Abstract
Nanodisks (ND) are nanoscale, disk-shaped phospholipid bilayers whose edge is stabilized by apolipoproteins. In the present study, ND were formulated with the bioactive polyphenol, curcumin, at a 6:1 phospholipid:curcumin molar ratio. Atomic force microscopy revealed that curcumin-ND are particles with diameters <50 nm and thickness of a phospholipid bilayer. When formulated in ND, curcumin is water-soluble and gives rise to a characteristic absorbance spectrum with a peak centered at 420 nm. Fluorescence spectroscopy of curcumin-ND provided evidence of self-quenching. Incubation of curcumin-ND with empty-ND relieved the self-quenching, indicating redistribution of curcumin between curcumin loaded- and empty-ND. In HepG2 cells, curcumin-ND mediated enhanced cell growth inhibition compared to free curcumin. In a cell culture model of mantle cell lymphoma, curcumin-ND were a more potent inducer of apoptosis than free curcumin. The nanoscale size of the complexes, combined with their ability to solubilize curcumin, indicates ND may have in vivo therapeutic applications.
Keywords: Curcumin, Nanodisk, Phospholipid, Apolipoprotein, Delivery
Background
Curcumin, known chemically as diferuloylmethane, is a hydrophobic polyphenol derived from rhizome of turmeric (Curcuma longa), an East Indian plant. Curcumin possesses diverse pharmacologic effects including anti-inflammatory, anti-oxidant and anti-proliferative activities.1–4 Indeed, accumulating experimental evidence indicates curcumin has intrinsic anticancer activity. Furthermore, curcumin is non-toxic, even at relatively high doses.5 Despite this, clinical advancement of this promising molecule has been hindered by poor water solubility, short biological half-life, and low bioavailability following oral administration.6,7 Attempts to overcome these limitations include synthesis of curcumin analogues,8 the use of adjuvants as well as development of improved delivery platforms such as liposomes and nanoparticles.9–12
Based on the structural properties of curcumin, it was identified as a candidate for formulation into nanodisks (ND), nanoscale particles comprised of a disk-shaped lipid bilayer that is stabilized by an apolipoprotein scaffold.13 ND self assemble in solution upon presentation of the scaffold protein to a phospholipid vesicle substrate to which an appropriate hydrophobic bioactive agent has been introduced. Apolipoprotein mediated re-organization of the phospholipid/bioactive agent substrate generates the ND product. ND are stabilized by interaction of the hydrophobic face of amphipathic α-helix segments of the apolipoprotein scaffold with fatty acyl moieties at the edge of the bilayer disk. At the same time, the hydrophilic face of these helices is directed toward the aqueous milieu, conferring water solubility to the complexes. Previous studies with the polyene antibiotic, amphotericin B14 and the bioactive isoprenoid, all trans retinoic acid,15 showed efficient incorporation into ND, with retention or enhancement of biological activity. In the present study curcumin was formulated in ND and characterized with respect to structural properties and biological activity.
Methods
Materials
Curcumin was obtained from Cayman Chemical (Ann Arbor, MI) and used without further purification. Dimyristoylphosphatidylcholine (DMPC) was obtained from Avanti Polar Lipids Inc (Alabaster, AL). The ND scaffold protein, recombinant apolipoprotein (apo) A-I, was expressed in E. coli and isolated as described previously.16 CellTiter 96 Non-Radioactive Cell Proliferation (MTT) Assay kit was obtained from Promega (Madison, WI). FITC-annexin-V Apoptosis kit was obtained from Invitrogen (Carlsbad, CA).
