Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. Due to its high prevalence, MS is the leading cause of non-traumatic neurological disability in young adults in the United States and Europe. The clinical disease course is variable and starts with reversible episodes of neurological disability in the third or fourth decade of life. This transforms into a disease of continuous and irreversible neurological decline by the sixth or seventh decade. Available therapies for MS patients have little benefit for patients who enter this irreversible phase of the disease. It is well established that irreversible loss of axons and neurons are the major cause of the irreversible and progressive neurological decline that most MS patients endure. This review discusses the etiology, mechanisms and progress made in determining the cause of axonal and neuronal loss in MS.
Keywords: Multiple sclerosis, neurons, axons, myelin
1. Introduction
Multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS), is the leading cause of non-traumatic neurological disability in young adults in North America and Europe, affecting more than two million people worldwide (Hauser and Oksenberg, 2006; Noseworthy, 1999; Noseworthy et al., 2000; Trapp and Nave, 2008; Weinshenker, 1998). Although descriptions of putative MS date back as early as the middle ages, the first pathological report was published by Jean-Martin Charcot, Professor of Neurology at the University of Paris, in 1868 in the Leçons du mardi (Charcot, 1868). He documented characteristic `plaques' thereby coining the definition of `la sclerose en plaques' upon examination of a young woman's brain. His diagnostic criteria based on nystagmus, intention tremor and scanning speech are still helpful in recognizing the disease. Although considerable scientific progress has been obtained through over a century of subsequent research, the underlying cause of MS is still unknown.
Pathologically, the diagnosis of MS is confirmed by the presence of multifocal inflammatory demyelinated plaques distributed over time and space within the CNS. Thus, identification of multiple foci of demyelination in the CNS of patients clinically diagnosed with MS is one of the cardinal pathological findings for confirming the MS diagnosis. The International Panel on diagnosis of MS presented several guidelines (McDonald et al., 2001) termed “McDonald Criteria” for detection of MS. The most recent recommendations of the panel are listed in Table I (Polman et al., 2005). However, in the absence of standardized equipment, analysis and interpretation, diagnosis of MS can be made reliably a by a knowledgeable physician using clinical data.
Table I.
Diagnostic criteria for MS (adapted from the ″Revisions to McDonald Diagnostic criteria for Multiple Sclerosis″ (Polman et al., 2005)
| Clinical Presentation | Additional Data Needed for MS Diagnosis |
|---|---|
|
No additional tests are recommended |
|
Two or more MRI-detected lesions and positive CSF analysis (detection of oligoclonal bands) OR Dissemination in space demonstrated by MRI: Fulfilling at least three of following:
OR Await further clinical attack |
|
Dissemination in space demonstrated by MRI with previous criteria OR Await second clinical attack |
|
Dissemination in space demonstrated by MRI with previous criteria OR Two or more MRI-detected lesions consistent with positive CSF analysis And Dissemination in space demonstrated by MRI with previous criteria OR Second clinical attack |
|
One year disease progression and Any two of the following
|
The majority (~85%) of MS patients have a biphasic disease course, beginning with the primary phase termed relapsing-remitting MS (RR-MS). During this disease course, patients experience alternating episodes of neurological disability and recovery that can last for many years (Hauser and Oksenberg, 2006; Noseworthy, 1999; Noseworthy et al., 2000; Trapp and Nave, 2008). Within 25 years, ~90% of RR-MS patients transform into a secondary-progressive disease course (SP-MS) which is characterized by steady neurological decline (Noseworthy et al., 2000; Trapp and Nave, 2008; Weinshenker et al., 1989). About 10% of MS patients also exhibit a disease course with steady decline in neurological function without recovery and are classified as primary progressive MS (PPMS). A small minority of MS patients (~5%) suffer from a disease course with progressive neurological decline accompanied by well demarcated acute attacks with or without recovery. This disease course is classified as progressive-relapsing MS (PRMS).
Typically, MS lesions include breakdown of the blood-brain barrier, multifocal inflammation, demyelination, oligodendrocyte loss, reactive gliosis, and axonal degeneration (Dutta and Trapp, 2007; Prineas, 2001; Trapp and Nave, 2008). While immune-mediated destruction of CNS myelin and oligodendrocytes are considered the primary pathology of MS, it is well established that progressive axonal loss is the major cause of neurological disability in MS (Stadelmann et al., 2008; Trapp and Nave, 2008). Various approaches including magnetic resonance imaging (MRI) (Bakshi et al., 2008; Filippi et al., 2003; Filippi and Rocca, 2007), magnetic resonance spectroscopy (MRS) (De and Filippi, 2007; Narayana, 2005; Tartaglia and Arnold, 2006) functional magnetic resonance imaging (fMRI) (Bakshi et al., 2008; Filippi et al., 2003; Rocca et al., 2003; Rocca and Filippi, 2007; Tartaglia and Arnold, 2006), and morphological analysis of MS tissue (Anthony et al., 2000; Bruck, 2005; Stadelmann et al., 2008; Trapp et al., 1998; Trapp and Nave, 2008) have provided evidence for axonal loss as the major cause of irreversible neurological disability in MS.
2. Neurological Disability in RRMS
The majority of RR-MS patients have alternating episodes of neurological disability and recovery with formation of new lesions. Brain imaging shows new lesion areas as enhanced with gadolinium (GAD), which reflects breakdown of the blood-brain barrier, infiltration of hematogeneous leukocytes, demyelination, and oligodendrocyte death. The edema associated with “MS lesions” is a major contributor to neurological relapses, blocking conduction of action potentials. Demyelination, which occurs throughout the CNS, is another contributor to the transient disability. The brain employs a number of adoptive mechanisms that restore function to demyelinated white matter. Recent fMRI studies have identified cortical areas which are activated after a white matter lesion which can be transient and may reflect recovery of function within the lesion. A classical example of such a phenomenon is the redistribution of Na+ channels following demyelination (Waxman, 1982). The demyelinated axon responds to the loss of myelin by re-distributing its voltage-gated Na+ channels along the demyelinated axolemma (Waxman, 2006). These axons slowly regain the ability to conduct action potentials, albeit at a reduced velocity. Subsequently, the edema resolves, leading to remyelination and the restoration of nerve conduction. Understanding the cellular and molecular mechanisms of axonal transection with disease progression is imperative for the development of neuroprotective therapies.
2.1. Axonal Transection during Inflammatory Demyelination
A series of papers in the late 1990s described a variety of axonal changes in actively demyelinating lesions present in postmortem MS brains. Axonal accumulation of amyloid precursor protein (APP) in acutely demyelinated lesions was documented by Ferguson and colleagues (Ferguson et al., 1997). APP is present at undetectable levels in normally myelinated axons, but can accumulate at detectable levels following demyelination. The pore-forming subunit of N-type calcium channels (Kornek et al., 2001) and metabotropic glutamate receptors (Geurts et al., 2003) also accumulate in acutely demyelinated axons (Bitsch et al., 2000), and if inserted into the axolemma they may also contribute to axonal dysfunction and transection. One of the best characterized axonal changes is the phosphorylation of axonal neurofilaments (Sanchez et al., 1996). Phosphorylation increases the extension of sidearms from the neurofilaments and this in turn increases interfilament spacing and axonal diameter. Using antibodies to non-phosphorylated neurofilaments to examine axonal changes in demyelinated MS lesions, a dramatic increase in non-phosphorylated neurofilament epitopes (Trapp et al., 1998) in demyelinated axons was shown. Using confocal microscopy and three-dimensional reconstructions, many of the non-phosphorylated neurofilament-positive ovoids were detected at the transected ends of axons (Trapp et al., 1998) (Fig 1a). Formation of axonal ovoid is a hallmark of a transected axon. Following transection, part of the axon still connected to the neuronal cell body can survive and mend the transection, however there is disruption of transport resulting in formation of an ovoid (Fig 1b–c). Three dimensional reconstruction of the ovoids established that most were connected to a single axon and thus represented the cut end of the transected axons. Transected axons in acute MS lesions were abundant and exceeding 11,000 per mm3 of lesion area, with disease durations ranging from 2 weeks to 27 years (Trapp et al., 1998). The identification of significant axonal transection in patients with short disease duration when inflammatory demyelination is predominant established the concept that axonal loss occurs at disease onset in MS. Positive correlations between inflammatory activity of MS lesions and axonal damage suggests that inflammation modulates axonal pathology in MS patients (Bitsch et al., 2000; Geurts et al., 2003; Geurts et al., 2009; Kornek et al., 2000; Kornek et al., 2001; Trapp et al., 1998; Trapp et al., 1999a; Trapp et al., 1999b).
