.03). The results from the longitudinal analysis of whether or not someone attended a clinic visit shows that the odds ratio for STYLE is 2.58 (95% CI 1.34–4.98) compared to the years pre-STYLE cohort. For both the pre-STYLE and STYLE cohorts there was a decreased likelihood of attending visits as time passed, for both groups, the odds of attending the next 4-month visit decreased 31% (95% CI 0.61, 0.79). The effect of STYLE did not change across time.
Conclusions
Prior studies have identified predictors of missed clinic visits and poor retention in care to include being young, non-white, and having a history of substance abuse or mental illness.33–35 Using a conservative measure of retention, two-thirds of our cohort was retained in care; an impressive achievement considering our population was young (mean age of 21), 83% African American, and nearly half reported drug use and/or depressive symptoms. Furthermore, when compared to the 3-year period prior to STYLE, there was a 75% increase the number of new diagnoses among black and Latino MSM aged 17–24 in the 12-county region of the state surrounding where the STYLE intervention occurred (Communicable Disease Branch, NC Division of Public Health, personal communication). While we cannot conclude that STYLE was responsible for the increased number of infections detected in YMSM of color during that time period, the increased awareness around HIV, provision of outreach and testing events on college campuses and throughout the larger community as well as the development of community partnerships to foster immediate linkage to care cannot be discounted as insignificant.
A thorough review of the current HIV literature could not find a standard and consistent measure of what constitutes retention in care, thus it is hard to make comparisons across studies. Sherer et al.36 found that 55% of adult HIV-infected patients had at least one primary care visit in each 6-month period over a 2-year span. Other studies using this same definition found rates of 60–81% over a 1-year period when intensive case management and outreach strategies were utilized.15,37 We chose a conservative measure of visits every 4 months as our primary outcome, however, if a similar measure as described above is used, our retention rate increases from 63% to 85% (data not shown). Moreover, even people who did not meet our strict definition of regular care still attended the majority of their scheduled clinic appointments and maintained consistent contact with program staff through email, SMS texting or attendance at weekly support group meetings. Text messaging and other innovative technologies have been used successfully to increase clinic attendance,38,39 improve medication adherence,40 assist adults and adolescents with chronic disease management,41,42 and promote healthy behaviors such as diet, exercise and smoking cessation.43–45 Future research should evaluate the use of mobile phone and Internet-based interventions such as adherence counseling, and the ability to schedule walk-in appointments online as a way to maintain greater communication and connection between youth and their HIV care providers.
Consistent with other studies8,11 we found high overall levels of depression in these young men; with 50% having CES-D scores falling within ranges considered to be indicative for clinical depression and 15% having a history of attempting suicide. This is similar to rates seen in other large population-based studies in which 12–19% of their sample of MSM (including a rate of 8% in MSM younger than 25 years) had attempted suicide compared with rates of 1.5–4% among men in the general population.46–48 Higher rates of depression were observed in those reengaging in care clearly indicating the need to incorporate mental health evaluations and treatment early into the provision of HIV primary care. This difference in mental health status between the newly diagnosed and reengaging participants may partially explain the higher retention rate among the newly diagnosed. However, because CES-D scores were only assessed at baseline, we cannot establish causality, though we hypothesize that early engagement in care through STYLE for the newly diagnosed persons may have served as a buffer to lessen symptoms of depression perhaps through increasing their network of social support.11,49–51 Future research using qualitative methods should explore the relationship between early engagement and retention in care, depression, and other ongoing risk behaviors in HIV-infected youth.