Cell lines
HepG2 cells were obtained from American Type Culture Collection. HepG2 cells were cultured in minimal essential media supplemented with 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Jeko cells, obtained from Dr. Steven Rosen (Northwestern University, Chicago, IL) were cultured in RPMI-1640 containing 10% fetal bovine serum, 1% sodium pyruvate and in the presence of penicillin, streptomycin, and glutamine at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Curcumin-ND preparation
Ten mg DMPC was dissolved in chloroform/methanol (3:1 v/v) and dried under a stream of N2 gas, forming a thin film on the vessel wall. Residual organic solvent was removed under vacuum. The prepared lipids were then dispersed in 1.2 ml phosphate buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.0) and 1.0 mg curcumin (from a 4 mg/ml stock solution in dimethylsulfoxide; DMSO) was added. Following this, 4 mg apoA-I (6.5 – 7 mg/ml in PBS) was added and the solution (2.0 ml final volume) subjected to bath sonication under a N2 atmosphere, with the sample temperature maintained between 22°C and 25°C. After sonication the turbid mixture became clear, indicating apolipoprotein/phospholipid complexes (e.g. ND) had formed. For solubilization efficiency calculations, the curcumin-ND preparation was centrifuged at 14,000 rpm for 10 min to pellet unincorporated curcumin, followed by determination of curcumin content in the supernatant. The preparation was further dialyzed (MWCO 6 – 8 kDa) against PBS for 16 h to remove DMSO, followed by 0.22 μm filter sterilization to remove particles larger than 220 nm. The phospholipid and curcumin content of the preparation was determined to calculate the final DMPC/curcumin mole ratio. Empty ND were prepared as described above except that curcumin was omitted.
Quantitation of curcumin
A 4 mg/ml stock solution of curcumin was prepared in DMSO. A standard curve, generated by serial dilution of the stock solution in a 96-well microplate format, was used to determine curcumin concentrations in unknown samples following transfer of an aliquot to DMSO. Sample absorbance was measured at 430 nm on SpectraMax 340 plate reader (Molecular Devices, CA).
Analytical procedures
Protein concentrations were determined by the bicinchoninic acid assay (Pierce Chemical Co.) with bovine serum albumin as standard. DMPC was quantified by enzyme based colorimetric Phospholipids C assay (Wako Chemicals USA).
Atomic force microscopy (AFM)
AFM was used to determine size and morphology of curcumin loaded- and empty- ND. Two μL of solution containing ND (10 ng/mL) was incubated for two min on atomically flat Muscovite mica surface in imaging buffer (phosphate buffered saline plus 10 mM MgCl2), then lightly rinsed. Topographical images were obtained with silicon nitride cantilever probes (MSCT, Veeco, Santa Barbara, CA) with a spring constant of 0.05 N/m. Images were taken in alternate contact mode in liquid, with amplitudes below 20 nm and an amplitude set point at 50% tapping amplitude. Scan rates were below 1.0 Hz. Height and amplitude of images were recorded. Diameters of particles in images were determined by full width half maximum analysis of contiguous particles in the slow scan direction, using IgorPro Wavemetrics software routines. Experiments were carried out at 22 +/− 1°C.
UV/Vis absorbance Spectroscopy
Absorbance spectroscopy was performed on a Perkin-Elmer Lambda 20 spectrophotometer. Samples were scanned from 300 – 700 nm.
Fluorescence spectroscopy
Fluorescence spectra were obtained on a Perkin-Elmer LS 50B luminescence spectrometer. Curcumin samples were excited at 420 nm and emission was monitored from 430 – 600 nm (2.5 nm slit width). Tryptophan residues in the ND scaffold protein (apoA-I) were excited at 280 nm and emission monitored from 300 – 450 nm (2.5 nm slit width).
Cell proliferation assay
HepG2 cells were plated in 96 well culture plates at 3000 cells per well and allowed to attach overnight. After 24 h, the media was replaced with fresh media supplemented with specified concentrations of free curcumin (in DMSO), curcumin-ND or empty ND. Twenty four and 48 h after treatment, cell proliferation assays were performed as described by the manufacturer. Briefly, cells were incubated with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) for 2 h at 37°C. MTT is reduced to the water insoluble, colored product, formazan, by metabolically active cells. Formazan is dissolved in solubilization buffer provided by the manufacturer and absorbance read at 570 nm. Values expressed are the mean ± S.D. (n = 4) percent cell viability relative to untreated cells.
