Abstract
HIV clinical care now involves prevention and treatment of age-associated comorbidity. Although physical function is an established correlate to comorbidity in older adults without HIV infection, its role in aging of HIV-infected adults is not well understood. To investigate this question we conducted cross-sectional analyses including linear regression models of physical function in 3227 HIV-infected and 3240 uninfected patients enrolled 2002–2006 in the Veterans Aging Cohort Study-8-site (VACS-8). Baseline self-reported physical function correlated with the Short Form-12 physical subscale (ρ = 0.74, p < 0.001), and predicted survival. Across the age groups decline in physical function per year was greater in HIV-infected patients (βcoef −0.25, p < 0.001) compared to uninfected patients (βcoef −0.08, p = 0.03). This difference, although statistically significant (p < 0.01), was small. Function in the average 50-year old HIV-infected subject was equivalent to the average 51.5-year-old uninfected subject. History of cardiovascular disease was a significant predictor of poor function, but the effect was similar across groups. Chronic pulmonary disease had a differential effect on function by HIV status (Δβcoef −3.5, p = 0.03). A 50-year-old HIV-infected subject with chronic pulmonary disease had the equivalent level of function as a 68.1-year-old uninfected subject with chronic pulmonary disease. We conclude that age-associated comorbidity affects physical function in HIV-infected patients, and may modify the effect of aging. Longitudinal research with markers of disease severity is needed to investigate loss of physical function with aging, and to develop age-specific HIV care guidelines.
Introduction
Evidence for increased risk of age-associated conditions in HIV-infected patients, including coronary artery disease and cardiovascular risk factors,1–4 chronic pulmonary disease,5–7 low bone mineral density,8 and frailty9 supports the concern that chronic HIV infection and prolonged antiretroviral therapy might be associated with an accelerated aging process.2,5,7,10,11 In adults without HIV, history of these age-associated conditions is an independent risk factor for decline in physical function with aging.12–16 The purpose of this study was to investigate the effect of age-associated conditions on physical function in HIV-infected patients compared to uninfected patients with similar demographic characteristics and medical care setting. Prior to combination antiretroviral therapy (cART) physical function in HIV-infected patients was studied in younger adults with AIDS.17 Limitation with activities of daily living (ADL) was common, and was associated with leg muscle atrophy and weakness in the setting of AIDS wasting syndrome.18 Research in the cART era shows that ADL capacity is preserved in more than 90% of the HIV-infected patients, but these patients remain limited in vigorous activities (exercise, walking hills, heavy work).19,20 The prevalence of age-associated conditions, such as chronic lung disease and coronary artery disease, was associated with decreased self-reported physical function in a 2001–2002 cross-sectional study of 889 HIV-infected and 647-uninfected veterans.20 Association of the conditions with function was independent of age, race, and smoking history and was not affected by HIV status. However, only 88 (10%) of the HIV-infected patients were 60 years of age and older. We hypothesized that older HIV-infected patients would have greater limitations in function compared to older uninfected patients, and that this effect would be amplified by cardiac and pulmonary disease. We conducted a cross-sectional study to investigate the relationship of self-reported physical function with prevalent age-associated conditions defined by ICD-9 codes at the baseline visit for HIV-infected and -uninfected patients enrolled in the Veterans Aging Cohort Study- 8 site (VACS-8), an ongoing longitudinal study.21 Mortality data currently available during follow-up were used to test construct validity of function survey items.
Methods
VACS-8 participants are enrolled prospectively from eight VA Medical Centers and include patients with HIV infection followed in infectious disease clinics, and age and race group-matched uninfected patients followed in general medicine clinics.21 All participants provided written informed consent for the protocol, which is approved by the respective Institutional Review Board (IRB) and VA research and development committees at each site. Enrollment began in 2002 and is ongoing. Patients provide information on physical activity, smoking, alcohol use, illicit drug use, health-related quality of life (SF-12), and demographic characteristics at study entry and then yearly. Clinical and administrative data are abstracted from the VA electronic medical record using methodology validated by chart review21 (details available, www.vacohort.org). Comorbid conditions are defined by ICD-9 diagnostic codes and require at least one inpatient or two outpatient encounters within 12 months prior to or 6 months after study enrollment to be considered present at baseline.22 For this analysis comorbid conditions were chosen based on available ICD-9 data, and plausible relationship with physical function in HIV-infected patients20 and uninfected patients.12 For instance, despite the increased risk of low bone mineral density8,23 and certain cancers in HIV-infected patients, and the probable impact on function, these conditions were not included since VACS-8 data are not yet available. Patients were classified with chronic obstructive lung disease if they had a diagnosis of chronic obstructive pulmonary disease (COPD; including ICD-9 codes for bronchitis and emphysema) or asthma, based on VACS research focused on obstructive lung disease and relationship to smoking and mortality.24,25 The analytic set includes baseline data collected from 2002–2006 in 3227 HIV-infected patients and 3240 uninfected patients. Only 213 individuals were exluded because of incomplete survey items for physical function. In the HIV group CD4 cell count, HIV-1 viral load, and hemoglobin concentration at baseline were available for 90% of the patients.
