Abstract
Objective
In systemic inflammatory diseases such as rheumatoid arthritis, pain and inflammation exhibit reciprocal inter-relationships. However, the nature of the association between pain and inflammation in osteoarthritis (OA) is not clear. We assessed experimental pain sensitivity and compared the inflammatory response to pain in 26 OA patients and 33 age-and sex-matched controls from the general population.
Methods
Participants underwent psychophysical pain testing to assess pain sensitivity in response to heat, cold and mechanical stimuli. Blood samples were taken at baseline and four time points after testing to determine the effect of acute pain on C-reactive protein (CRP), interleukin 6 (IL-6), interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α).
Results
OA patients had lower pressure pain thresholds (P ≤ 0.003) and higher heat pain ratings (P ≤ 0.04) than controls across multiple body sites. OA patients had higher CRP levels than controls (P = 0.007). CRP levels did not change in response to pain testing. Although not statistically significant, OA patients tended to have higher IL-6 levels than controls (P = 0.12). IL-6 levels increased after pain testing in OA patients and controls (P < 0.0001), but the amount of increase was not different between the two groups. Among OA patients, heightened pain sensitivity was associated with elevated CRP and IL-6 (P ≤ 0.05).
Conclusions
Compared to controls, OA patients are more sensitive to experimental pain at multiple body sites. IL-6 levels in OA patients and controls exhibited reactivity to acute painful stimuli, increasing at similar rates after psychophysical pain testing.
Pain, stress and inflammation are intricately interrelated (1). Among patients with systemic inflammatory diseases, such as rheumatoid arthritis (RA), the stress response is altered (2). RA patients not taking anti-TNF agents have significantly greater stress-induced increases in TNF-α production compared to controls and RA patients taking anti-TNF agents (3). Similarly, RA patients exhibit increases in serum IL-6 after mental stress associated with anticipation of surgery (4) and increases in serum CRP after mental stress from paced auditory serial addition (5); however, no changes in CRP or IL-6 were noted among osteoarthritis (OA) patients in these studies.
The association between stress and inflammation in chronic pain syndromes with low levels of systemic inflammation, such as OA, is not clear. To our knowledge, the two studies cited above are the only two that have assessed the inflammatory stress response in OA. These studies suggest that the inflammatory response to stress may not be as robust among OA patients compared to RA patients (4, 5), but neither study compared the stress response between OA patients and healthy controls. It is not clear whether other stressors (e.g., painful stimuli) may be more effective inducers of the inflammatory response.
The potential role of pain as a stressor that enhances the inflammatory response is intriguing because this relationship could be a critical link in the cycle of pain and inflammation that perpetuates chronic painful conditions. Although the effect of acute experimental pain as a stressor in healthy individuals is well-documented (6–8), the data suggesting that pain induces an inflammatory response are limited. A few studies have shown that experimental pain stimuli induce elevations in serum IL-6 among healthy individuals, chronic low back pain patients and RA and juvenile RA patients (9). Our group also recently reported an increase in serum TNF-α among RA patients in response to acute experimental pain stimuli (10). Although these studies have been small and these data are not conclusive, they suggest that the relationship between pain and inflammation should be studied further.
In this study, we used psychophysical tests to assess experimental pain sensitivity in 26 knee OA patients and 33 participants from the general population. We examined whether OA patients are more sensitive to experimental pain stimuli than healthy controls and whether the inflammatory response to pain is altered in OA patients compared to controls. These questions were asked because previous studies have shown that OA is a disease involving both peripheral and low-grade systemic inflammation, and inflammation may be associated with central pain processing. Human genetic studies have identified inflammation-relevant genotypes (e.g. polymorphisms in genes encoding IL-1 receptor antagonist) as potential risk factors for OA pain severity (11), and animal studies have shown that peripheral application of inflammatory cytokines induces hyperalgesia (12–14). Little, however, is known about the association between deficits in central pain processing and systemic inflammation (15).