Apoptosis measurement
Cellular apoptosis was measured by flow cytometry.17–18 In brief, cells were incubated with free curcumin, curcumin-ND or empty ND for 24 h, washed with ice cold PBS and re-suspended in binding buffer containing 2.5 μl FITC-annexin V and 5.0 μl propidium iodide for 15 min at 37°C in a CO2 incubator. Subsequently, flow cytometry measurements were obtained on a Beckman Coulter EPICS XL-MCL Cytometer. All experiments were performed in triplicate.
Statistics
Data from cell proliferation and apoptosis assays were expressed as percent viable cells (viability after treatment with either free curcumin or curcumin-ND over viability after treatment with media only) and percent apoptotic cells (annexin V positive and PI positive over total cells) respectively. For cell proliferation assay, differences between groups were analyzed by the unpaired Student’s t-test using Prism 5 software (Graphpad software, La Jolla, CA). P<0.05 was considered as significant (*P<0.05; **P<0.01; ***P<0.001). For apoptosis assay, statistical analysis was performed by one-way ANOVA and Newman-Keuls multiple comparison test (GraphPad Software, Inc., San Diego, CA).
Results
Curcumin-ND formulation
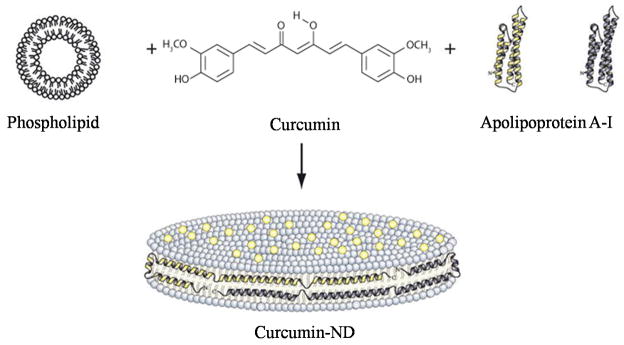
To explore the utility of ND as a vehicle for solubilization and transport of curcumin, experiments were designed to incorporate this lipophilic polyphenol into ND. The formulation strategy takes advantage of the unique ability of amphipathic apolipoproteins to transform specific phospholipid vesicle substrates into ND.19 By introducing curcumin into the reaction mix, it was hypothesized that a ternary ND complex, comprised of phospholipid, apolipoprotein and curcumin, would be generated. A general scheme describing curcumin-ND formulation process and ND particle structure is depicted in Figure 1.
Figure 1. Diagram of curcumin-ND formulation.
Phospholipid vesicles, curcumin and apoA-I were combined to form curcumin-ND. Curcumin-ND are organized as disk-shaped phospholipid bilayers whose periphery is circumscribed by apoA-I “scaffold” protein. Curcumin molecules (yellow dots) are integrated into ND bilayer.
Characterization of curcumin-ND
When 10 mg DMPC and 1 mg curcumin (DMPC/curcumin mole ratio of 5.5:1) were incubated with apoA-I at 24°C with bath sonication, the sample changed from an opaque, turbid suspension to a clarified solution. After centrifugation, 70% of the original curcumin was recovered in the supernatant fraction (Table 1). Although phospholipid alone was able to solubilize 60% of the added curcumin, the solution remained turbid. By contrast, only 9% of the original curcumin was recovered in the supernatant when incubated in buffer alone under identical conditions. Overnight dialysis and filtration of the curcumin-ND preparation resulted in negligible loss of curcumin, indicating stable incorporation of this polyphenol into ND. Compositional analysis of the product particles yielded a final DMPC/curcumin mole ratio of 6:1.
Table 1.
Effects of phospholipid (PL) and ApoA-I on curcumin solubility.
| Components* | Solubilization efficiency† (%) |
|---|---|
| Curcumin + PL + ApoA-I | 70 |
| Curcumin + PL | 60 |
| Curcumin + Buffer only | 9 |
The individual components were added to PBS and processed as described in Materials and Methods. Sample containing curcumin, PL and ApoA-I yielded curcumin-ND.
Solubilization efficiency (%) = the amount of curcumin in supernatant after centrifugation ÷ curcumin added to the incubation × 100.