Physical function was quantified using the VACS function scale, a measure derived by summation of patient report on 12 questions included in the VACS-5 survey20 and originally adapted from the HIV Cost and Services Utilization Study (HCSUS; available online, www.vacohort.org). The questions span the continuum from basic activities of daily living (feeding, bathing, and dressing one's self) to instrumental activities of daily living (light, moderate, and heavy types of work), mobility (walking a few steps, walking inside, and walking one block) and vigorous activity (walking uphill, running, sports). Patients report their current ability to perform each physical activity. For analysis of VACS-8 data, we refined the previously used scale20 to facilitate comparison with physical function scales in the HIV literature.26,27 First, we scored the items so that higher numbers represented greater capability to perform the activity (unable to do = 0, yes but slowly = 1, yes = 2). Then, we transformed the raw scale to a scale of zero to 100 by dividing the sum by the maximum possible score (24) and multiplying by 100. The interitem consistency of the scale and domains were tested using Cronbach α and factor analysis. To assess the content validity we tested the VACS function scale and the physical component scale of the SF-12 by Spearman's ρ. To assess construct validity, we performed survival analyses to determine whether baseline physical function measured by the VACS function scale predicted death through the most recent follow-up visit.
Differences in clinical characteristics between HIV-infected and uninfected patients were assessed using log-linear models adjusted for demographic factors after initial univariate comparisons by t-test or chi-squared test. The function scale was used as the main outcome measure in linear regression models. Parallel models stratified by HIV status tested the association of function with independent variables (demographics, lifestyle factors, comorbidity) for each patient group. Differences in the β coefficient of the independent variables between HIV-positive and HIV-negative models were tested using a z statistic. To test for effect modifiers, independent variables that were significantly different (p < 0.05) in the stratified analysis were included in a single combined multivariable model with all subjects and an HIV-interaction term. In order to provide a clinical context for the impact of effect modifiers (x), age equivalent function in an uninfected subject was calculated for a 50-year-old HIV-infected subject. First we computed the predicted function in HIV-infected subjects alone (γHIV = β0 + β1age + β2x) using age = 50 years. Then using this function outcome (yHIV) we solved for age of uninfected subjects using coefficients derived from the model in uninfected subjects alone.
Cox regression was used to test the association of mortality with baseline physical function, categorized as impaired (lowest tertiary, score < 67), moderate limitations (score 67–99), and no limitations (score = 100). Eight subjects did not have any follow-up data. The remaining subjects were included in the survival analysis that included event data through follow-up visit 4. Subjects were censored at the date of their last survey. Cox proportional hazards models were tested for proportionality assumption and were valid. Significance was defined as a two-tailed α of 0.05.
Results
Study population
The age distribution was similar in HIV-infected and uninfected patients (mean years ± standard deviation [SD], 49.5 ± 8.7 versus 50.7 ± 10.0). Differences in lifestyle factors and comorbid conditions between groups adjusted for demographic factors (age, race, gender) are summarized in Table 1. HIV-infected patients reported exercising less frequently than uninfected patients (p = 0.03). When this analysis was stratified by age, exercise frequency remained lower among older HIV-infected patients (age ≥ 55 years, p = 0.05), but was similar between younger HIV-infected and uninfected patients (age ≤ 44 years, p = 0.1).
Table 1.