Long distances to care and lack of provision of transportation services are associated with less access to and retention in care.52,53 Patients in this study traveled a substantial distance for their HIV medical care, which is not uncommon for those living in rural settings accessing services associated with stigmatizing diseases like HIV, substance abuse, or mental health. This problem was likely accentuated by the low availability of public transportation systems and record high gas prices seen in our state during the course of the study. Additionally, newly diagnosed persons reported lower levels of financial distress compared to those who were reengaging. Previous studies have shown the importance of reducing financial barriers for successful retention in care.15 Future studies should assess whether provision of reimbursement for travel through taxi vouchers or gas cards could result in overall cost savings through improved long term health outcomes and decreased utilization of emergency rooms and a reduction in hospitalizations.54
National data highlight the need to link MSM of color and other HIV-infected populations to care to improve their overall health and to stem further infections.55–58 The DHHS HIV guidelines now recommend earlier initiation of ART to prevent long-term complications and preserve immune function.25,59–61 In our study, youth achieved viral suppression rates greater than 75%, which compares favorably to levels of virologic success ranging from 51% to 79% for currently utilized NNRTI, or boosted PI regimens in adult cohorts.62 While we tried to ensure that prior to initiating therapy, youth enrolled in our cohort were deeply committed to the process of attending regular clinic visits and comfortable with both the notion and the process of taking medications on a daily basis, there is still significant room for improvement. Having the youth as active and willing participants in making decisions regarding their health care—an act that requires a multidisciplinary team model of HIV care that addresses in a comprehensive and culturally sensitive manner all of the developmental, physical and mental health issues—is relevant to this population.
The study has the following limitations. The generalizability of our findings to other populations and regions of the United States may be limited as our sample size was relatively small and participants were located in one geographic area. However, our population is reflective of the current epidemic of HIV infection within this country, where a significant burden of disease falls upon minorities, youth, and those residing in the southern United States.63,64 Furthermore, as one of the main goals of the overall SPNS initiative was to increase diagnoses within our target population, the youth in our study were followed for different periods of time based on when they were diagnosed. Although the measure of retention we utilized accounted for time enrolled in STYLE, since participants enrolled at different times throughout the study period it does not control for possible effects specific to any given month or year. Furthermore, we did not measure exposure to or uptake of our outreach materials or participation in events and are thus unable to assess whether this component of STYLE had an effect on retention in care. Additionally, there is no ideal control group to which STYLE can be compared. We utilized data from similar aged Black and Latino MSM in the same clinic in the period directly preceding STYLE, but there is always a possibility that a temporal effect, and not STYLE, was responsible for differences in retention. Furthermore, the limited information we were able to collect on this comparison group did not allow us to statistically control for other variables that might also be related to retention. Finally, we relied on self-report and the survey was administered face-to-face, thus bias may have been introduced.
While there has been a significant focus on increasing testing for HIV, the importance of timely linkage to and engagement in care, and knowing one's CD4 count, viral load, and other clinical parameters once diagnosed have not received such high priority national attention. Media campaigns that promote the value of not just knowing one's HIV status but the importance of being in regular care if infected are needed. Clinic support staff should make it a priority to spend extra time with new patients helping them understand the significance of learning and interpreting all of their laboratory results and being engaged with providers in an interactive conversation about their health.
We conclude that STYLE was able to provide efficient and timely engagement in care for both those newly diagnosed and those who had fallen out of care and improved overall retention compared to a pre-STYLE cohort. We believe that the results of our study demonstrate that successful interventions should promote HIV counseling, testing, and referral services at venues that youth frequent or use outreach to make testing easily accessible and linkage to care seamless. Future research should investigate both the barriers that preclude full engagement in care as well as the resiliency factors present among HIV-infected youth that promote consistent care over an extended follow-up interval.