We hypothesize that OA patients are: 1) more sensitive to pain in a widespread distribution, indicative of deficits in central pain processing and 2) have an increased systemic inflammatory response to painful stimuli. Previous studies have established that OA patients have lower pain thresholds than healthy controls (16–18), and we recently reported that RA patients have elevated TNF-alpha levels in response to pain (10). If OA patients also have a heightened systemic inflammatory response to pain, then this study may provide a crucial link in the cycle of chronic pain and inflammation, suggesting a model in which peripheral inflammation leads to deficits in central pain processing and widespread pain sensitivity. This, in turn, may lead to an enhanced systemic inflammatory response that may reinforce central pain processing deficits, leading to a cycle of chronic pain and inflammation.
Patients and Methods
Participants
Participants were 26 patients with clinically diagnosed knee OA and 33 control participants from the general population who did not have a clinical diagnosis of OA. OA patients were recruited from the outpatient clinics at Johns Hopkins Hospital. Controls were recruited from the Baltimore community. Inclusion criteria for OA patients were: clinically diagnosed knee OA, documented by a physician in the medical record; pain attributed to knee OA documented in the medical record; no current mood or anxiety disorder; no current infection; no current pregnancy; no history of autoimmune disorders, cardiovascular disease, peripheral neuropathy, Raynaud syndrome, or peripheral vascular disease; and no recent history of substance abuse. OA patients were not required to have an x-ray to confirm OA diagnosis, nor were they assessed for OA at other sites. Controls met the same inclusion criteria. They were not screened radiographically for the presence of OA at the knees or other joints. Exclusion criteria included a diagnosis of OA and a history of joint pain, thereby excluding patients with clinical OA.
Consistent with other studies examining the role of acute pain stimuli on pro-inflammatory cytokines (3, 10), participants were asked not to take non-steroidal anti-inflammatory drugs (NSAIDs) within 24 hours of the study visit. Most common NSAIDs have half-lives less than five hours (19), so the effect after 24 hours should be minimal. Participants taking opioids, antidepressants, or corticosteroids were excluded. Participants were allowed to continue all other medications. The study was approved by the Johns Hopkins Institutional Review Board. All participants provided written informed consent.
Session protocol
Study visits started between 12:00 and 12:30 pm. Participants were asked not to smoke, drink caffeine, use over-the-counter medications or participate in strenuous exercise on the day of the visit. Participants completed questionnaires, including the Short Form Health Survey 36 (SF-36) (20). Participants then underwent psychophysical pain testing. Participants were seated in a reclining chair, and blood samples were drawn from an IV line, a small catheter inserted into the vein. Two baseline blood samples were drawn 15 minutes after IV placement, separated by five minutes between draws. Blood samples were taken immediately after pain testing and 15, 30 and 60 minutes after testing.
Psychophysical pain testing
Pressure pain thresholds were assessed first, followed by contact heat and the cold pressor task. Five minutes were allotted between tests for equilibration. The cold pressor task was done last because it has the longest effects on subsequent pain responses.
Pressure pain thresholds were assessed at the trapezius muscle, the first metacarpophalangeal joint, and the quadriceps muscle, using an algometer (Somedic Production AB, Sollentuna, Sweden) with a 0.5 cm2 probe. The investigator increased the pressure at a rate of 30 kPa/second until the participant indicated that the pressure was “first perceived as painful.” Measurements were assessed two times at each site, on both sides of the body.
A Medoc Thermal Sensory Analyzer (TSA-2001; Medoc Ltd., Ramat Yishai, Israel) was used to apply contact heat stimuli. To assess heat pain thresholds, the investigator placed the probe against the ventral forearm, and the temperature was increased at 0.5°C per second until the participant indicated pain. This procedure was repeated a total of three times. To assess suprathreshold heat pain ratings, four series of ten rapid heat pulses were applied to the forearm (10). Temperatures were increased at 10°C/second to a target temperature of 49°C (two sequences) or 51°C (two sequences). The target temperature was maintained for 0.5 seconds, and the temperature was decreased back to baseline at 10°C/second. The time between series was two minutes. A numeric rating scale was used to rate the painfulness of each pulse from 0 to 100.
A cold pressor task, involving immersion of the right hand in a 4°C water bath, was used to assess responses to cold. During the first four trials, the hand was submerged in the water bath for 30 seconds, with two minutes between immersions. At the end of each trial, participants rated pain severity on a 0-to-100 scale. During the fifth trial, participants were asked to keep their hands in the cold water until they could not tolerate the pain. Cold pain tolerance was defined as the length of time participants’ hands remained submerged in water. If participants did not remove their hands after three minutes, the trial was ended.