Description of Study Population by Demographic and Clinical Characteristics
| |
HIV uninfected N = 3147 |
HIV infected N = 3107 |
|
||
|---|---|---|---|---|---|
| Characteristic | n | % Total | n | % Total | p Valuea |
| Age, years | |||||
| ≤ 44 | 785 | 25.0 | 849 | 27.3 | <0.001 |
| 45–49 | 699 | 21.3 | 728 | 23.4 | |
| 50–54 | 701 | 22.3 | 696 | 22.4 | |
| ≥ 55 | 992 | 31.5 | 834 | 26.8 | |
| Gender, male | 2897 | 92.1 | 3028 | 97.5 | <0.001 |
| Race | |||||
| Black | 1951 | 62.0 | 2070 | 66.6 | <0.001 |
| White | 776 | 24.7 | 625 | 20.1 | |
| Hispanic | 313 | 10.0 | 295 | 9.5 | |
| Other | 107 | 3.4 | 117 | 3.8 | |
| Lifestyle factors: | |||||
| Weekly exerciseb | 0.03 | ||||
| Never | 330 | 10.5 | 395 | 12.8 | |
| < 1 times | 569 | 18.2 | 526 | 17.1 | |
| 1–2 times | 831 | 26.5 | 759 | 24.7 | |
| 3–4 times | 781 | 24.9 | 822 | 26.7 | |
| ≥ 5 times | 623 | 19.9 | 575 | 18.7 | |
| Body mass index (BMI, kg/m2) | <0.001 | ||||
| Underweight (BMI < 18.5) | 38 | 1.2 | 106 | 3.4 | |
| Normal (BMI 18.6–24.9) | 703 | 22.5 | 1446 | 46.9 | |
| Overweight (BMI 25.0-29.9) | 1225 | 39.2 | 1149 | 37.3 | |
| Obese (BMI ≥ 30) | 1161 | 37.1 | 380 | 12.3 | |
| Smoker, current | 1380 | 43.8 | 1,650 | 53.1 | <0.001 |
| Substance abusec | |||||
| History of alcohol disorders | 724 | 23.0 | 583 | 18.7 | <0.001 |
| History of injection drug use | 483 | 15.5 | 1032 | 33.6 | <0.001 |
| Comorbidity: | |||||
| Congestive heart failure | 105 | 3.3 | 61 | 2.0 | <0.001 |
| Coronary artery disease | 318 | 10.1 | 158 | 5.1 | 0.006 |
| Diabetes | 716 | 22.8 | 397 | 12.8 | <0.001 |
| Hepatitis C | 482 | 15.3 | 968 | 31.2 | <0.001 |
| Hypertension | 1668 | 53.0 | 933 | 30.0 | <0.001 |
| Major depression | 465 | 14.8 | 420 | 13.5 | 0.3 |
| Chronic pulmonary diseased | 351 | 11.2 | 295 | 9.5 | 0.2 |
| Peripheral vascular disease | 116 | 3.7 | 60 | 1.9 | 0.003 |
| Stroke | 119 | 3.8 | 66 | 2.1 | 0.001 |
p Value for difference between HIV-uninfected and HIV-infected patients, χ2 tests used for demographic characteristics, log linear regression models adjusted for demographics used for lifestyle and clinical characteristics.
How often engage in regular activities (e.g., brisk walking, jogging, etc.) long enough to work up a sweat?
History of alcohol disorders by ICD-9 codes; Injection drug use, prior self-reported use.
Pulmonary disease included chronic obstructive pulmonary disease (bronchitis and emphysema) or asthma.
Function scale
Factor analysis showed that the VACS physical function scale had a single domain with high degree of interitem consistency (Cronbach α = 0.9). A ceiling effect was present; 35% of the subjects reported no limitations in physical activities (score of 100). The VACS physical function score correlated with the SF-12 physical subscale (ρ = 0.74 p < 0.001), which was unchanged when stratified by HIV group. Physical function score (adjusted mean ± standard error [SE]), was significantly higher in patients who exercised weekly 5 or more times (86.0 ± 0.8), 3–4 times (85.0 ± 0.7), 1–2 times (81.8 ± 0.8), and less than once (77.0 ± 0.8) compared to patients who never exercised (68.0 ± 0.9; all p < 0.001). The median follow-up time was 5.2 years (interquartile range [IQR] 4.2–5.7 years). During this time, 560 (18.0%) of the HIV-infected patients and 232 (7.4%) of the uninfected patients died. Patients with functional impairment (those scoring below 67, lowest tertile) had a two-fold increased risk of death compared to patients without any functional impairment (score = 100), adjusted for effects of demographic and baseline clinical characteristics (Table 2).
Table 2.
Baseline Physical Function Independently Predicts Mortality
| |
|
Death |
||||
|---|---|---|---|---|---|---|
| |
Total |
|
Unadjusted |
Adjusteda |
||
| Physical function at baseline | N | N (%) | HR | 95% CI | HR | 95% CI |
| No limitations (score = 100) | 2217 | 169 (7.6) | 1.0 | 1.0 | ||
| Intermediate limitations | 2535 | 327 (12.9) | 1.68 | 1.40, 2.03 | 1.37 | 1.13, 1.65 |
| Severe limitations (score < 67, lowest 20%) | 1494 | 296 (19.8) | 2.67 | 2.21, 3.22 | 1.96 | 1.60, 2.39 |
| Total | 6246 | 792 (12.7) | ||||
Adjusted for demographic characteristics, baseline lifestyle factors, and comorbid conditions.
HR, hazard ratio; CI, confidence interval.