Acknowledgments
Funding was provided by a Health Resources and Services Administration Special Projects of National Significance (HRSA SPNS) grant, #1 H97HA03789-01-00; and a National Institute of Mental Health (NIMH) K23 award, #5K23MH075718-02. We would like to thank the Men of STYLE for their sharing their time and for their participation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.CDC. Trends in HIV/AIDS diagnoses among men who have sex with men—33 states, 2001–2006. MMWR. 2008;57:681–686. [PubMed] [Google Scholar]
- 2.Valleroy LA. MacKellar DA. Karon JM, et al. HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group. JAMA. 2000;284:198–204. doi: 10.1001/jama.284.2.198. [DOI] [PubMed] [Google Scholar]
- 3.Cherry DK. Woodwell DA. Rechtsteiner EA. Advance data from vital and health statistics. Hyattsville, Maryland: National Center for Health Statistics; 2007. National Ambulatory Medical Care Survey: 2005 Summary. [PubMed] [Google Scholar]
- 4.Fortuna RJ. Robbins BW. Halterman JS. Ambulatory care among young adults in the United States. Ann Intern Med. 2009;151:379–385. doi: 10.7326/0003-4819-151-6-200909150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Harris SK. Samples CL. Keenan PM. Fox DJ. Melchiono MW. Woods ER. Outreach, mental health, and case management services: Can they help to retain HIV-positive and at-risk youth and young adults in care? Matern Child Health J. 2003;7:205–218. doi: 10.1023/a:1027386800567. [DOI] [PubMed] [Google Scholar]
- 6.Flicker S. Skinner H. Read S, et al. Falling through the cracks of the big cities: Who is meeting the needs of HIV-positive youth? Can J Public Health. 2005;96:308–312. doi: 10.1007/BF03405172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LK. Lourie KJ. Pao M. Children and adolescents living with HIV and AIDS: A review. J Child Psychol Psychiatry. 2000;41:81–96. [PubMed] [Google Scholar]
- 8.Pao M. Lyon M. D'Angelo LJ. Schuman WB. Tipnis T. Mrazek DA. Psychiatric diagnoses in adolescents seropositive for the human immunodeficiency virus. Arch Pediatr Adolesc Med. 2000;154:240–244. doi: 10.1001/archpedi.154.3.240. [DOI] [PubMed] [Google Scholar]
- 9.Giordano TP. White AC., Jr. Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Park WB. Choe PG. Kim SH, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: A predictor of clinical progress in HIV patients. J Intern Med. 2007;261:268–275. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 11.Lam PK. Naar-King S. Wright K. Social support and disclosure as predictors of mental health in HIV-positive youth. AIDS Patient Care STDs. 2007;21:20–29. doi: 10.1089/apc.2006.005. [DOI] [PubMed] [Google Scholar]
- 12.Giordano TP. Gifford AL. White AC, Jr, et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 13.Mugavero MJ. Lin HY. Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naar-King S. Green M. Wright K. Outlaw A. Wang B. Liu H. Ancillary services and retention of youth in HIV care. AIDS Care. 2007;19:248–251. doi: 10.1080/09540120600726958. [DOI] [PubMed] [Google Scholar]
- 15.Naar-King S. Bradford J. Coleman S. Green-Jones M. Cabral H. Tobias C. Retention in care of persons newly diagnosed with HIV: Outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21(Suppl 1):S40–48. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 16.Ashman JJ. Conviser R. Pounds MB. Associations between HIV-positive individuals' receipt of ancillary services and medical care receipt and retention. AIDS Care. 2002;14(Suppl 1):S109–118. doi: 10.1080/09540120220149993a. [DOI] [PubMed] [Google Scholar]
- 17.Kochhar R. Suro R. Tafoya S. The New Latino South: The context and consequences of rapid population growth. Washington DC: PEW Hispanic Center; Jul, 2005. pp. 1–91. [Google Scholar]
- 18.Rhodes SD. Hergenrather KC. Griffith DM, et al. Sexual and alcohol risk behaviours of immigrant Latino men in the South-eastern USA. Cult Health Sex. 2009;11:17–34. doi: 10.1080/13691050802488405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones KT. Gray P. Whiteside YO, et al. Evaluation of an HIV prevention intervention adapted for Black men who have sex with men. Am J Public Health. 2008;98:1043–1050. doi: 10.2105/AJPH.2007.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightow LB. MacDonald PD. Pilcher CD, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: Transmission among college students. J Acquir Immune Defic Syndr. 2005;38:531–537. doi: 10.1097/01.qai.0000155037.10628.cb. [DOI] [PubMed] [Google Scholar]