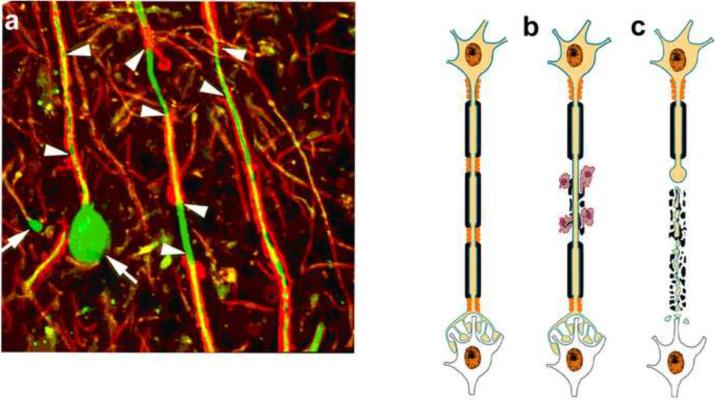
Figure 1. Immune-mediated demyelination and axonal transection.
Axonal ovoids are hallmark of transected axons. Abundant axonal ovoids were detected in MS tissue (a) when stained for myelin protein (red) and axons (green). There are areas of demyelination (arrowheads), mediated by microglia and hematogenous monocytes. One of the axons ends in a large swelling (arrow) or axonal retraction bulb (arrow). (b-c) Schematic of axonal response during and following transection. Demyelination is an immune-mediated or immune cell assisted process leading to axonal transaction. When transected, the distal end of the axon rapidly degenerates while the proximal end connected to the neuronal cell body survives and transported organelles accumulate at the transection site and form an ovoid (arrows). (Reproduced from(Trapp and Nave, 2008)
Despite extensive axonal loss occurring in acute MS lesions, relapses are reversible, as the human brain has a remarkable ability to compensate for neuronal loss. For example, it has been estimated that Parkinson's patients lose over 70% of dopaminergic neurons before they show clinical signs (Trapp et al., 1999b). If it takes a 60–70% loss of neurons or axons to produce irreversible neurological disability, an acute demyelinated lesion is unlikely to exceed this threshold. About 22% axonal loss at sites distal to a fatal brain stem lesion (Bjartmar et al., 2001) was reported in a MS patient. Similarly neurological disability correlated with axonal loss only in the chronic-EAE animal models (Papadopoulos et al., 2006; Wujek et al., 2000; Wujek et al., 2002). Initial axonal loss, therefore, does not have an immediate substantial clinical impact during early stages of RRMS. With time and additional lesions, however, axonal loss can drive the clinical aspects of MS. The conversion of RR-MS to SP-MS is therefore thought to occur when the brain exhausts its capacity to compensate for further axonal loss (Nave and Trapp, 2008; Trapp et al., 1999a). Axonal loss in MS patients is supported by variety of analyses including whole brain atrophy (De Stefano et al., 2003a; De Stefano et al., 2003b; Fisher et al., 2000; Fisher et al., 2007; Stevenson and Miller, 1999) and reductions in the neuronal-specific amino acid N-acetyl aspartate acid (NAA) (De et al., 2001; Matthews et al., 1996; Narayana, 2005; Narayanan et al., 1997; Stefano et al., 2000).
2.2. Immune-Mediated Axonal Loss
As axon pathology and frequency of transected axons in acute MS lesions correlates with the degree of inflammation (number of immune cells) (Ferguson et al., 1997; Trapp et al., 1998), early axonal transection is therefore thought to occur due to vulnerability of demyelinated axons to inflammation. Activated immune and glial cells release a plethora of substances including proteolytic enzymes, matrix metalloproteases, cytokines, oxidative products, and free radicals that can damage axons (Hohlfeld, 1997; Nave and Trapp, 2008). Inducible nitric oxide synthase (iNOS), a key enzyme required for synthesis of nitric oxide (NO), is significantly increased in acute MS lesions (Bo et al., 1994; Liu et al., 2001). Additionally, glutamate-mediated excitotoxicity is observed in many acute and chronic neurodegenerative conditions (Lipton and Rosenberg, 1994). Activated immune cells, axons and astrocytes are potential sources for excessive levels of glutamate in acute MS lesions (Lassmann, 2003; Matute et al., 2001; Parpura et al., 2004; Steinman, 2001; Ye et al., 2003) and magnetic resonance spectroscopy studies of MS brains have shown elevated glutamate levels in acute MS lesions(Srinivasan et al., 2005). Based on glutamate receptor expression, excess glutamate may damage oligodendrocytes, myelin and axons (Micu et al., 2006; Ouardouz et al., 2009a; Ouardouz et al., 2009b). When released in excess, glutamate activates ionotropic and metabotropic receptors, resulting in toxic cytoplasmic Ca++ accumulation and cell death. Studies have demonstrated NMDA receptor-dependent signaling in oligodendrocytes (Karadottir et al., 2005) their processes (Salter and Fern, 2005) and the mature myelin sheath (Micu et al., 2006). As activation of AMPA and/or kainate (but not NMDA) receptors can damage axons, antagonists to these receptors can be axon-protective under certain conditions (Li and Stys, 2000; Tekkok and Goldberg, 2001).
Another possible mechanism of axonal degeneration in MS is a specific immunologic attack on the axon, suggested by the strong correlation between inflammation and axonal transection (Trapp and Nave, 2008; Trapp and Stys, 2009; Weiner, 2009). The terminal axonal ovoids are often surrounded by macrophages and activated microglia in acute MS lesions (Trapp et al., 1998). Whether these cells are directly attacking axons, protecting axons or removing debris remains to be determined. Direct immunological targeting of axons is not without precedence. In acute motor axonal neuropathy (AMAN), a variant of Guillain-Barré syndrome (GBS) (Ho et al., 1998), primary immune-mediated attack occurs against gangliosides on peripheral nervous system (PNS) axons. Antibodies to axonal components in the CNS have not, however, been localized to MS lesions (Hafer-Macko et al., 1996; Ho et al., 1998). T-cell repertoire in individual MS patients change depending on disease activity (Khoury et al., 2000), CD4+ T cells are the most prominent cells in active but absent in chronic MS lesions (Bennett and Stuve, 2009). In some cases CD8+ cells out number the CD4+ T cells thereby suggesting the former driving the cytotoxicity (Crawford et al., 2004). Both CD4+ and CD8+ T-cells have been identified as possible mediators of axonal transection in MS lesions (Babbe et al., 2000; Skulina et al., 2004), in EAE mice (Huseby et al., 2001), and in vitro (Giuliani et al., 2003; Medana et al., 2001). Despite the lack of a distinct TH1-TH2 dichotomy in the human system, there is focus on the role of CD4+ subsets and their respective cytokines on the pathogenesis of the disease (Gor et al., 2003). Further, some reports indicate that axonal subpopulations may be targeted by immune-mediated mechanisms (Evangelou et al., 2001; Ganter et al., 1999; Lovas et al., 2000). Additionally, since most axons survive the acute demyelinating process, it seems unlikely there is a specific immunological attack against axons. Despite the current paucity of direct evidence supporting a specific immunological attack on axons in MS, the possibility of cell-mediated mechanisms of axon loss is still necessary to investigate.