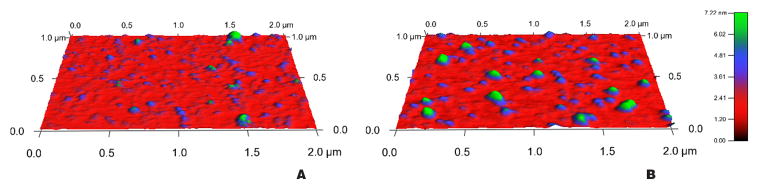
AFM
To examine the shape, morphology and polydispersity of curcumin-ND, AFM experiments were conducted (Figure 2). Both empty and curcumin-loaded ND were discoidal in shape. Fully assembled curcumin-ND were uniformly nanoscale in size, with a diameter of 48 +/− 9 nm and thickness of approximately 5.3 +/− 0.8 nm, consistent with the thickness of a single phospholipid bilayer. Control empty ND had a diameter of 49 +/− 15 nm and height of 4.9 +/− 0.7 nm. No particles were found above 60 nm in diameter. Nondenaturing polyacrylamide gel electrophoresis of curcumin-ND revealed a single major population (data not shown). Taken together, these results indicate that curcumin incorporates into ND, generating a uniform population of discoidal nanoparticles.
Figure 2. Atomic force microscopy of curcumin-ND.
Samples were incubated on an atomically flat Muscovite mica surface in imaging buffer (PBS with 10 mM MgCl2) then lightly rinsed. Topographical images were obtained with silicon nitride cantilever probes with a spring constant of 0.05 N/m. (A) Empty ND. (B) Curcumin-ND. Scale at right represents a color-coded measurement of height (nm).
Absorbance spectroscopy
Curcumin is a yellow colored compound that, upon dissolution in a polar organic solvent (e.g. DMSO) absorbs light in the visible wavelength range. On the contrary, curcumin is insoluble in aqueous media at neutral pH and, as a result, exhibits low absorbance. In agreement with previous studies,20–22 a UV-Vis absorbance spectrum of curcumin in DMSO gave rise to a single major peak centered at 430 nm while the corresponding spectrum of free curcumin in PBS displayed far less absorbance (Figure 3). In comparison, the spectrum of curcumin-ND in PBS was of similar intensity to that of free curcumin in DMSO, indicating solubilization of curcumin. The spectrum of curcumin-ND also displayed more fine structure, with distinct shoulders on both sides of the absorbance maximum. Furthermore, the absorbance maximum for curcumin-ND was blue shifted to 420 nm indicating curcumin present in ND experiences a relatively non-polar environment.
Figure 3. UV-Vis Absorbance spectra of curcumin.
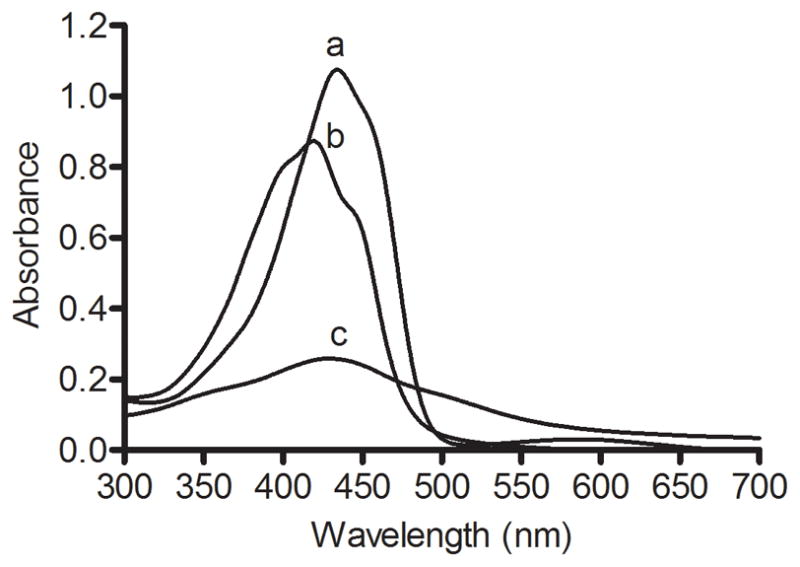
Twenty μM curcumin as a) free curcumin in DMSO, b) curcumin-ND in PBS and c) free curcumin in PBS were scanned from 300 to 700 nm.