HIV infection, age, and physical function
There was no significant difference in the mean physical function score between HIV-infected patients (mean ± SD; 82.6 ± 0.4) and uninfected patients (82.4 ± 0.3). In the combined multivariable model including demographic and clinical factors for all subjects, physical function was significantly lower in HIV-infected patients compared to uninfected patients, but the effect was very modest (βHIV −1.3, 95% confidence interval [CI] [−2.3, −0.2] p = 0.02). In the stratified HIV model higher HIV-1 viral load (log10 copies per milliliter) was associated with worse function but the effect was small and did not remain significant in the multivariable model (Table 3). Patients with a hemoglobin ≤ 12 g/dL had on average a 7-point lower score than those with higher hemoglobin. Patients who were within 180 days of starting combination antiretroviral therapy (cART, 3 or more antiretroviral medications) at baseline had worse function compared to patients not receiving cART. However, there was no significant difference for patients who had been on cART for greater than 180 days.
Table 3.
Association of Physical Function with HIV-Related Factors Based on a Multivariable Linear Regression Model Including Demographic and Clinical Variables Listed in Table 1
| Characteristic | N | b | 95% CI | p Value |
|---|---|---|---|---|
| CD4 count: | ||||
| ≤ 200 cells/mm3 | 687 | −2.6 | −5.0, −0.2 | 0.03 |
| 201–500 cells/mm3 | 1338 | 0.5 | −1.3, 2.4 | 0.6 |
| > 500 cells/mm3 | 880 | — | — | — |
| HIV-1 RNA, per log10 copies/mL | 2535 | −0.05 | −0.75, 0.66 | 0.8 |
| Hemoglobin | ||||
| ≤ 12 gm/dL | 444 | −4.9 | −7.2, −2.6 | < 0.001 |
| > 12 gm/dL | 2470 | — | — | |
| cART history in prior year: | ||||
| Cumulative use, days | ||||
| None | 525 | — | — | — |
| 1–180 | 622 | −3.4 | −6.07, −0.67 | 0.02 |
| 181–364 | 1001 | −1.7 | −4.21, 0.71 | 0.1 |
| 365 | 801 | −1.2 | −3.94, 1.46 | 0.4 |
cART, combination antiretroviral therapy; CI, confidence interval.
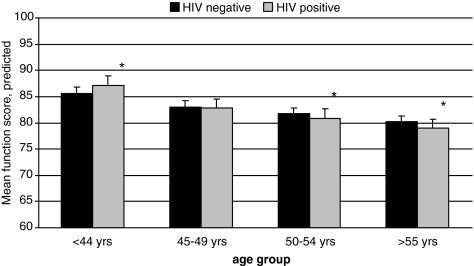
The decline in physical function score associated with each additional year of age was three times greater for HIV-infected patients (βHIV+ −0.25, 95% CI [−0.33, −0.17]) compared to uninfected patients (βHIV- = −0.08, 95% CI [−0.16, −0.01]; Table 4). Stratified analysis by age group and HIV status showed better function in the younger (≤44 years) HIV-infected patients compared to the uninfected (p < 0.01), and worse function in the older (>55 years) HIV-infected patients compared to the uninfected (p < 0.01; Fig. 1). Although the HIV-age interaction was statistically significant (HIV*age ßcoeff = −0.17, p < 0.01), function differences were small. In terms of equivalent years without adjustment for comorbidity, the average 50-year-old HIV-infected subject had the same level of function as the average 51.5-year-old uninfected subject.
Table 4.
