Abstract
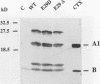
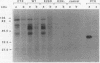
Cholera toxin (CTX) is composed of two subunits, subunit A, which possesses ADP-ribosyltransferase activity, and subunit B, which is responsible for receptor binding. It has previously been shown that agents that increase cyclic AMP (cAMP) levels in cells induce differentiation of PC12 cells into neurite-like cells. In this report, we show that as little as 100 pg of CTX per ml induces such changes. CTX was found to ADP-ribosylate at least four membrane proteins of PC12 cells in vitro and in vivo and to increase intracellular cAMP levels. We have developed an inducible ctx gene expression system in Vibrio cholerae by using the tac promoter. The culture medium of the CTX-producing bacteria was able to induce the morphological changes and the ADP-ribosylation of the PC12 cell membrane proteins. We have constructed two CTX-cross-reactive mutant proteins (CTX-CRM) by site-directed mutagenesis. The choice of glutamic acid 29 as the target amino acid was based on sequence similarities with other bacterial toxins. CTX-CRM-E29 delta, in which the Glu-29 of the A subunit was deleted, showed strongly reduced ADP-ribosyltransferase activity and did not induce significant morphological changes of PC12 cells. In contrast, CTX-CRM-E29D, in which the Glu-29 was replaced by an aspartic acid, was as active as the wild-type protein. We conclude that the ADP-ribosylation activity of CTX is important for the toxin-induced differentiation of PC12 cells. Pertussis toxin, which had no visible effect on PC12 cell morphology, was also able to ADP-ribosylate a membrane-bound protein(s) in vitro and in vivo. Pertussis toxin alone did not significantly increase cAMP levels in PC12 cells, but it acted synergistically with CTX.