- 21.Hightow LB. Leone PA. Macdonald PD. McCoy SI. Sampson LA. Kaplan AH. Men who have sex with men and women: a unique risk group for HIV transmission on North Carolina College campuses. Sex Transm Dis. 2006;33:585–593. doi: 10.1097/01.olq.0000216031.93089.68. [DOI] [PubMed] [Google Scholar]
- 22.CDC. A Community-Based Strategy for Identifying Persons with Undiagnosed HIV Infection. Interim Guide for HIV Counseling, Testing, and Referral Programs. 2006. www.cdcgov/hiv/resources/guidelines/snt/ [Aug 19;2010 ]. www.cdcgov/hiv/resources/guidelines/snt/
- 23.Magnus M. Jones K. Phillips G, et al. Characteristics Associated With Retention Among African American and Latino Adolescent HIV-Positive Men: Results From the Outreach, Care, and Prevention to Engage HIV-Seropositive Young MSM of Color Special Project of National Significance Initiative. J Acquir Immune Defic Syndr. 2010;53:529–536. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 24.Pilcher CD. Fiscus SA. Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 25.DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Dec, 2009. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolsecentGL.pdf. [Aug 19;2010 ]. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolsecentGL.pdf
- 26.Reisner SL. Mimiaga MJ. Skeer M, et al. Clinically significant depressive symptoms as a risk factor for HIV infection among black MSM in Massachusetts. AIDS Behav. 2009;13:798–810. doi: 10.1007/s10461-009-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voss J. Portillo CJ. Holzemer WL. Dodd MJ. Symptom cluster of fatigue and depression in HIV/AIDS. J Prev Interv Community. 2007;33:19–34. doi: 10.1300/J005v33n01_03. [DOI] [PubMed] [Google Scholar]
- 28.Raudenbush SW. Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 29.Teti M. Bowleg L. Cole R, et al. A mixed methods evaluation of the effect of the protect and respect intervention on the condom use and disclosure practices of women living with HIV/AIDS. AIDS Behav. 2010;14:567–579. doi: 10.1007/s10461-009-9562-x. [DOI] [PubMed] [Google Scholar]
- 30.McGuigan WM. Katzev AR. Pratt CC. Multi-level determinants of retention in a home-visiting child abuse prevention program. Child Abuse Negl. 2003;27:363–380. doi: 10.1016/s0145-2134(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 31.Pilcher CD. Wohl DA. Hicks CB. Diagnosing primary HIV infection. Ann Intern Med. 2002;136:488–9. doi: 10.7326/0003-4819-136-6-200203190-00016. author reply 489. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DE. Camacho RJ. Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugavero MJ. Lin HY. Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr. 2009;50:100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napravnik S. Eron JJ., Jr. McKaig RG. Heine AD. Menezes P. Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(Suppl 1):S45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- 35.Katz MH. Cunningham WE. Mor V, et al. Prevalence and predictors of unmet need for supportive services among HIV-infected persons: Impact of case management. Med Care. 2000;38:58–69. doi: 10.1097/00005650-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Sherer R. Stieglitz K. Narra J, et al. HIV multidisciplinary teams work: support services improve access to and retention in HIV primary care. AIDS Care. 2002;14(Suppl 1):S31–44. doi: 10.1080/09540120220149975. [DOI] [PubMed] [Google Scholar]
- 37.Gardner LI. Metsch LR. Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 38.da Costa TM. Salomao PL. Martha AS. Pisa IT. Sigulem D. The impact of short message service text messages sent as appointment reminders to patients' cell phones at outpatient clinics in Sao Paulo, Brazil. Int J Med Inform. 2010;79:65–70. doi: 10.1016/j.ijmedinf.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Leong KC. Chen WS. Leong KW, et al. The use of text messaging to improve attendance in primary care: A randomized controlled trial. Fam Pract. 2006;23:699–705. doi: 10.1093/fampra/cml044. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds NR. Testa MA. Su M, et al. Telephone support to improve antiretroviral medication adherence: A multisite, randomized controlled trial. J Acquir Immune Defic Syndr. 2008;47:62–68. doi: 10.1097/QAI.0b013e3181582d54. [DOI] [PubMed] [Google Scholar]
- 41.Franklin V. Waller A. Pagliari C. Greene S. "Sweet Talk": text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technol Ther. 2003;5:991–996. doi: 10.1089/152091503322641042. [DOI] [PubMed] [Google Scholar]
- 42.Hazelwood AJ. Using text messaging in the treatment of eating disorders. Nurs Times. 2008;104:28–29. [PubMed] [Google Scholar]