Multivariable Linear Regression Models of Physical Function in HIV-Infected and Uninfected Subjects
| |
HIV uninfected N = 3126 |
HIV infected N = 3081 |
Difference (HIV infected-uninfected) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| b | 95% CI | p Value | b | 95% CI | p Value | Delta | SE | p Valuea | |
| Age, per year | −0.08 | (−0.16, −0.01) | 0.03 | −0.2 | (−0.33, −0.17) | < .0001 | −0.17 | 0.06 | < 0.01 |
| Gender, female | −1.4 | (−3.87,1.14) | 0.29 | −4.7 | (−8.97, −0.35) | 0.03 | −3.30 | 2.54 | 0.19 |
| Race | |||||||||
| Black | 1.4 | (−0.19,3.01) | 0.09 | 0.8 | (−1.01,2.54) | 0.40 | −0.64 | 1.22 | 0.60 |
| Hispanic | −0.2 | (−2.67,2.27) | 0.87 | −2.7 | (−5.34,0.03) | 0.05 | −2.45 | 1.86 | 0.19 |
| Lifestyle factors: | |||||||||
| Body mass index (BMI, kg/m2) | |||||||||
| Underweight (BMI < 18.5) | −1.8 | (−7.89,4.26) | 0.56 | −6.2 | (−9.99, −2.41) | < 0.01 | −4.39 | 3.65 | 0.23 |
| Overweight (BMI 25.0–29.9) | 0.5 | (−1.26,2.24) | 0.58 | 1.3 | (−0.21,2.78) | 0.09 | 0.79 | 1.17 | 0.50 |
| Obese (BMI ≥ 30) | −2.6 | (−4.39, −0.73) | 0.01 | −0.9 | (−3.12,1.38) | 0.45 | 1.69 | 1.48 | 0.25 |
| Current smoker | −4.0 | (−5.4, −2.51) | < 0.0001 | −2.4 | (−3.8, −0.96) | < 0.01 | 1.58 | 1.03 | 0.13 |
| History of alcohol disorders | 0.6 | (−1.1,2.39) | 0.47 | −1.4 | (−3.28,0.48) | 0.14 | −2.04 | 1.31 | 0.12 |
| Comorbid conditions: | |||||||||
| Congestive heart failure | −6.1 | (−9.95, −2.32) | < 0.001 | −9.5 | (−14.49, −4.51) | < 0.001 | −3.37 | 3.20 | 0.29 |
| Coronary artery disease | −5.6 | (−8, −3.29) | < 0.0001 | −7.8 | (−10.99, −4.53) | < .0001 | −2.11 | 2.04 | 0.30 |
| Diabetes | −5.2 | (−6.88, −3.56) | < 0.0001 | −1.8 | (−3.88,0.38) | 0.11 | 3.47 | 1.37 | 0.01 |
| Hepatitis C | −2.2 | (−4.08, −0.26) | 0.03 | −3.8 | (−5.37, −2.25) | < 0.0001 | −1.64 | 1.26 | 0.19 |
| Hypertension | −1.6 | (−3.04, −0.1) | 0.04 | −0.02 | (−1.65,1.61) | 0.98 | 1.55 | 1.12 | 0.17 |
| Major depression | −8.2 | (−10.14, −6.32) | < 0.0001 | −4.9 | (−6.97, −2.85) | < 0.0001 | 3.32 | 1.44 | 0.02 |
| Pulmonary diseaseb | −3.8 | (−5.9, −1.69) | < 0.0001 | −7.3 | (−9.61, −4.93) | < 0.0001 | −3.48 | 1.60 | 0.03 |
| Peripheral vascular disease | −6.7 | (−10.28, −3.18) | < 0.01 | −7.2 | (−12.22, −2.13) | 0.01 | −0.44 | 3.15 | 0.89 |
| Stroke | −6.3 | (−9.85, −2.83) | < 0.001 | −6.7 | (−11.49, −1.97) | 0.01 | −0.39 | 3.02 | 0.90 |
p value between groups.
Pulmonary disease includes chronic obstructive pulmonary disease (bronchitis and emphysema) or asthma.
Reference groups: white race, normal BMI (18.6-24.9), former/never smoker, and for each comorbidity, patients without the condition based on ICD-9 codes.
CI, confidence interval.
FIG. 1.
Mean (standard error [SE]) of predicted physical function score by HIV status and age group based on multivariable models. *p < 0.05.
Comorbid conditions and physical function
HIV-infected and uninfected patients with a history of cardiovascular disease, including congestive heart failure, coronary artery disease, peripheral vascular disease, and stroke had on average function scores 9–12 points lower than patients without the condition (Table 4). Diagnosis of hypertension was significantly associated with worse physical function controlled for demographic factors (βHTN −2.8, 95% CI [−3.9, −1.8]) but was no longer significant in the multivariable model. These relationships were similar in HIV-infected and uninfected patients.
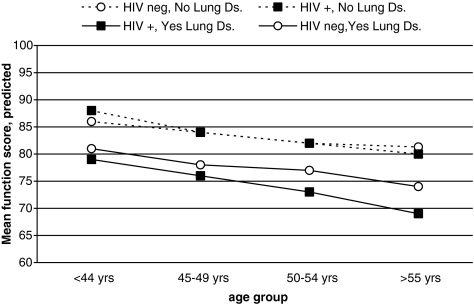
The independent effect of the history of chronic obstructive lung disease on function was greater in HIV-infected patients (Fig. 2). Physical function adjusted for demographic, lifestyle, and comorbidity factors was comparable in HIV-infected and uninfected patients without pulmonary disease across all age groups. However, among patients with pulmonary disease, HIV-infected patients had significantly worse function compared to uninfected patients; a 50-year old HIV-infected subject had the equivalent level of function as a 68.1-year old uninfected subject. These results were confirmed in the full multivariable model; the interaction term for HIV and pulmonary disease was significant (HIV*pulmonary ßcoeff = −3.6, 95% CI [−6.6, −0.4], p = 0.03).