Full text
PDF

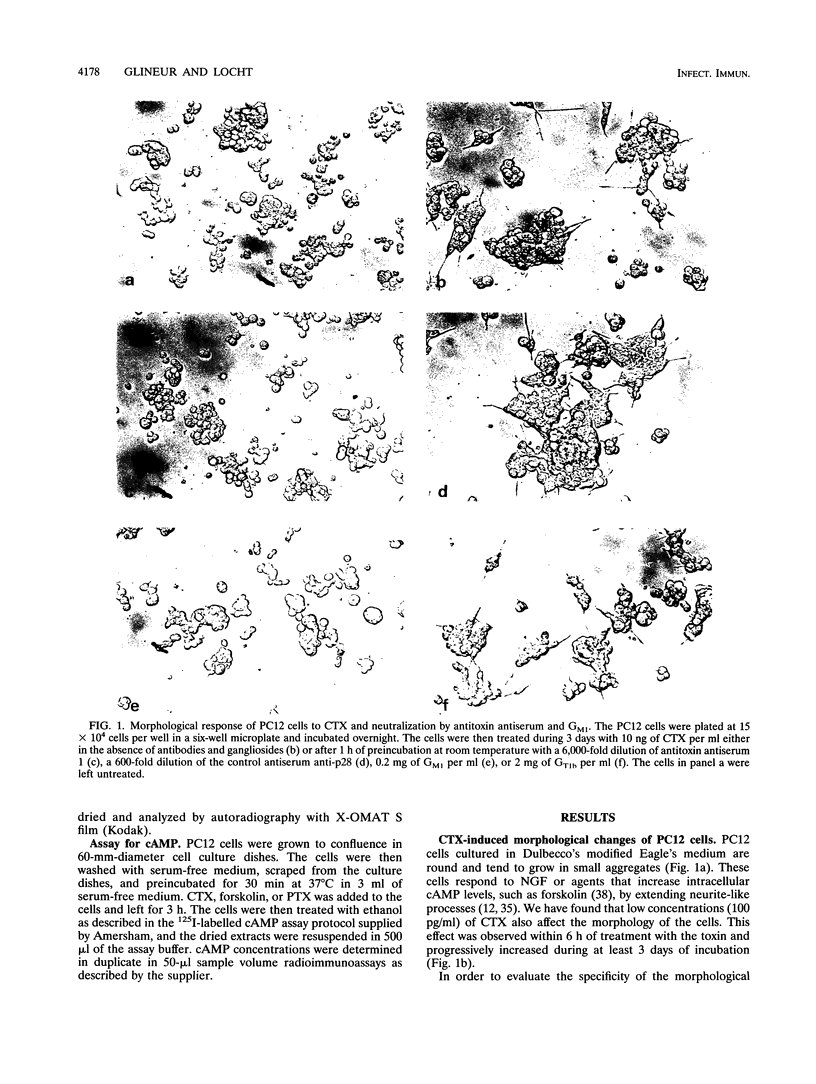
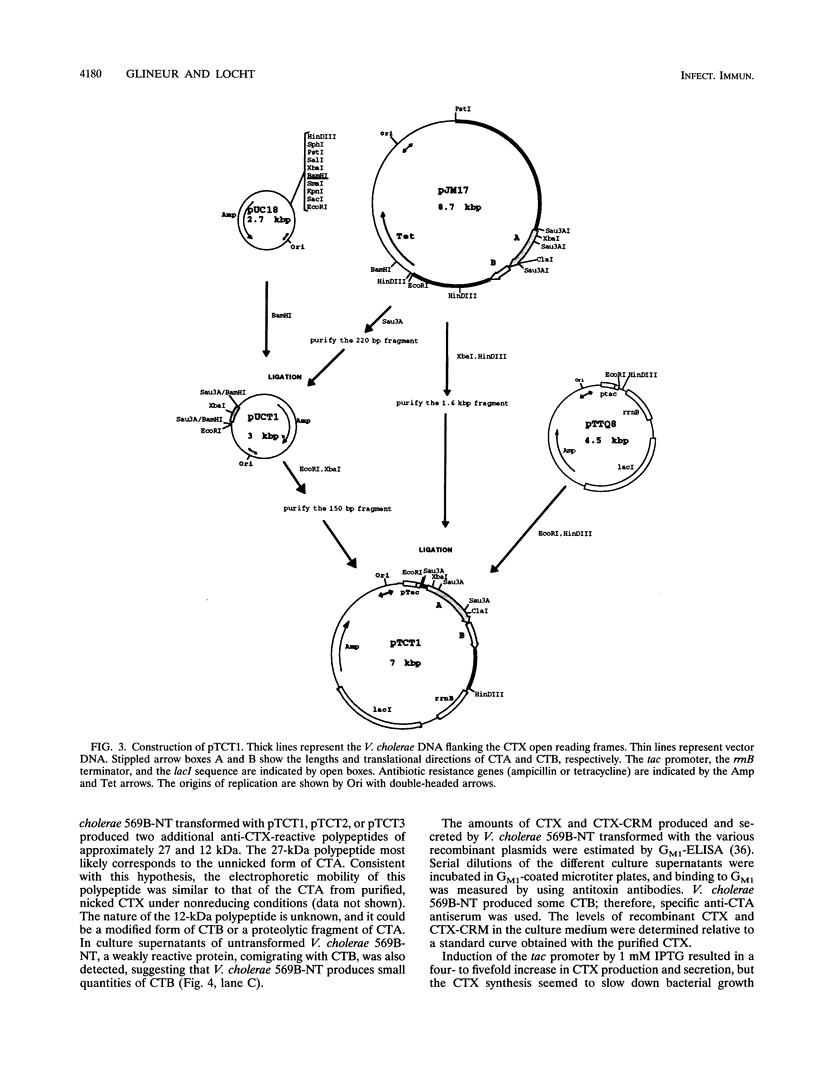

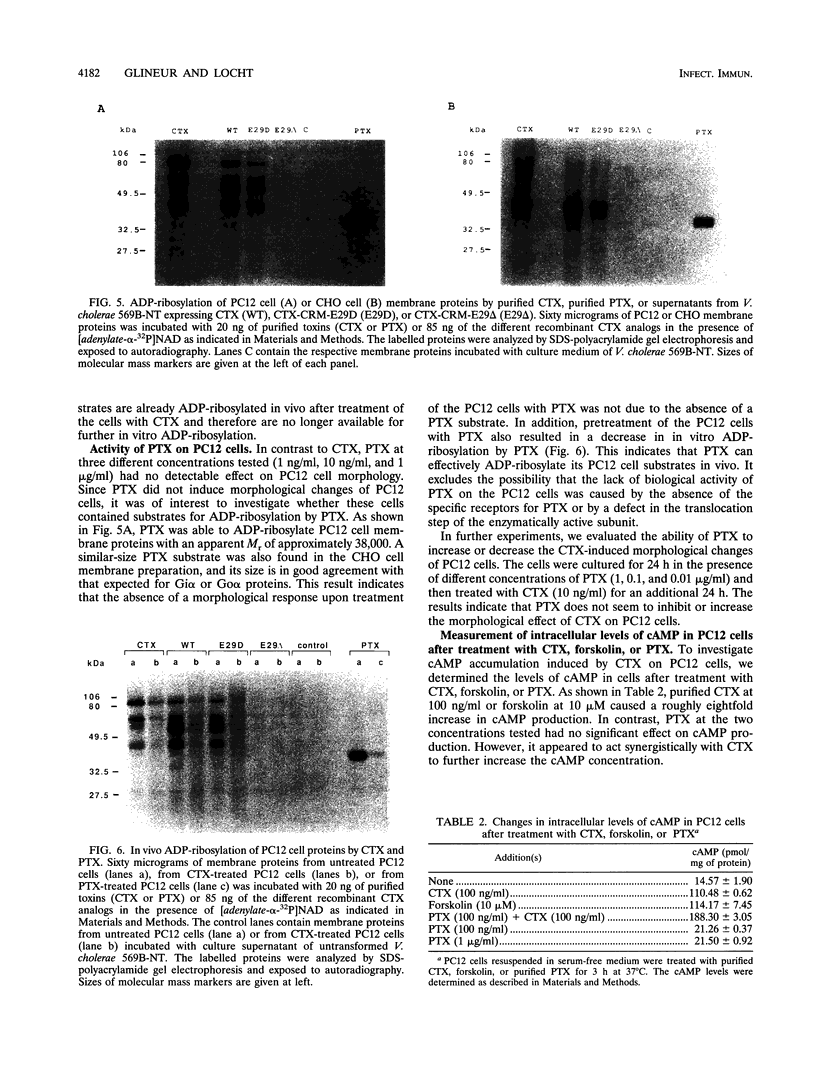



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine R., Tallett A., van Heyningen S., Locht C. Evidence for a catalytic role of glutamic acid 129 in the NAD-glycohydrolase activity of the pertussis toxin S1 subunit. J Biol Chem. 1993 Nov 15;268(32):24149–24155. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplak W., Jr, Locht C., Mar V. L., Burnette W. N., Keith J. M. Photolabelling of mutant forms of the S1 subunit of pertussis toxin with NAD+. Biochem J. 1990 Jun 15;268(3):547–551. doi: 10.1042/bj2680547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina G., Barbieri J. T. Role of tryptophan 26 in the NAD glycohydrolase reaction of the S-1 subunit of pertussis toxin. J Biol Chem. 1989 Oct 15;264(29):17322–17328. [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell. 1991 Jan 11;64(1):29–37. doi: 10.1016/0092-8674(91)90206-e. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Smith D. M. Stimulation of steroidogenesis in tissue culture by enterotoxigenic Escherichia coli and its neutralization by specific antiserum. Infect Immun. 1974 Mar;9(3):500–505. doi: 10.1128/iai.9.3.500-505.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Pseudomonas aeruginosa exotoxin A: alterations of biological and biochemical properties resulting from mutation of glutamic acid 553 to aspartic acid. Biochemistry. 1990 May 29;29(21):5043–5049. doi: 10.1021/bi00473a007. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P. W., Landreth G. E., Bothwell M. A., Shooter E. M. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981 May;89(2):240–245. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