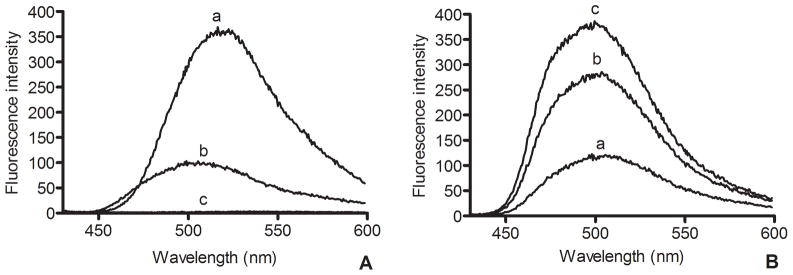
Fluorescence spectroscopy
Excitation of free curcumin in DMSO gave rise to an emission peak centered at 520 nm (excitation 420 nm) (Figure 4, panel A). Whereas free curcumin in PBS did not fluoresce under these conditions, curcumin-ND in PBS gave rise to an emission spectrum whose intensity was significantly attenuated, compared to curcumin in DMSO. Similar to the absorbance maximum, the fluorescence maximum for curcumin-ND was blue shifted to 500 nm. To examine if the low fluorescence intensity of curcumin-ND was due to self-quenching by curcumin when concentrated in the ND milieu, curcumin-ND were incubated with increasing amounts of empty ND. Consistent with transfer of curcumin from curcumin-ND to the empty ND pool, curcumin fluorescence emission intensity increased as a function of increasing empty ND concentration (Figure 4, panel B).
Figure 4.
(A) Fluorescence spectroscopy of curcumin Fifteen μM curcumin as a) free curcumin in DMSO, b) curcumin-ND in PBS and c) free curcumin in PBS were excited at 420 nm and emission scanned from 430 to 600 nm. (B) Effect of empty ND on the fluorescence intensity of curcumin-ND. Samples included a) curcumin-ND, b) curcumin-ND plus an equivalent amount of empty ND and c) curcumin-ND plus 2 fold excess empty ND.
Given the characteristic fluorescence properties of curcumin and its apparent localization in the ND particle milieu, experiments were conducted to assess whether it may affect the intrinsic tryptophan fluorescence properties of the ND scaffold protein, apoA-I. When curcumin-ND were excited at 280 nm and fluorescence emission monitored from 300 – 450 nm, compared to empty ND, apoA-I tryptophan fluorescence was quenched (Supplementary Materials Figure S1). Control experiments with free curcumin showed no fluorescence upon excitation at 280 nm. This result suggests that when incorporated into ND, curcumin resides in close proximity to the ND scaffold protein, apoA-I.
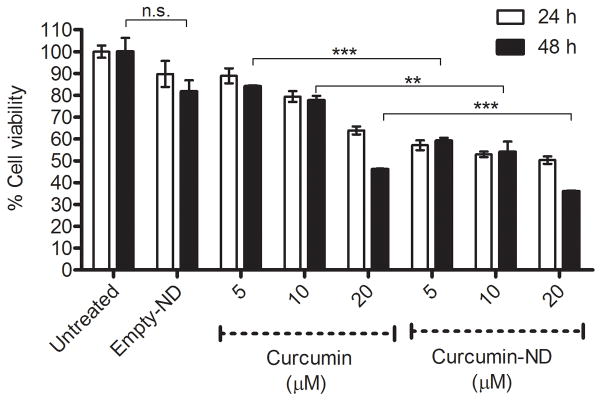
Effect of curcumin on HepG2 cell growth
To assess the biological activity of curcumin when formulated in ND, hepatoma cells were exposed to culture media supplemented with buffer (control), free curcumin (in DMSO) or curcumin-ND (in PBS) and cell viability determined at 24 and 48 h (Figure 5). Whereas empty ND had no effect on cell viability, curcumin-ND inhibited hepatoma cell growth more effectively than free curcumin. At both time points and for all three concentrations tested, the difference between curcumin-ND and free curcumin was found to be statistically significant (P<0.05).