3. Neurological Disability in Secondary Progressive MS
While new inflammatory demyelinating lesions may contribute to disability in RR-MS, the majority of SP-MS patients continue to decline neurologically without evidence of new inflammatory demyelinating lesions as measured by MRI. SP-MS patients do not respond to current anti-inflammatory therapies. One logical and accepted explanation for the continuous neurological decline in SP-MS is degeneration of chronically demyelinated axons. This is a difficult phenomenon to unequivocally demonstrate, as one would have to sample and quantify demyelinated axons in the same location at multiple time points. There are, however, a convincing number of correlations and “proofs of principle” that supports continuous loss of chronically demyelinated axons in MS brain.
3.1. Axonal loss due to loss of myelin-derived trophic support
“Proof-of-principle” for the degeneration of chronic demyelinated axons is derived from mice that lack individual myelin proteins (Nave, 2010; Nave and Trapp, 2008; Nave, 1996). Myelin-associated glycoprotein (MAG), 2',3'cyclic nucleotide 3'-phosphodiesterase (CNP), and proteolipid protein (PLP) can be removed from oligodendrocytes without major effects on the process of myelination (Klugmann et al., 1997; Lappe-Siefke et al., 2003; Yin et al., 1998). All three lines of mice, however, developed a late onset, slowly progressing axonopathy and axonal degeneration (Nave, 2010; Nave and Trapp, 2008; Nave, 1996). These studies established that in addition to axonal insulation, myelin/oligodendrocytes provide trophic support that is essential for long-term axonal survival (Nave, 2010; Nave and Trapp, 2008). The axonal atrophy precedes axonal degeneration in MAG-null mice when compared to the PLP- and CNP-null mice. There is a reduction in axonal caliber, most prominent in paranodal regions, due in part to reduced phosphorylation on neurofilaments (Yin et al., 1998). In PLP-and CNP-null mice, axonal swelling occurs at distal paranodes, suggesting that a defect in retrograde axonal transport at nodes of Ranvier (Griffiths et al., 1998; Klugmann et al., 1997) precedes axonal degeneration. Compared to PLP-null mice, CNP-null mice have a more severe axonal phenotype (Lappe-Siefke et al., 2003). In addition, axonal degeneration was prominent in PLP-null mice when their compact myelin phenotype was rescued by the peripheral myelin protein P0 (Yin et al., 2006). While the MAG- and CNP-null mice segregate the role of oligodendrocytes in myelin formation and axonal survival, the mechanisms by which these proteins provide this support are currently unknown.
These studies provide evidence that alterations in single myelin proteins can cause axonal degeneration. It is therefore not surprising that loss of myelin as it occurs in MS can also cause axonal degeneration. Several findings support axonal loss during the latter stages of MS. Postmortem studies have identified axonal retraction bulbs, the histological hallmark of transected axons, in chronic inactive lesions (Kornek et al., 2000). These ovoids are transient structures and their accumulation over decades could contribute substantially towards the degeneration of chronically demyelinated axons. Estimates of total axonal loss in spinal cord, corpus callosum and optic nerve lesions approached almost 70% (Bjartmar et al., 2000; Ganter et al., 1999; Lovas et al., 2000). The remaining 30% of demyelinated axons that remain in these chronic lesions have significant structural and molecular changes that are detrimental to normal function and survival (Dutta et al., 2006) These observations implicate axonal degeneration as a cause of irreversible neurological impairment during chronic progressive stages of MS.
3.2. Degeneration of Chronically Demyelinated Axons
Indirect evidence supports general mechanisms by which chronically demyelinated axons degenerate. These mechanisms are difficult to test directly as demyelinated axons do not persist for extended periods of time in animal models. The central hypotheses of degeneration of chronically demyelinated axons involve an imbalance between energy demand and energy supply (Bechtold and Smith, 2005; Trapp and Stys, 2009; Waxman, 1982). In normal myelinated fibers, Na+ channels are concentrated at nodes of Ranvier, allowing saltatory conduction of action potentials. When Na+ enters nodal axoplasm, the Na+/K+ ATPase exchanges it rapidly for extracellular K+. Na/K ATPases which maintain the ionic gradients necessary for neurotransmission are the largest consumers of ATP in the CNS (Ames, III, 2000). This continuous energy-dependent ion exchange is required for maintenance of axonal polarization to support the repetitive axonal firing essential for many neuronal functions. Thus, myelination not only promotes rapid nerve conduction, but it is an effective way to conserve energy (Nave, 2010; Nave and Trapp, 2008). While demyelination per se may not kill axons, it renders them more vulnerable to physiological stress and degeneration by substantially increasing the energy requirements for nerve conduction as have been demonstrated in rodent models of anoxic injury (Stys, 2004; Stys, 2005; Trapp and Stys, 2009; Waxman et al., 1992). Following demyelination, Na+ channels are diffusely distributed along the denuded axolemma. If axonal Na+ rises above its nominal concentration (Stys et al., 1997), the Na+/Ca++ exchanger, which exchanges axoplasmic Ca++ for extracellular Na+, will operate in the reverse Ca++-import mode. With increasing electrical traffic, axoplasmic Ca++ will rise and eventually a Ca++-mediated degenerative response will be initiated (Trapp and Stys, 2009). An underlying mechanism of Ca++-mediated axonal degeneration is reduced axoplasmic ATP production, which impairs Na+/K+ ATPase function causing axoplasmic ionic imbalance. Excessive axoplasmic Ca++ accumulation will cause a vicious cycle of impaired mitochondrial operation, reduced energy production and compromised axonal transport (Mahad et al., 2008a; Smith, 2007; Trapp and Stys, 2009; Waxman, 2006). This vulnerability to degeneration is compounded by several additional factors. Due to the redistribution of Na+ channels and the resulting increased influx of sodium, ATP consumption is greatly increased in demyelinated axons (Peterson et al., 2005; Waxman, 2006). The mitochondria that reach chronically demyelinated axoplasm are likely to be compromised and have a reduced capacity for ATP production caused by decreased neuronal transcription of nuclear encoded mitochondrial genes (Dutta et al., 2006). In microarray comparisons of control and MS motor cortex, 25% of nuclear encoded mitochondrial genes were decreased together with ≈40 –50% reduction in mitochondrial function of complex I and III. Mitochondrial respiratory chain complex I activity was also found to be reduced in chronic active MS lesions (Mahad et al., 2008a; Mahad et al., 2008b). Localization and quantification of mitochondrial gene transcripts by in situ hybridization and microarray comparisons of control and MS white matter support decreased mitochondrial gene transcripts in neurons, but not in glia, in chronic MS brains (Dutta et al., 2006).