FIG. 2.
Mean predicted physical function score by HIV status for patients with and without lung disease (lung ds.) by subject age group based on multivariate models.
Obesity, based on body mass index (BMI) ≥ 30, was associated with poor function in uninfected patients but not in HIV-infected patients (Table 4). Being underweight (BMI < 18.5) was associated with poor function in HIV-infected patients, but not in uninfected patients. However, these differences in the relationship of BMI group and function by HIV status were small and were not statistically significant (Table 4).
Among HIV-infected patients, history of diabetes was not associated with function when adjusted for effect of BMI and comorbidity (Table 4). However, in uninfected patients diabetes was significantly related to function. This differential effect of diabetes on function by HIV status was statistically significant, and reflected the finding that a 50-year-old diabetic HIV-infected subject had the equivalent level of function as a 36-year-old diabetic uninfected subject.
Discussion
In this cross-sectional study we compare physical function of HIV-infected patients to uninfected patients who are demographically similar and under care in the same medical system. The baseline function score, based on patient reported ability to perform a range of physical activities, correlates with the established SF-12 physical subscale, and is associated with differential survival. The majority of patients in this clinic-based cohort are 50 years of age and older in both patients groups, a frequently used benchmark to designate older HIV-infected adults. This opportunity allowed us to investigate the relationship of age and function with comorbidity between HIV-infected and uninfected patients.
Within the limits of a cross-sectional study, the difference in function between younger and older patients was greater in HIV-infected patients compared to the uninfected patients, adjusted for comorbidity. The magnitude of the rate of decline in function across the age groups was greater in the HIV-infected patients. In both the 50–54 and 55 + age groups physical function was worse in the HIV-infected patients. These results are supported by exercise performance testing that shows significantly lower aerobic capacity among older HIV-infected patients compared to age-matched uninfected adults.28 It should be noted in the younger (age ≤ 44 years) age group that HIV-infected patients reported higher function than uninfected patients. Only this age group of HIV-infected patients had similar frequency of exercise compared to the uninfected patients. This finding raises the question of the role of physical inactivity in worse physical function among older HIV-infected patients.
In the general medical literature, poor physical function is strongly associated with cardiovascular disease (CVD), including coronary artery disease, congestive heart failure, peripheral vascular disease, and stoke.12,13,15,16 For all of these conditions we found a significant independent association with function in HIV-infected patients that was similar to uninfected patients. Given that HIV-infected patients may have increased risk of coronary heart disease and cardiac dysfunction,1,3,29 CVD will likely become a significant source of physical disability in HIV-infected patients who are otherwise stable on cART, and thus provides additional incentive to reduce cardiac risk factors.4 Although none of the CVD conditions in our study were associated with worse function in HIV-infected patients compared to uninfected patients, our function scale may be unable to distinguish these differences given the scale's ceiling effect. In addition, self-report in general may be limited in its capacity to measure specific functional performance parameters, such as endurance, which are related to cardiovascular disease. For instance, exercise treadmill testing has shown that aerobic capacity is reduced 16% in older HIV-infected men with hypertension compared to those without hypertension.30 Further research is needed to investigate the specific mechanisms underlying poor function for different types of CVD, and whether differences exist between HIV-infected and uninfected patients.
In contrast, the VACS function score clearly showed an additive effect of chronic obstructive lung disease and HIV on physical function. The results were consistent across the age groups with adjustment for other comorbid conditions and smoking history. Chronic pulmonary disease is independently related to functional limitations in uninfected adults,31,32 and may occur more frequently in HIV-infected adults.5–7 Our results suggest that among those with chronic obstructive lung disease, HIV-infected patients have worse physical function compared to uninfected patients. However, conclusions should be tempered given the lack of information on lung function. Physical function in HIV-infected patients among those with chronic pulmonary disease could be worse due to either accelerated progression33 or longer duration of lung disease. A third possibility is a confounding factor related to both chronic pulmonary disease and function. Recent research shows that the adjusted risk of lung cancer, pulmonary hypertension and pulmonary fibrosis is greater in HIV-infected compared to uninfected patients.6 Although these conditions may be less common, they are associated with chronic lung disease and were not considered in our analyses. While understanding these mechanisms is beyond the scope of this study, the results support an HIV aging interaction driven by comorbidity that warrants further investigation. With regard to HIV care, this finding supports the importance of smoking cessation.24,34