- 43.Gerber BS. Stolley MR. Thompson AL. Sharp LK. Fitzgibbon ML. Mobile phone text messaging to promote healthy behaviors and weight loss maintenance: A feasibility study. Health Informatics J. 2009;15:17–25. doi: 10.1177/1460458208099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haug S. Meyer C. Schorr G. Bauer S. John U. Continuous individual support of smoking cessation using text messaging: A pilot experimental study. Nicotine Tob Res. 2009;11:915–923. doi: 10.1093/ntr/ntp084. [DOI] [PubMed] [Google Scholar]
- 45.Fjeldsoe BS. Marshall AL. Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36:165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 46.Cochran SD. Mays VM. Lifetime prevalence of suicide symptoms and affective disorders among men reporting same-sex sexual partners: Results from NHANES III. Am J Public Health. 2000;90:573–578. doi: 10.2105/ajph.90.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul JP. Catania J. Pollack L, et al. Suicide attempts among gay and bisexual men: Lifetime prevalence and antecedents. Am J Public Health. 2002;92:1338–1345. doi: 10.2105/ajph.92.8.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman MM. Bland RC. Canino GJ, et al. Prevalence of suicide ideation and suicide attempts in nine countries. Psychol Med. 1999;29:9–17. doi: 10.1017/s0033291798007867. [DOI] [PubMed] [Google Scholar]
- 49.Berkman LF. The role of social relations in health promotion. Psychosom Med. 1995;57:245–254. doi: 10.1097/00006842-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kelly JA. Murphy DA. Psychological interventions with AIDS and HIV: Prevention and treatment. J Consult Clin Psychol. 1992;60:576–585. doi: 10.1037//0022-006x.60.4.576. [DOI] [PubMed] [Google Scholar]
- 51.Murphy DA. Moscicki AB. Vermund SH. Muenz LR. Psychological distress among HIV(+) adolescents in the REACH study: Effects of life stress, social support, and coping. The Adolescent Medicine HIV/AIDS Research Network. J Adolesc Health. 2000;27:391–398. doi: 10.1016/s1054-139x(00)00158-0. [DOI] [PubMed] [Google Scholar]
- 52.Magnus M. Schmidt N. Kirkhart K, et al. Association between ancillary services and clinical and behavioral outcomes among HIV-infected women. AIDS Patient Care STDs. 2001;15:137–145. doi: 10.1089/108729101750123607. [DOI] [PubMed] [Google Scholar]
- 53.Arcury TA. Preisser JS. Gesler WM. Powers JM. Access to transportation and health care utilization in a rural region. J Rural Health. 2005;21:31–38. doi: 10.1111/j.1748-0361.2005.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 54.Whetten K. Reif S. Ostermann J, et al. Improving health outcomes among individuals with HIV, mental illness, and substance use disorders in the Southeast. AIDS Care. 2006;18(Suppl 1):S18–26. doi: 10.1080/09540120600839330. [DOI] [PubMed] [Google Scholar]
- 55.Auvert B. Males S. Puren A. Taljaard D. Carael M. Williams B. Can highly active antiretroviral therapy reduce the spread of HIV?: A study in a township of South Africa. J Acquir Immune Defic Syndr. 2004;36:613–621. doi: 10.1097/00126334-200405010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Cock KM. Crowley SP. Lo YR. Granich RM. Williams BG. Preventing HIV transmission with antiretrovirals. Bull World Health Organ. 2009;87:488-A. doi: 10.2471/BLT.09.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granich R. Crowley S. Vitoria M, et al. Highly active antiretroviral treatment for the prevention of HIV transmission. J Int AIDS Soc. 2010;13:1. doi: 10.1186/1758-2652-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granich RM. Gilks CF. Dye C. De Cock KM. Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 59.Lundgren JD. Babiker A. El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: Role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 60.Phillips AN. Carr A. Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: Exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 61.Siegfried N. Uthman OA. Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 3:CD008272. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartlett JA. Buda JJ. von Scheele B, et al. Minimizing resistance consequences after virologic failure on initial combination therapy: A systematic overview. J Acquir Immune Defic Syndr. 2006;41:323–331. doi: 10.1097/01.qai.0000197070.69859.f3. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report: 2007. 2008. p. 19.
- 64.Rangel MC. Gavin L. Reed C. Fowler MG. Lee LM. Epidemiology of HIV and AIDS among adolescents and young adults in the United States. J Adolesc Health. 2006;39:156–163. doi: 10.1016/j.jadohealth.2006.02.011. [DOI] [PubMed] [Google Scholar]