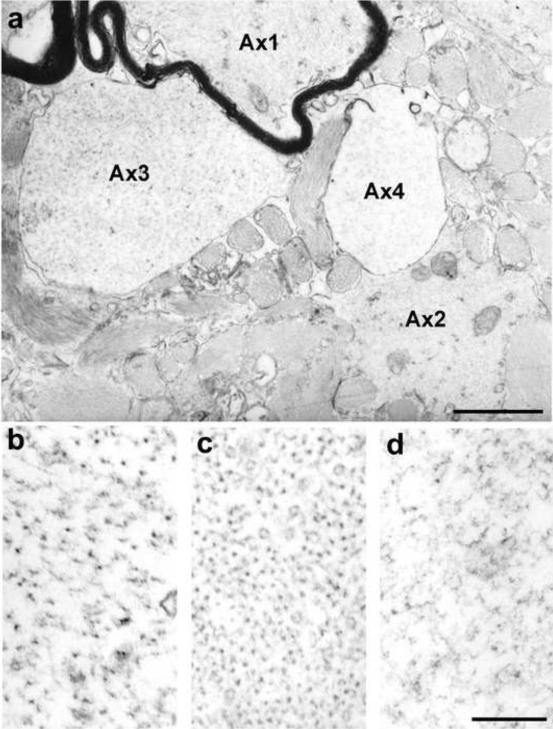
Axonal integrity was analyzed in spinal cords of ten severely disabled (EDSS> 7.0) MS patients using electron microscopy (Dutta et al., 2006). Samples retained significant ultrastructural integrity as indicated by preservation of cytoplasmic organelles and extracellular spaces (AX1 in Fig 2a) within myelinated axons. Greater than 95% of the myelinated axons had normal appearing axoplasm with well-preserved cellular structures like microtubules, mitochondria and neurofilaments with appropriate side-arm extensions (Fig 2b). Fifty percent of the demyelinated axons also had well-preserved axoplasm with reduced neurofilament spacing (Fig 2c). The remaining 50% of demyelinated axons had abnormal axoplasm with reduced organelle content and varying degrees of neurofilament fragmentation (Fig 2d) and a dramatic reduction in numbers of mitochondria and microtubules. These observations support the possibility that half of the demyelinated axons in spinal cord lesions from chronic MS patients have activated Ca2+-dependent enzymes that are known to fragment neurofilaments (Stys and Jiang, 2002).
Figure 2. Ultrastructural changes in MS axons.
The electron micrograph in (a) contains four axons (Ax1-Ax4) at the edge of a demyelinated lesion. The myelinated axon (Ax1) has normal-appearing axoplasm (b), with intact and appropriately oriented neurofilaments. Axoplasm of demyelinated Ax2 (c) is also intact, but neurofilament spacing is significantly reduced. Neurofilaments in demyelinated Ax3 (d) are fragmented and they are barely detectable in demyelinated Ax4. Scale Bar b-d = 200 nm. (Reproduced from (Dutta et al., 2006)
Another feature of chronic MS lesions is axonal swelling. Histological comparison of axons in normal appearing white matter, acute MS lesions and chronic MS lesions detected a statistically significant increase in axonal diameters in chronic MS lesions (Fisher et al., 2007). Altered T1 and MTR sequences identify chronic lesions with severe axonal loss and swelling, whereas T2-only changes correlated with breakdown of the blood-brain barrier, with or without acute demyelination. Axonal swelling correlated with T1 and MTR changes on MRI (but not T2 only MRI changes) (Fisher et al., 2007). Axoplasmic swelling, therefore, is a pathological hallmark of chronically demyelinated CNS axons that is likely to reflect, in part, increased axoplasmic Ca++ (Trapp and Stys, 2009).
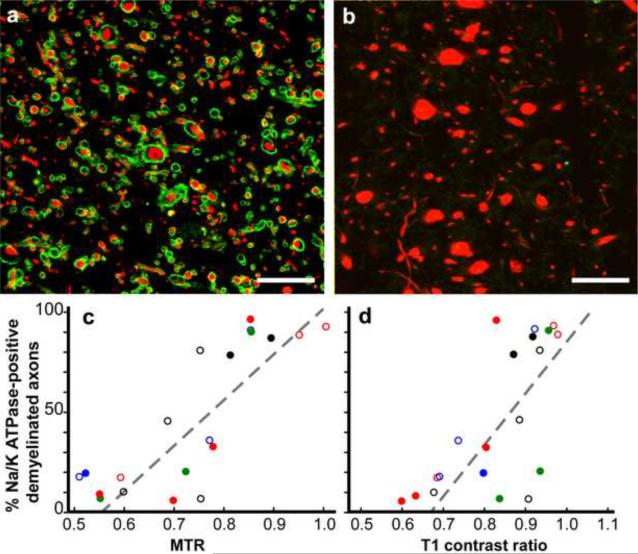
Recent studies also support the notion that chronically demyelinated axolemma eventually loose critical molecules that are essential for propagation of action potentials. Thus, many chronically demyelinated axons may be dysfunctional prior to degeneration because they lack voltage-gated Na+ channels (Black et al., 2007b) and/or Na+/K+ ATPase (Young et al., 2008). This observation was extended by T1MTR analysis of demyelinated axons with and without Na+/K+ ATPase(Young et al., 2008) (Fig 3). In acutely demyelinated lesions, Na+/K+ ATPase was detectable on demyelinated axolemma while 58% of chronic lesions contained less than 50% Na+/K+ ATPase-positive demyelinated axons. Chronically demyelinated axons that lack Na+/K+ ATPase therefore cannot exchange axoplasmic Na+ for K+ and are incapable of repolarizing the axolemma. Reduced exchange of axonal Na+ for extracellular K+ will also increase axonal Na+ concentrations, which will, in turn, reverse the Na+/Ca++ exchanger and lead to increase in axonal Ca++ and contribute to Ca++-mediated axonal degeneration as mentioned earlier. These data support the concept that many chronically demyelinated axons are non-functional before degeneration. Loss of axonal Na+ channels and/or Na+/K+ ATPase therefore is likely to be a contributor to continuous neurological decline in chronic stages of MS and the quantitative MRI may provide a valuable predictor of this process in longitudinal studies of MS patients
Figure 3. Magnetization transfer ratios (MTR) and T1 contrast ratios linearly correlate with the percentage of Na+/K+ ATPase-positive axons in chronic MS lesions.
Chronically demyelinated lesions stained for Na+/K+ ATPase (green) varied from nearly 100% (a) to zero (b), in neurofilament (red). Many axons without Na+/K+ ATPase had increased diameters (b). A comparison of the percentage of Na+/K+ ATPase-positive axons in chronically demyelinated MS lesions correlated with quantitative postmortem magnetization transfer ratio (MTR, p<0.0001, c) and T1 contrast ratios, (p<0.0006, d). Each data point is from a single lesion and each unique color-symbol combination denotes one of the brains studied. Scales bars = 5μm. (Reproduced from (Young et al., 2008)
4. Neuronal Compensation
A number of adaptive and neuroprotective mechanisms repress or delay the neuronal degeneration and neurological decline in MS patients. As mentioned earlier in this chapter, functional MRI studies have identified the activation of cortical areas that compensate for functional loss caused by new MS lesions (Buckle, 2005; Pantano et al., 2006; Reddy et al., 2000; Rocca et al., 2005) and compensate for damage caused by inflammatory demyelination of white matter (Pantano et al., 2002). It is also possible that neurogenesis can occur in the adult mammalian brain including the production of interneurons in the human hippocampus (Altman and Das, 1965; Alvarez-Buylla and Garcia-Verdugo, 2002; Eriksson et al., 1998; Kaplan and Hinds, 1977; Kornack and Rakic, 2001; Kuhn et al., 1996; Luskin, 1993; Pencea et al., 2001). We recently reported generation of new neurons in chronic MS lesions (Chang et al., 2008) which can be viewed as a neuronal compensation towards disease pathogenesis. Other compensatory mechanisms also include remyelination (reviewed in (Franklin et al., 1997; Franklin and Ffrench-Constant, 2008; Trapp and Nave, 2008), redistribution of sodium channels on demyelinated axons (Smith, 2007; Trapp and Stys, 2009; Waxman, 2006), and expression of neurotrophic factors by immune and CNS resident cells (Dutta et al., 2007; Martino, 2004; Nave, 2010; Nave and Trapp, 2008; Stadelmann et al., 2002; Stadelmann et al., 2008; Trapp and Nave, 2008).