Figure 5. Effect of curcumin on HepG2 cell growth.
Cells were incubated with specified concentrations of free curcumin (in DMSO) or curcumin-ND for 24 and 48 h. Percent cell viability, relative to untreated cells, was measured by MTT assay. Values are reported as mean ± S.D. (n=4). At both time-points, for all three concentrations tested, the difference between curcumin-ND and free curcumin was statistically significant. Differences between groups for the 48 h time point are shown in the figure. **P<0.01; *** P<0.001; n.s. not significant.
Effect of curcumin on Jeko cells
The apoptotic response of cultured Jeko cells to incubations with medium alone (control), free curcumin, empty ND and curcumin-ND was analyzed by flow cytometry (Figure 6). Compared to medium alone, empty ND had no effect on the number of apoptotic cells. By contrast, curcumin-ND produced a concentration-dependent increase in apoptosis. Indeed, at 20 μM, curcumin-ND induced a much greater apoptotic response than free curcumin.
Figure 6. Curcumin induced apoptosis in Jeko cells.
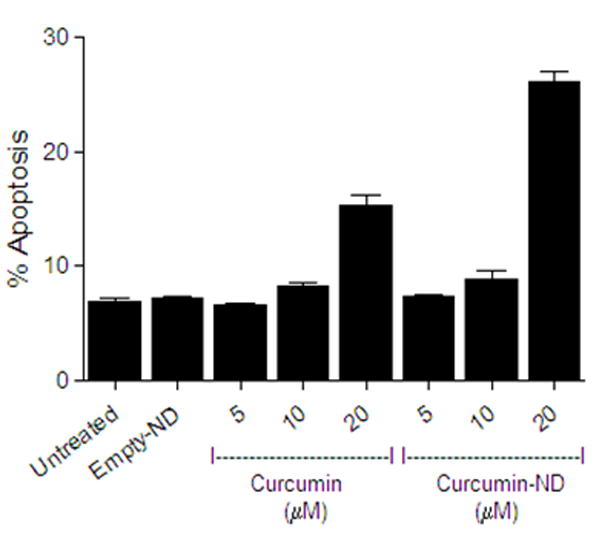
Cells were incubated for 24 h with different concentrations of free curcumin (in DMSO) or curcumin-ND. Apoptosis was measured by flow cytometry. Values shown are the mean ± SD (n = 3). At 20 μM concentration, P<0.001 for curcumin-ND versus free curcumin.
Discussion
The results presented indicate that incorporating curcumin into ND overcomes the poor water solubility of this bioactive molecule. The particles generated are nanoscale, disk-shaped complexes of curcumin, DMPC and apoA-I. As such, these data extend previous studies of ND as a drug delivery vehicle. For example, incorporation of the water insoluble, polyene antibiotic, amphotericin B, into ND generates similar sized particles that show efficacy in mice infected with the pathogenic fungus, Candida albicans14 as well as the protozoal parasite, Leishmania major.23 Likewise, ND formulated with the lipophilic retinoid, all trans retinoic acid, induce apoptosis in cell models of Mantle cell lymphoma.18 When considered in the light of the present data, it may be concluded that ND provide a broad platform for hydrophobic bioactive agent delivery. Further studies will be required to evaluate curcumin-ND as an in vivo delivery vehicle for curcumin that retains, or enhances, its potent anti-proliferative and pro-apoptotic effects.
When taken orally, poor absorption/bioavailability severely limits the clinical utility of curcumin.24 The data presented herein show that curcumin can be solubilized by stable integration into ND. The observation that curcumin-ND not only retains, but potentiates, the biological activity of curcumin in hepatoma cells and Jeko lymphoma cells, indicates that curcumin itself has not been altered during formulation or as a result of its association with ND.