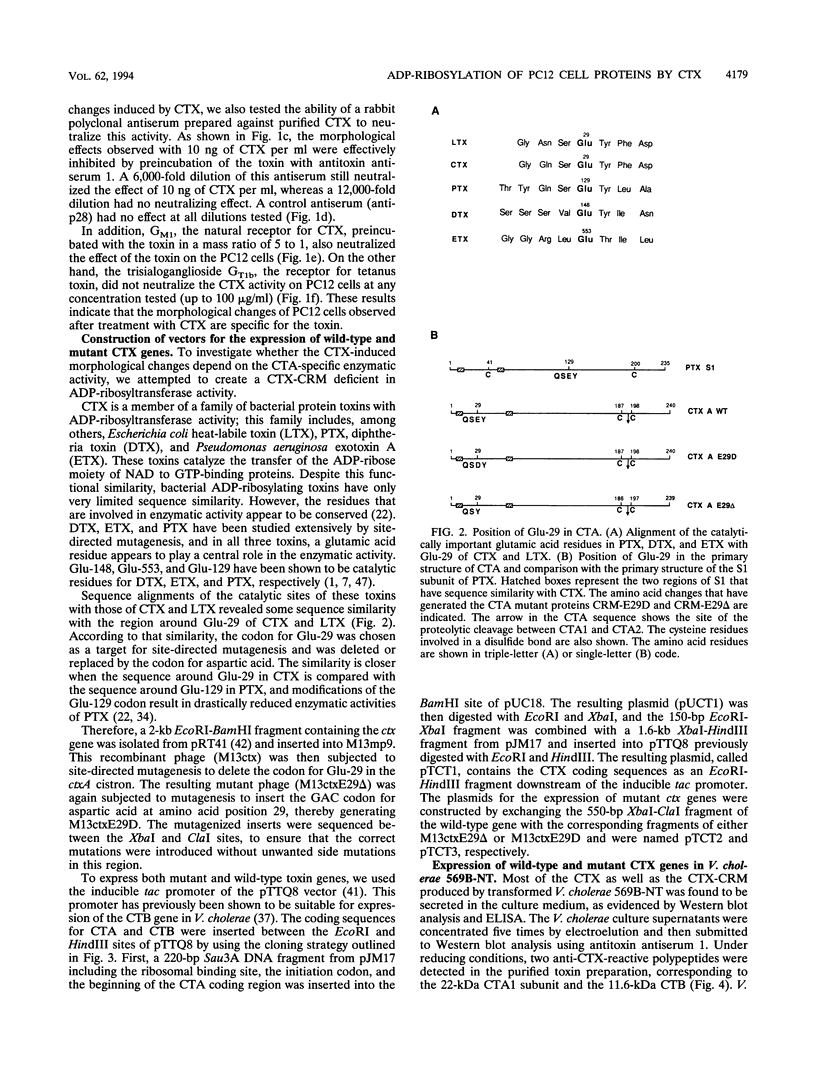
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J. A., Tsai S. C., Adamik R., Yost D. A., Hewlett E. L., Moss J. Amino acid-specific ADP-ribosylation. Sensitivity to hydroxylamine of [cysteine(ADP-ribose)]protein and [arginine(ADP-ribose)]protein linkages. J Biol Chem. 1985 Dec 25;260(30):16187–16191. [PubMed] [Google Scholar]
- Jobling M. G., Holmes R. K. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun. 1992 Nov;60(11):4915–4924. doi: 10.1128/iai.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis S., Hagmann J., Fishman P. H., Chang P. P., Moss J. Mechanism of action of cholera toxin on intact cells. Generation of A1 peptide and activation of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):12148–12152. [PubMed] [Google Scholar]
- Katada T., Ui M. In vitro effects of islet-activating protein on cultured rat pancreatic islets. Enhancement of insulin secretion, adenosine 3':5'-monophosphate accumulation and 45Ca flux. J Biochem. 1981 Apr;89(4):979–990. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locht C., Capiau C., Feron C. Identification of amino acid residues essential for the enzymatic activities of pertussis toxin. Proc Natl Acad Sci U S A. 1989 May;86(9):3075–3079. doi: 10.1073/pnas.86.9.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Cieplak W., Marchitto K. S., Sato H., Keith J. M. Activities of complete and truncated forms of pertussis toxin subunits S1 and S2 synthesized by Escherichia coli. Infect Immun. 