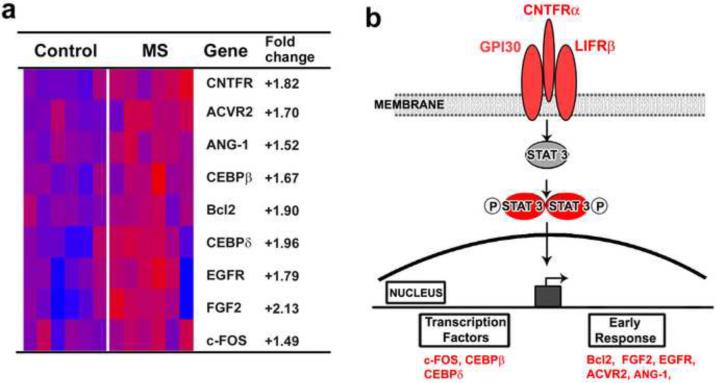
While the role of myelin in regulating neuronal molecules has not been investigated, myelin-axonal interactions can modulate neuronal gene expression together with providing trophic support (Nave, 2010; Nave and Trapp, 2008) Other mechanisms of neuronal compensation at the cellular level also lead to alteration in neuronal gene expression. These gene changes were first identified by unbiased comparisons of 33,000 mRNA transcripts in motor cortices from control and MS patients. Among the 555 significantly altered transcripts, 488 were decreased and 67 were increased in MS cortex (Dutta et al., 2006; Dutta et al., 2007). When grouped into ontology-based biological processes, altered genes show decreases in two gene families, oxidative phosphorylation and synaptic transmission. Reductions in mRNA and protein levels were confirmed by RT PCR and western blotting. Of the 103 nuclear encoded mitochondrial genes, 26 were decreased in MS cortices. In addition, mitochondria isolated from MS cortex had reduced function of respiratory chain complexes I and III. Since reduced ATP production can protect cells from noxious stress and reduce apoptosis, reduction of mitochondrial gene expression may therefore be a part of a neuroprotective response putting their demyelinated axonal segments at risk for degeneration. The neurotransmitter changes were restricted to pre- and post-synaptic components of inhibitory neurotransmitters. Reduced inhibitory innervation leads to up-regulation of neuroprotective pathways (Hardingham et al., 2002) (Jalabi et al, 2010, under review). Increased transcripts were searched for possible neuroprotective pathways. Using gene classification methods, we found 9 out of the 67 increased transcripts to be members of the ciliary neurotrophic factor (CNTF) family (Fig 4a). CNTF is an established neurotrophic factor, which enhances neuronal survival during development and in disease. Translational and transcriptional products of CNTF-related genes were quantified and localized in control and MS cortices (Dutta et al., 2007). CNTF, the tripartite CNTF receptor complex and downstream CNTF signaling molecules, including the anti-apoptotic molecule Bcl2, were increased in neurons in MS cortex (Fig 4b). An active and functionally significant role of CNTF in MS patients is supported by the report that MS patients with CNTF-null mutations have an earlier disease onset and more aggressive disease course (Giess et al., 2002). Hoffman and colleagues (Hoffmann et al., 2002), however, failed to detect differences in disease onset or progression in MS patients with CNTF-null mutations and suggested that other neurotrophic factors may compensate for loss of CNTF. Increased expressions of neurotrophic genes represent part of the endogenous defense mechanisms mounted by the MS brain to maintain neurons and combat progressive neurological decline. The gray matter of MS patients however is not immune to demyelination, as cortical MS lesions are a prominent but underappreciated feature of the disease.
Figure 4. Upregulation of a CNTF-mediated Neuroprotection in MS Motor Cortex.
mRNA encoding CNTF and multiple members of the CNTF signaling pathway are increased in MS cortex (a). (b) Schematic representation of the CNTF signaling pathway members which were increased in MS cortex. Red denotes increased in MS cortex. (Reproduced from (Dutta et al., 2007)
5. Cortical Demyelination
Cortical demyelination is a prominent feature of postmortem MS brains (Bo et al., 2003a; Bo et al., 2003b; Brownell and Hughes, 1962; Kidd et al., 1999; Kutzelnigg and Lassmann, 2005; Peterson et al., 2001). Demyelinated cortices are not evident macroscopically in postmortem brain slices because they do not change color like white matter lesions. Estimates of cortical lesion load have been limited to immunocytochemical analysis of postmortem brains and may equal or exceed white matter lesion load (Bo et al., 2003a; Kutzelnigg and Lassmann, 2005). In four cortical regions of 20 postmortem MS brains, over 25% of the total cortical area was demyelinated compared to 6% demyelination in the subcortical white matter (Bo et al., 2003b). Compared to white matter lesions, cortical lesions contain few infiltrating leukocytes, less CD68-positive microglia/macrophages (Peterson et al., 2001) and CD3-positive T-cells (Bo et al., 2003b; Peterson et al., 2001). Neuronal degeneration is a prominent feature of cortical lesions accompanied by neuritic transection, neuronal apoptosis (Fig. 5)(Peterson et al., 2001) and reduced neuronal and synaptic density (Vercellino et al., 2005).
Figure 5. Cortical Demyelination and neuronal pathology.
Three patterns of cortical demyelination (orange) occur in MS brains. Type I lesions occur at the leukocortical junction and demyelinate both white and gray matter (a). Type II lesions are small perivascular lesions (b). Type III lesions extended into the cortex from the pial surface and often involve multiple gyri (c).
(d) Cortical demyelination occurs without significant infiltration of hematogenous leukocytes, which is schematically depicted in a Type I lesion (ctx, cortex; wm, white matter). (e) Axons and dendrites are transected (arrowheads) during cortical demyelination. (f) Apoptotic neurons (arrows), identified by tunnel staining, are increased in demyelinated cortex. (Reproduced from (Peterson et al., 2001; Peterson et al., 2005; Trapp and Nave, 2008)
There is no reliable mechanism to identify cortical demyelination by routine brain imaging because cortical demyelination occurs without significant infiltration of hematogenous leukocytes (Bo et al., 2003a; Kidd et al., 1999; Peterson et al., 2001) and significant break down of the BBB. Because of the paucity of inflammatory cell infiltrates, the criteria used to stage white matter lesions also cannot be directly applied to cortical lesions (Lucchinetti et al., 2000). Based upon the distribution of demyelination, three types of cortical lesions have been described (Fig 5a)(Peterson et al., 2001). Type I lesions are leukocortical areas of demyelination that contiguously occupy subcortical white matter and cortex. Type II lesions are small, perivascular areas of demyelination. Type III lesions are strips or bands of subpial demyelination that can transverse several gyri and often stop at cortical layer III or IV (Peterson et al., 2001). Based upon current estimates, Type III and to some extent Type I lesions contribute significantly to the total lesion load. However, it is unknown whether subgrouping patients with variable pathogenesis (Lucchinetti et al., 2000) will identify patients with particular cortical lesion subtypes. Mechanisms of demyelination and characteristics of the immune response or demyelinating inflammatory environment may be different for each cortical lesion subtype. Demyelination without significant participation of immune cells from the blood, however, questions the basic premises of MS pathogenesis. Despite the paucity of immune cells, neuronal and axonal pathology are prominent features of cortical lesions.
Neuronal damage in motor and sensory cortex would negatively impact ambulation in MS patients and thus cortical pathology must contribute to neurological decline. In addition to motor and sensory deficits, gray matter lesions may provide the pathological correlate for the cognitive and executive dysfunction that arises in 40–70% of MS patients (Beatty et al., 1995; Rao et al., 1991). Loss of lower motor neurons has also been reported in postmortem MS brains (Schirmer et al., 2009; Vogt et al., 2009). Demyelination has been reported in other gray matter areas including basal ganglia, hippocampus, cerebellar cortex, and spinal gray matter (Geurts et al., 2009). Demyelinated lesions in MS hippocampus have been reported to be common and could cause memory deficits (Geurts et al., 2007; Sicotte et al., 2008). Survival of neurons in thalamus and hippocampus in MS patients are also reduced due to loss of myelin (Cifelli et al., 2002; Geurts et al., 2007; Papadopoulos et al., 2009). Interestingly, neocortical synaptic densities were reduced at a greater rate in comparison to neuronal loss (Wegner et al., 2006), suggesting that cortical demyelination causes a “dying back” axonopathy. Loss of axons, dendrites, and neurons is therefore an important contributor of the irreversible permanent neurological disability experienced by MS patients.