Finally, the contrasting results for BMI and diabetes in HIV-infected versus uninfected patients highlights the challenge of differentiating effects of medication, HIV infection, and aging. HIV-infected patients classified as obese by BMI likely represent a heterogeneous group, which includes those experiencing a restoration to health phenomenon that comes with successful antiretroviral therapy. This supposition is supported by the Nutrition for Healthy Living study, a prospective longitudinal study that showed that HIV-infected men with a five kilogram or larger increase in total body weight reported improvement in physical function.26 Importantly, HIV-infected individuals have experienced the effects of obesity for a shorter period of time than uninfected subjects since they were likely thinner prior to receiving cART treatment. The attenuated negative association of diabetes with function in the HIV-infected group supports this possibility. However, our findings are limited without data on duration or severity of diabetes, and need to be investigated further. In addition, anthropometrics may provide information that is missed by measure of BMI alone.35
Our findings confirm that advanced HIV disease is associated with worse physical function. However, in comparison to earlier studies which focused on the effect of AIDS on function,17 the majority of HIV-infected VACS participants receive cART and have high CD4 cell counts. Our findings demonstrate that age-related comorbidity should be considered an important risk factor for poor physical function in this clinical setting. For example, history of congestive heart failure is independently associated with a 10-point lower function score, compared to a low CD4 cell count (<200 cells/cm3), which is associated with a 3-point lower score. Unlike our preliminary study,20 in this larger cohort with over 1450 patients with hepatitis C infection, the relationship between hepatitis C and function was similar in HIV-infected and uninfected patients (Table 4). The absence of a significant interaction was confirmed in the full multivariable model (HIV*HCV ßcoeff = −1.7, p = 0.1). However, further work is needed to investigate this relationship as we defined hepatitis C infection by ICD-9 code and did not differentiate cases by ongoing viral replication, nor severity of liver disease.
The primary limitation of the study is related to the cross-sectional design. We report a decline in function with age that that compares individuals at different ages, not a within-person difference in rate of decline. Therefore, findings could reflect selection or cohort effects, and require confirmation in longitudinal analysis. An additional limitation is the definition of comorbid conditions by history only, without data on disease severity and duration. While most cross-sectional studies are limited to prevalent cases, it is possible that duration and severity of some comorbid conditions may be greater in HIV-infected patients and then translate to worse function. Self-reported limitations in physical activities allow for measure of function within the social context, but can be affected by reporting bias. This may be evident given the large proportion of patients that denied any physical limitations. Although this ceiling effect is very similar to a survey study on function in community dwelling HIV-infected patients,26 it limits the ability to investigate higher level of functioning. While the study results are subject to these limitations in terms of causal inference, they provide important direction for future research in aging and physical function.
In summary, age-associated comorbidity affects physical function in HIV-infected patients. Longitudinal research with measure of disease incidence and severity is needed to determine if there is an accelerated loss of function with aging. However, our results highlight the potential role of comorbidity as an effect modifier in the relationship of HIV and aging. The study supports further integration of primary health care and prevention into HIV care with increased focus on age-associated comorbidity.36
Acknowledgments
Supported by the National Institutes of Health (NIH) K23AG024896 (K.K.O.); U01AA13566 (A.C.J.); R01HL090342 (K.C.); R01MH058984 (S.C.); University of Maryland Claude D. Pepper Older Americans Independence Center P60AG 028747 (K.K.O., L.I.K., J.D.S., K.F.), and Department of Veterans Affairs Baltimore Geriatric Research, Clinical and Education Center (L.I.K., J.D.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friis-Moller N. Reiss P. Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA. Lee H. Hadigan C. Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunfeld C. Delaney JA. Wanke C, et al. Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adeyemi O. Rezai K. Bahk M. Badri S. Thomas-Gossain N. Metabolic syndrome in older HIV-infected patients: Data from the CORE50 cohort. AIDS Patient Care STDs. 2008;22:941–945. doi: 10.1089/apc.2008.0119. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K. Butt AA. Gibert CL. Rodriguez-Barradas MC. Crystal S. Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 6.Crothers K. Huang L. Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. doi: 10.1164/rccm.201006-0836OC. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz PT. King MA. Pacht ER. Wewers MD. Gadek JE. Nagaraja HN et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten JH. Freeman R. Howard AA. Floris-Moore M. Lo Y. Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21:617–623. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desquilbet L. Jacobson LP. Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 10.Effros RB. Fletcher CV. Gebo K. Halter JB. Hazzard WR. Horne FM et al. Aging and infectious diseases: Workshop on HIV infection and aging: What is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips AN. Carr A. Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: Exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 12.Markides KS. Stroup-Benham CA. Goodwin JS. Perkowski LC. Lichtenstein M. Ray LA. The effect of medical conditions on the functional limitations of Mexican-American elderly. Ann Epidemiol. 1996;6:386–391. doi: 10.1016/s1047-2797(96)00061-0. [DOI] [PubMed] [Google Scholar]