1987 Nov;55(11):2546–2553. doi: 10.1128/iai.55.11.2546-2553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Marcus H., Ketley J. M., Kaper J. B., Holmes R. K. Effects of DNase production, plasmid size, and restriction barriers on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):149–154. doi: 10.1111/j.1574-6968.1990.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Mekalanos J. J., Moseley S. L., Murphy J. R., Falkow S. Isolation of enterotoxin structural gene deletion mutations in Vibrio cholerae induced by two mutagenic vibriophages. Proc Natl Acad Sci U S A. 1982 Jan;79(1):151–155. doi: 10.1073/pnas.79.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza M., Bartoloni A., Prugnola A., Silvestri S., Rappuoli R. Subunit S1 of pertussis toxin: mapping of the regions essential for ADP-ribosyltransferase activity. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7521–7525. doi: 10.1073/pnas.85.20.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C., Jastorff B. The role of cAMP in nerve growth factor-promoted neurite outgrowth in PC12 cells. J Cell Biol. 1986 Mar;102(3):821–829. doi: 10.1083/jcb.102.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Huda S., Neogi P. K., Daniel R. R., Spira W. M. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980 Jan;11(1):35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J., Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Wilson B. A., Reich K. A., Weinstein B. R., Collier R. J. Active-site mutations of diphtheria toxin: effects of replacing glutamic acid-148 with aspartic acid, glutamine, or serine. Biochemistry. 1990 Sep 18;29(37):8643–8651. doi: 10.1021/bi00489a021. [DOI] [PubMed] [Google Scholar]
- Young D. B., Broadbent D. A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982 Sep;37(3):875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]