6. Future Challenges and development of therapies
The major challenge for MS researchers is to develop therapies that not only prevent the neurological disability associated with MS, but that also are able to stop it. Two classes of therapeutics, Interferon B (IFNB) and Glatiramer Acetate (GA), are commonly used to treat RR-MS. GA and three slightly different recombinant IFNBs reduce relapses, decrease MRI activity and possibly slow, but do not stop the progression of permanent neurological disability (Trapp and Nave, 2008). As inflammation is one of the major factors contributing to axonal pathology, aggressive anti-inflammatory treatment during early stages of RR-MS directly reduces new inflammatory lesions and indirectly prevents axonal injury. It remains to be determined if more aggressive anti inflammatory therapies effect long-term disease progression. In Phase III trials, Tysabri showed great promise for the treatment of RRMS by reducing new gad-enhancing MS lesion by 90% (O'Connor et al., 2004; Polman et al., 2006). The occurrence of progressive multifocal leukoencephalopathy (PML), a rare and most often fatal virus-induced demyelinating disease of immunocompromised individuals was reported in MS patients receiving Tysabri (Kleinschmidt-DeMasters and Tyler, 2005). Alemtuzumab, which targets the mature lymphocyte marker CD52, leads to depletion of CD4+ and CD8+ lymphocytes (Cox et al., 2005) and the development of thyroid autoimmunity (Buttmann, 2010) in treated patients. The B-cell-depleting, chimeric anti-CD20 mAb, also did not significantly reduce the risk of disease progression, despite a favorable trend during initial testing (Hawker et al., 2009). One should therefore be cautious in application of strong immunosupressants to MS patients because of potential fatal side effects (Clifford et al., 2010; Goodin et al., 2008; Menge et al., 2008).
In order to develop new effective therapies for MS patients, we need to elucidate and understand the cause of the disease, which may be multi-factorial. As genetic associations with disease risk are modest and not Mendelian, gene linkage studies, therefore, are not likely to directly point to the cause of MS as has been the case in inherited CNS diseases. One can argue that the concept of MS as an autoimmune disease induced by molecular mimicry has little direct support despite decades of searching for the initiating environmental agent. The past decade has seen renewed interest in the role of the axon and axon-myelin interactions in the pathogenesis of MS (Nave, 2010). Axonal protection in MS has been deemed the therapeutic challenge for the next decade (Rieckmann and Maurer, 2002). The development of surrogate markers for axonal loss is needed to monitor neurodegeneration. Further elucidation of the molecular mechanisms behind axonal injury and their relation to disease stage in MS is essential for the development and refinement of therapeutic strategies. Among the existing strategies of axonal protections, remyelination and ion channel blockers have generated immense interest as potential therapeutic strategies.
Repair of myelin restores conduction and prevents axonal degeneration. It is therefore one of the best axon-protective mechanisms in myelin disease (Dubois-Dalcq, 1995; Dubois-Dalcq et al., 2008; Franklin and Ffrench-Constant, 2008; Lassmann et al., 1997; Trapp and Nave, 2008). Current remyelination therapies focus on transplantation of oligodendrocyte-producing cells and manipulation of endogenous remyelination (Gallo and Armstrong, 2008; Miller and Mi, 2007). Studies are also beginning to unravel the molecular mechanisms by which myelin-forming cells provide trophic support to axons (for review, see (Nave and Trapp, 2008). Some MS lesions successfully remyelinated and production of new oligodendrocytes that remyelinate MS lesions would be the best characterized and most abundant adult human brain repair phenomenon.
Persistent demyelinated axonal Na+ accumulation is thought to contribute to Ca++ - mediated axonal degeneration in MS brain (Smith et al., 1998; Waxman, 2006). Another viable therapeutic approach therefore would be preventing axonal degeneration. Inhibition of Na+ channel and Ca++-mediated activators are thus logical therapeutic targets that may delay axonal degeneration and permanent neurological disability in MS patients (Waxman, 2005; Waxman, 2006). In animal models of MS, systemic administration of the class I anti-arrhythmic flecainide (Bechtold et al., 2004) or Na+ channel-blocking anticonvulsants (lamotrigine, phenytoin, carbamazepine) (Bechtold et al., 2004; Bechtold et al., 2006; Black et al., 2006; Black and Waxman, 2008; Lo et al., 2002; Lo et al., 2003) reduced neurological disability. Two of these drugs, phenytoin and carbamazepine although being protective in EAE, also show acute exacerbation of disease and an increase in inflammatory markers following withdrawal of these agents after disease induction (Black et al., 2007a). In a phase II clinical trials, lamotrigine also did not have significant effects between placebo and secondary progressive multiple sclerosis patients (Kapoor et al., 2010).
Excitotoxicity mediated by glutamate is involved in tissue damage in acute MS lesions (Pitt et al., 2000) and treatment with the AMPA/kainate glutamate receptor antagonist NBQX decreased neurological disability, increased oligodendrocyte survival and reduced axonal damage in experimental autoimmune encephalomyelitis (EAE) (Groom et al., 2003; Pitt et al., 2000; Smith et al., 2000). It is therefore possible that a combinatorial approach towards blocking both AMPA/kainate and NMDA class of receptors may be an effective target for protecting glia and axons (Trapp and Stys, 2009).
7. Conclusion
Neurodegeneration is a fundamental aspect of MS pathogenesis as loss of axons, dendrites, and neurons is a major cause of permanent neurological disability in MS patients. Current hypotheses support primary inflammatory demyelination as the underlying cause of axonal loss during earlier stages in MS. The transition from RR-MS to SP-MS is thought to occur when a threshold of axonal loss is reached and the compensatory capacity of the CNS is surpassed, resulting in steady progression of permanent neurological symptoms. This is one of the reasons for the limited success of immunomodulatory therapies which delay the progression of MS, but do not succeed in preventing neurological decline. Elucidation of the molecular mechanisms responsible for neuronal injury and determining whether axonal or neuronal pathology precedes demyelination are essential for the development of therapies that will stop neurological decline in MS patients. This represents a significant challenge to the MS research community. Multi-disciplinary approaches, new animal models, a better understanding of the natural history of MS and a mindset to look for novel aspects of MS pathogenesis will aid in this important and hopefully soon to be productive endeavor.
Research Highlights.