- 13.Kriegsman DM. Deeg DJ. Stalman WA. Comorbidity of somatic chronic diseases and decline in physical functioning: The Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57:55–65. doi: 10.1016/S0895-4356(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP. Tangen CM. Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs Z. Blumstein T. Novikov I, et al. Morbidity, comorbidity, and their association with disability among community-dwelling oldest-old in Israel. J Gerontol A Biol Sci Med Sci. 1998;53:M447–M455. doi: 10.1093/gerona/53a.6.m447. [DOI] [PubMed] [Google Scholar]
- 16.Kiely DK. Morris JN. Morris SA, et al. The effect of specific medical conditions on functional decline. J Am Geriatr Soc. 1997;45:1459–1463. doi: 10.1111/j.1532-5415.1997.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 17.Crystal S. Fleishman JA. Hays RD. Shapiro MF. Bozzette SA. Physical and role functioning among persons with HIV: Results from a nationally representative survey. Med Care. 2000;38:1210–1223. doi: 10.1097/00005650-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon S. Corcoran C. Rosenthal D, et al. Quantitative assessment of cross-sectional muscle area, functional status, and muscle strength in men with the acquired immunodeficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 1999;84:201–206. doi: 10.1210/jcem.84.1.5375. [DOI] [PubMed] [Google Scholar]
- 19.Rusch M. Nixon S. Schilder A. Braitstein P. Chan K. Hogg RS. Impairments, activity limitations and participation restrictions: Prevalence and associations among persons living with HIV/AIDS in British Columbia. Health Qual Life Outcomes. 2004;2:46. doi: 10.1186/1477-7525-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oursler KK. Goulet JL. Leaf DA, et al. Association of comorbidity with physical disability in older HIV-infected adults. AIDS Patient Care STDs. 2006;20:782–791. doi: 10.1089/apc.2006.20.782. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC. Dombrowski E. Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC. Lasky E. McGinnis K, et al. Comorbid disease and alcohol use among veterans with HIV infection: A comparison of measurement strategies. Med Care. 2006;8(Suppl 2):S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 23.Womack J. Goulet J. Gibert C, et al. HIV-infection and Fragility Fracture Risk among Male Veterans [Paper#129]. 17th Conference on Retroviruses and Opportunistic Infections (CROI); 2010; [Google Scholar]
- 24.Crothers K. Goulet JL. Rodriguez-Barradas MC, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(3 Suppl):40–53. doi: 10.1521/aeap.2009.21.3_supp.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crothers K. Griffith TA. McGinnis KA, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson IB. Jacobson DL. Roubenoff R. Spiegelman D. Knox TA. Gorbach SL. Changes in lean body mass and total body weight are weakly associated with physical functioning in patients with HIV infection. HIV Med. 2002;3:263–270. doi: 10.1046/j.1468-1293.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu AW. Revicki DA. Jacobson D. Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6:481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 28.Oursler KK. Sorkin JD. Smith BA. Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22:1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 29.Hsue PY. Hunt PW. Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oursler KK. Katzel LI. Smith BA. Scott WB. Russ DW. Sorkin JD. Prediction of cardiorespiratory fitness in older men infected with the human immunodeficiency virus: Clinical factors and value of the six-minute walk distance. J Am Geriatr Soc. 2009;57:2055–2061. doi: 10.1111/j.1532-5415.2009.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannino DM. Ford ES. Redd SC. Obstructive and restrictive and functional limitation: Data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254:540–547. doi: 10.1111/j.1365-2796.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 32.Simpson CF. Punjabi NM. Wolfenden L. Shardell M. Shade DM. Fried LP. Relationship between lung function and physical performance in disabled older women. J Gerontol A Biol Sci Med Sci. 2005;60:350–354. doi: 10.1093/gerona/60.3.350. [DOI] [PubMed] [Google Scholar]
- 33.Meehan PS. Nagaraja H. Drake J. Martin K. Diaz T. Analysis of pulmonary function decline in two cohorts of hiv-infected subjects. Is HAART making a difference? Am J Respir Crit Care Med. 2010;181:A5202. [Google Scholar]
- 34.Drach L. Holbert T. Maher J. Fox V. Schubert S. Saddler LC. Integrating smoking cessation into HIV care. AIDS Patient Care STDs. 2010;24:139–140. doi: 10.1089/apc.2009.0274. [DOI] [PubMed] [Google Scholar]
- 35.Brown T. Wang Z. Chu H, et al. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2006;43:356–362. doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 36.Weiss JJ. Osorio G. Ryan E. Marcus SM. Fishbein DA. Prevalence and patient awareness of medical comorbidities in an urban AIDS clinic. AIDS Patient Care STDs. 2010;24:39–48. doi: 10.1089/apc.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]