ND represent a potentially novel means for effective delivery of curcumin to specific tissues or cell types. Given the water solubility of this formulation, it is amenable to intravenous infusion, thereby bypassing the poor bioavailability of oral curcumin. The possibility of targeting ND by engineering its apolipoprotein scaffold merits further investigation.25
In summary, the results indicate that incorporation of curcumin into ND enhances its ability to inhibit HepG2 cell growth and induce apoptosis in Jeko cells. At present it is not known if this effect may be attributed to facilitated entry of ND associated curcumin into the cells or whether other factors, such as increased curcumin solubility when presented as ND, improves its effectiveness. Given the potent induction of apoptosis in Jeko cells incubated with curcumin-ND, further investigation of the in vivo efficacy of curcumin-ND, in isolation or as a combination therapy, is warranted.
Supplementary Material
Acknowledgments
Grant support: HL64159 and 1R43CA141904
Abbreviations List
- ApoA-I
apolipoprotein A-I
- ND
nanodisks
- DMPC
dimyristoylphosphatidylcholine
- AFM
atomic force microscopy
- DMSO
dimethylsulfoxide
- PBS
phosphate buffered saline
Footnotes
Conflicts of Interest: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative Medicine Review. 2009;14:141–53. [PubMed] [Google Scholar]
- 2.Epstein J, Sanderson IR, Macdonald TTX. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 1994;26:1–13. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 3.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Letters. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants and Redox Signaling. 2008;10:511–45. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Molecular pharmaceutics. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 7.Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol. 2009;6:192–9. doi: 10.2174/157016309789054933. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal DN, Mishra PK. Curcumin and its analogues: potential anticancer agents. Medicinal Research Reviews. 2009 doi: 10.1002/med.20188. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. Journal of Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 12.Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32:1119–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan RO. Nanodisks: hydrophobic drug delivery vehicle. Expert Opin Drug Deliv. 2008;5:343–51. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- 14.Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high-density lipoprotein enriched with the polyene antibiotic, amphotericin B. J Lipid Res. 2006;47:260–7. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Redmond KA, Nguyen TS, Ryan RO. All-trans retinoic acid nanodisks. Int J Pharm. 2007;339:246–50. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 17.Singh ATK, Evens AM, Prachand SN, Gordon LI. Motexafin Gadolinium enhances p53-Mdm2 interactions, reducing p53 and downstream targets in lymphoma cell lines. Anticancer Research. 2010;30:1131–36. [PubMed] [Google Scholar]
- 18.Singh ATK, Evens AM, Anderson RJ, Beckstead JA, Sankar N, Bhalla S, Yang S, Forte TM, Ryan RO, Gordon LI. All trans retinoic acid nanodisks enhance retinoic acid receptor-mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Brit J Haemotol. 2010;150:158–69. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weers PM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–35. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- 20.Chignell CF, Bilski P, Reszka KJ, Motten AG, Sik RH, Dahl TA. Spectral and photochemical properties of curcumin. Photochemistry and Photobiology. 1994;59:295–302. doi: 10.1111/j.1751-1097.1994.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 21.Khopde SM, Priyadarsini I, Palit DK, Mukherjee T. Effect of solvent on the excited-state photophysical properties of curcumin. Photochemistry and Photobiology. 2000;72:625–31. doi: 10.1562/0031-8655(2000)072<0625:eosote>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Jasim F, Ali F. Measurement of some spectrophotometric parameters of curcumin in 12 polar and nonpolar organic solvents. Microchemical Journal. 1989;39:156–9. [Google Scholar]
- 23.Nelson KG, Bishop JV, Ryan RO, Titus R. Nanodisk-associated amphotericin B clears Leishmania major cutaneous infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2006;50:1238–44. doi: 10.1128/AAC.50.4.1238-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhillon N, Aggrawal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 25.Iovannisci DM, Beckstead JA, Ryan RO. Targeting nanodisks via a single chain variable antibody -apolipoprotein chimera. Biochem Biophys Res Commun. 2009;379:466–9. doi: 10.1016/j.bbrc.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.