Cause of Neurological Disability in Multiple Sclerosis
Axonal Transection during Inflammatory Demyelination
Immune-Mediated Axonal Loss
Axonal loss and degeneration due to loss of myelin-derived trophic support
Degeneration of Chronically Demyelinated Axons
Cortical Demyelination
Acknowledgements
The work is in part by supported by NMSS RG-4280 (RD), NIH NS38667 and NIH NS35058 (BDT). The authors would like to thank Dr. Christopher Nelson for assisting with the editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest None
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., III CNS energy metabolism as related to function. Brain Res. Brain Res. Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Hughes P, Perry VH. The evidence for primary axonal loss in multiple sclerosis. Rev. Neurol. 2000;30:1203–1208. [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, Inglese M, Guttmann CR, Horsfield MA, Filippi M. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–625. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Paul RH, Wilbanks SL, Hames KA, Blanco CR, Goodkin DE. Identifying multiple sclerosis patients with mild or global cognitive impairment using the Screening Examination for Cognitive Impairment (SEFCI) Neurology. 1995;45:718–723. doi: 10.1212/wnl.45.4.718. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Kapoor R, Smith KJ. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann. Neurol. 2004;55:607–616. doi: 10.1002/ana.20045. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Miller SJ, Dawson AC, Sun Y, Kapoor R, Berry D, Smith KJ. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J. Neurol. 2006;253:1542–1551. doi: 10.1007/s00415-006-0204-1. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Smith KJ. Sodium-mediated axonal degeneration in inflammatory demyelinating disease. J. Neurol. Sci. 2005;233:27–35. doi: 10.1016/j.jns.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Stuve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: therapeutic implications. Clin. Neuropharmacol. 2009;32:121–132. doi: 10.1097/WNF.0b013e3181880359. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduce N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57:1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Carrithers M, Carrithers LM, Waxman SG. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 2007a;62:21–33. doi: 10.1002/ana.21172. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Hains BC, Saab CY, Waxman SG. Long-term protection of central axons with phenytoin in monophasic and chronic-relapsing EAE. Brain. 2006;129:3196–3208. doi: 10.1093/brain/awl216. [DOI] [PubMed] [Google Scholar]
- Black JA, Newcombe J, Trapp BD, Waxman SG. Sodium channel expression within chronic multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 2007b;66:828–837. doi: 10.1097/nen.0b013e3181462841. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Phenytoin protects central axons in experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2008;274:57–63. doi: 10.1016/j.jns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann. Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult. Scler. 2003a;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 2003b;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- Brownell B, Hughes JT. Distribution of plaques in the cerebrum in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1962;25:315–320. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J. Neurol. 2005;252(Suppl 5):v3–v9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- Buckle GJ. Functional magnetic resonance imaging and multiple sclerosis: the evidence for neuronal plasticity. J. Neuroimaging. 2005;15:82S–93S. doi: 10.1177/1051228405284093. [DOI] [PubMed] [Google Scholar]
- Buttmann M. Treating multiple sclerosis with monoclonal antibodies: a 2010 update. Expert. Rev. Neurother. 2010;10:791–809. doi: 10.1586/ern.10.38. [DOI] [PubMed] [Google Scholar]
- Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD. Neurogenesis in the chronic lesions of multiple sclerosis. Brain. 2008;131:2366–2375. doi: 10.1093/brain/awn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcot M. Histologie de la sclerose en plaques. Gaz Hosp. 1868;141:554–5. 557–8. [Google Scholar]
- Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002;52:650–653. doi: 10.1002/ana.10326. [DOI] [PubMed] [Google Scholar]
- Clifford DB, De LA, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, Compston DA, Coles AJ. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur. J. Immunol. 2005;35:3332–3342. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Guidi L, Stromillo ML, Bartolozzi ML, Federico A. Imaging neuronal and axonal degeneration in multiple sclerosis. Neurol. Sci. 2003a;24(Suppl 5):S283–S286. doi: 10.1007/s10072-003-0175-2. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, Guidi L, Ghezzi A, Montanari E, Cifelli A, Federico A, Smith SM. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003b;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- De SN, Filippi M. MR spectroscopy in multiple sclerosis. J. Neuroimaging. 2007;17(Suppl 1):31S–35S. doi: 10.1111/j.1552-6569.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- De SN, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP, Matthews PM, Arnold DL. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch. Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M. Regeneration of oligodendrocytes and myelin. TINS. 1995;18:289–291. doi: 10.1016/0166-2236(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Williams A, Stadelmann C, Stankoff B, Zalc B, Lubetzki C. From fish to man: understanding endogenous remyelination in central nervous system demyelinating diseases. Brain. 2008;131:1686–1700. doi: 10.1093/brain/awn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Chang A, Swamy L, Siu A, Kidd GJ, Rudick R, Mirnics K, Trapp BD. Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain. 2007;130:2566–2576. doi: 10.1093/brain/awm206. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124:1813–1820. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A, Martinelli V, Grossman RI, Scotti G, Comi G, Falini A. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003;126:433–437. doi: 10.1093/brain/awg038. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA. Conventional MRI in multiple sclerosis. J. Neuroimaging. 2007;17(Suppl 1):3S–9S. doi: 10.1111/j.1552-6569.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- Fisher E, Chang A, Fox RJ, Tkach JA, Svarovsky T, Nakamura K, Rudick RA, Trapp BD. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann. Neurol. 2007;62:219–228. doi: 10.1002/ana.21113. [DOI] [PubMed] [Google Scholar]
- Fisher E, Rudick RA, Cutter G, Baier M, Miller D, Weinstock-Guttman B, Mass MK, Dougherty DS, Simonian NA. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult. Scler. 2000;6:373–377. doi: 10.1177/135245850000600602. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J. Neurosci. Res. 1997;50:337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gallo V, Armstrong RC. Myelin repair strategies: a cellular view. Curr. Opin. Neurol. 2008;21:278–283. doi: 10.1097/WCO.0b013e3282fd1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter P, Prince C, Esiri MM. Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol. Appl. Neurobiol. 1999;25:459–467. doi: 10.1046/j.1365-2990.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van, d. V. Extensive hippocampal demyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Stys PK, Minagar A, Amor S, Zivadinov R. Gray matter pathology in (chronic) MS: modern views on an early observation. J. Neurol. Sci. 2009;282:12–20. doi: 10.1016/j.jns.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Wolswijk G, Bo L, van, d. V, Polman CH, Troost D, Aronica E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain. 2003;126:1755–1766. doi: 10.1093/brain/awg179. [DOI] [PubMed] [Google Scholar]
- Giess R, Maurer M, Linker R, Gold R, Warmuth-Metz M, Toyka KV, Sendtner M, Rieckmann P. Association of a null mutation in the CNTF gene with early onset of multiple sclerosis. Arch. Neurol. 2002;59:407–409. doi: 10.1001/archneur.59.3.407. [DOI] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–379. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat. Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave K-A. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Groom AJ, Smith T, Turski L. Multiple sclerosis and glutamate. Ann. N. Y. Acad. Sci. 2003;993:229–275. doi: 10.1111/j.1749-6632.2003.tb07533.x. [DOI] [PubMed] [Google Scholar]
- Hafer-Macko C, Hsieh S-T, Li CY, Ho TW, Sheikh KA. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann. Neurol. 1996;40:635–644. doi: 10.1002/ana.410400414. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, Hauser S, Waubant E, Vollmer T, Panitch H, Zhang J, Chin P, Smith CH. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- Ho TW, McKhann GM, Griffin JW. Human autoimmune neuropathies. Annu. Rev. Neurosci. 1998;21:187–226. doi: 10.1146/annurev.neuro.21.1.187. [DOI] [PubMed] [Google Scholar]
- Hoffmann V, Pohlau D, Przuntek H, Epplen JT, Hardt C. A null mutation within the ciliary neurotrophic factor (CNTF)-gene: implications for susceptibility and disease severity in patients with multiple sclerosis. Genes Immun. 2002;3:53–55. doi: 10.1038/sj.gene.6363818. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R. Biotechnological agents for the immunotherapy of multiple sclerosis. Principles, problems and perspectives (invited review) Brain. 1997;120:865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J. Exp. Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, Chataway J, Hughes RA, Miller DH. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681–688. doi: 10.1016/S1474-4422(10)70131-9. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury SJ, Guttmann CR, Orav EJ, Kikinis R, Jolesz FA, Weiner HL. Changes in activated T cells in the blood correlate with disease activity in multiple sclerosis. Arch. Neurol. 2000;57:1183–1189. doi: 10.1001/archneur.57.8.1183. [DOI] [PubMed] [Google Scholar]
- Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Bauer J, Djamshidian A, Weissert R, Wallstroem E, Stefferl A, Zimprich F, Olsson T, Linington C, Schmidbauer M, Lassmann H. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2001;124:1114–1124. doi: 10.1093/brain/124.6.1114. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J. Neurol. Sci. 2005;233:55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J. Neurol. Sci. 2003;206:187–191. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti C, Rodriguez M. Remyelination in multiple sclerosis. Mult. Scler. 1997;3:133–136. doi: 10.1177/135245859700300213. [DOI] [PubMed] [Google Scholar]
- Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J. Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am. J. Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Black JA, Waxman SG. Neuroprotection of axons with phenytoin in experimental allergic encephalomyelitis. Neuroreport. 2002;13:1909–1912. doi: 10.1097/00001756-200210280-00015. [DOI] [PubMed] [Google Scholar]
- Lo AC, Saab CY, Black JA, Waxman SG. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J. Neurophysiol. 2003;90:3566–3571. doi: 10.1152/jn.00434.2003. [DOI] [PubMed] [Google Scholar]
- Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123:308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008a;34:577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008b;131:1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G. How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS disorders. Lancet Neurol. 2004;3:372–378. doi: 10.1016/S1474-4422(04)00771-9. [DOI] [PubMed] [Google Scholar]
- Matthews PM, Pioro E, Narayanan S, De Stefano N, Fu L, Francis G, Antel J, Wolfson C, Arnold DL. Assessment of lesion pathology in multiple sclerosis using quantitative MRI morphometry and magnetic resonance spectroscopy. Brain. 1996;119:715–722. doi: 10.1093/brain/119.3.715. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Medana I, Martinic MA, Wekerle H, Neumann H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am. J. Pathol. 2001;159:809–815. doi: 10.1016/S0002-9440(10)61755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge T, Weber MS, Hemmer B, Kieseier BC, von Budingen HC, Warnke C, Zamvil SS, Boster A, Khan O, Hartung HP, Stuve O. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs. 2008;68:2445–2468. doi: 10.2165/0003495-200868170-00004. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Miller RH, Mi S. Dissecting demyelination. Nat. Neurosci. 2007;10:1351–1354. doi: 10.1038/nn1995. [DOI] [PubMed] [Google Scholar]
- Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J. Neuroimaging. 2005;15:46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Fu L, Pioro E, De Stefano N, Collins DL, Francis GS, Antel JP, Matthews PM, Arnold DL. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann. Neurol. 1997;41:385–391. doi: 10.1002/ana.410410314. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Nave K-A. Myelin-specific genes and their mutations in the mouse. In: Jessen KR, Richardson WD, editors. Glial Cell Development. Basic Principles and Clinical Relevance. Oxford: Bios; Oxford: 1996. pp. 141–164. [Google Scholar]
- Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N. Engl. J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- O'Connor PW, Goodman A, Willmer-Hulme AJ, Libonati MA, Metz L, Murray RS, Sheremata WA, Vollmer TL, Stone LA. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology. 2004;62:2038–2043. doi: 10.1212/01.wnl.0000128136.79044.d6. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, Rehak R, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann. Neurol. 2009a;65:151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 2009b;65:160–166. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano P, Iannetti GD, Caramia F, Mainero C, Di Legge S, Bozzao L, Pozzilli C, Lenzi GL. Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain. 2002;125:1607–1615. doi: 10.1093/brain/awf164. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Caramia F. Functional brain reorganization in multiple sclerosis: evidence from fMRI studies. J. Neuroimaging. 2006;16:104–114. doi: 10.1111/j.1552-6569.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos D, Pham-Dinh D, Reynolds R. Axon loss is responsible for chronic neurological deficit following inflammatory demyelination in the rat. Exp. Neurol. 2006;197:373–385. doi: 10.1016/j.expneurol.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem. Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons and reduced inflammation in cortical MS lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Kidd GJ, Trapp BD. Axonal Degeneration in Multiple Sclerosis: The Histopathological Evidence. In: Waxman S, editor. Multiple Sclerosis as a Neuronal Disease. Elsevier; 2005. pp. 165–184. [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Prineas J. Pathology of Multiple Sclerosis. In: Cook S, editor. Handbook of Multiple Sclerosis. Marcel Dekker; 2001. pp. 289–324. [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Arnoutelis R, Jenkinson M, Antel J, Matthews PM, Arnold DL. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123(Pt 11):2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Maurer M. Anti-inflammatory strategies to prevent axonal injury in multiple sclerosis. Curr. Opin. Neurol. 2002;15:361–370. doi: 10.1097/00019052-200206000-00022. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Filippi M. Functional MRI in multiple sclerosis. J. Neuroimaging. 2007;17(Suppl 1):36S–41S. doi: 10.1111/j.1552-6569.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Mezzapesa DM, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M. Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage. 2003;18:847–855. doi: 10.1016/s1053-8119(03)00043-0. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J. Neurosci. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer L, Albert M, Buss A, Schulz-Schaeffer WJ, Antel JP, Bruck W, Stadelmann C. Substantial early, but nonprogressive neuronal loss in multiple sclerosis (MS) spinal cord. Ann. Neurol. 2009;66:698–704. doi: 10.1002/ana.21799. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl. Acad. Sci U. S. A. 2004;101:2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol. 2007;17:230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL. Functional analysis of the mouse Scn8a sodium channel. J. Neurosci. 1998;18:6093–6102. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Albert M, Wegner C, Bruck W. Cortical pathology in multiple sclerosis. Curr. Opin. Neurol. 2008;21:229–234. doi: 10.1097/01.wco.0000318863.65635.9a. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Kerschensteiner M, Misgeld T, Bruck W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- Stefano ND, Narayanan S, Matthews PM, Mortilla M, Dotti MT, Federico A, Arnold DL. Proton MR spectroscopy to assess axonal damage in multiple sclerosis and other white matter disorders. J. Neurovirol. 2000;6(Suppl 2):S121–S129. [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Stevenson VL, Miller DH. Magnetic resonance imaging in the monitoring of disease progression in multiple sclerosis. Mult. Scler. 1999;5:268–272. doi: 10.1177/135245859900500413. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Current Mol. Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol. 2005;223:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Stys PK, Jiang Q. Calpain-dependent neurofilament breakdown in anoxic and ischemic rat central axons. Neurosci. Lett. 2002;328:150–154. doi: 10.1016/s0304-3940(02)00469-x. [DOI] [PubMed] [Google Scholar]
- Stys PK, Lehning E, Saubermann AJ, Lopachin RM., Jr. Intracellular concentrations of major ions in rat myelinated axons and glia: calculations based on electron probe X-ray microanalyses. J. Neurochem. 1997;68:1920–1928. doi: 10.1046/j.1471-4159.1997.68051920.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Arnold DL. The role of MRS and fMRI in multiple sclerosis. Adv. Neurol. 2006;98:185–202. [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J. Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 1999a;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff RM, Fisher E, Rudick RA. Neurodegeneration in multiple sclerosis: Relationship to neurological disability. The Neuroscientist. 1999b;5:48–57. [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey matter pathology in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dorr J, Dorr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann. Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Membranes, myelin, and the pathophysiology of multiple sclerosis. N. Engl. J. Med. 1982;306:1529–1533. doi: 10.1056/NEJM198206243062505. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Sodium channel blockers and axonal protection in neuroinflammatory disease. Brain. 2005;128:5–6. doi: 10.1093/brain/awh353. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat. Rev. Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Stys PK, Ransom BR. Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Res. 1992;574:105–119. doi: 10.1016/0006-8993(92)90806-k. [DOI] [PubMed] [Google Scholar]
- Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–967. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann. Neurol. 2009;65:239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG. Natural history of multiple sclerosis. Ann. Neurol. 1998;36:S6–S11. doi: 10.1002/ana.410360704. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Yu M, Tuohy V, Trapp BD. Loss of spinal cord axons in the experimental allergic encephalomyelitis animal model of multiple sclerosis. J Neurochem. 2000;206:165–171. [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Baek RC, Kirschner DA, Peterson A, Fujii Y, Nave KA, Macklin WB, Trapp BD. Evolution of a neuroprotective function of central nervous system myelin. J. Cell Biol. 2006;172:469–478. doi: 10.1083/jcb.200509174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P-H, Lee VMY, Li C, Roder J, Trapp BD. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Fowler CD, Kidd GJ, Chang A, Rudick R, Fisher E, Trapp BD. Imaging correlates of decreased axonal Na+/K+ ATPase in chronic multiple sclerosis lesions. Ann. Neurol. 2008;63:428–435. doi: 10.1002/ana.21381. [DOI] [PubMed] [Google Scholar]