Physiologic measures
Enzyme-linked immunosorbent assays were used to measure serum levels of CRP (ALPCO kit: lower limit of detection 0.10 mg/L; sensitivity: 0.05 mg/L; intra-assay coefficient of variation: < 5%), IL-6 (R&D Systems kit: lower limit of detection: 0.16 pg/mL; sensitivity: 0.04 pg/mL; intra-assay coefficient of variation: < 5%), IL-1β (R&D Systems kit: lower limit of detection: 0.06 pg/mL; sensitivity: 0.06 pg/mL; intra-assay coefficient of variation: < 5%), and TNF-α (R&D Systems kit: lower limit of detection: 0.25 pg/mL; sensitivity: 0.06 pg/mL; intra-assay coefficient of variation: < 10%). CRP, IL-6 and IL-1β were chosen because a meta-analysis showed that these biomarkers were elevated following acute experimental stress (21). TNF-α was chosen because previous analyses indicated that TNF-α increased after exposure to acute experimental pain stimuli among RA patients (10). All tests were run in duplicate.
Data analysis
Univariate associations were assessed using Fisher’s Exact Tests, t-tests or Wilcoxon rank sum tests. Multivariable linear regression models were used to examine the association between OA diagnosis and baseline inflammatory markers, adjusted for body mass index (BMI). Baseline values for inflammatory marker concentrations were obtained by averaging the measurements from the two baseline blood samples. Logarithm transformations were performed for CRP and IL-6 due to deviations from normality.
General linear mixed models were used to evaluate between group differences in log CRP, log IL-6, IL-1β and TNF-α over the course of a session. Because the demographic data were similar between the two groups and sample sizes were small, we did not control for age, gender or race. The SF-36 general health subscale score was included as a covariate to adjust for differences in perceived health. BMI was included because it was significantly associated with CRP. General linear mixed models were used to assess the association between psychophysical pain measures and log CRP, log IL-6, IL-1β and TNF-α during the course of the study. Non-linear relationships were examined using quadratic terms. If these terms were significant (P ≤ 0.05), they were included in the model. If these terms were not significant, a linear model was assumed. Due to the small sample size, we did not adjust for covariates. All analyses were performed using the SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
OA patients (n = 26) did not differ from controls (n = 33) in age, gender, race or BMI (P > 0.1). OA patients scored significantly lower on all measures of physical health, assessed by the SF-36 (P < 0.05). OA patients also had lower scores on the SF-36 social functioning and role-emotional scales (P < 0.05) (Table 1).
Table 1.
Characteristics of osteoarthritis patients (n = 26) and healthy controls (n = 33).*
| Characteristic | Osteoarthritis | Controls | P value |
|---|---|---|---|
| Demographics | |||
| Mean age, years (SD) | 59.0 (7.5) | 57.7 (10.3) | 0.59† |
| Female (N, %) | 20 (76.9) | 23 (69.7) | 0.57‡ |
| Caucasian (N, %) | 20 (76.9) | 28 (84.9) | 0.73‡ |
| Median body mass index, kg/m2 (IQR) | 27.5 (24.8, 32.6) | 27.9 (24.3, 30.0) | 0.60§ |
| Median SF-36 Subscale Score | |||
| Physical function (IQR) | 65.3 (30.3, 80.3) | 90.0 (75.0, 95.0) | 0.0007§ |
| Role physical (IQR) | 50.0 (25.0, 100.0) | 100.0 (75.0, 100.0) | 0.002§ |
| Bodily pain (IQR) | 55.0 (45.0, 67.5) | 90.0 (70.0, 100.0) | <0.0001§ |
| General health (IQR) | 67.5 (45.0, 75.0) | 75.0 (70.0, 85.0) | 0.03§ |
| Vitality (IQR) | 65.0 (50.0, 75.0) | 65.0 (55.0, 75.0) | 0.61§ |
| Social functioning (IQR) | 87.5 (62.5, 100.0) | 100.0 (87.5, 100.0) | 0.03§ |
| Role emotional (IQR) | 100.0 (66.7, 100.0) | 100.0 (100.0. 100.0) | 0.04§ |
| Mental health (IQR) | 82.0 (68.0, 92.0) | 84.0 (65.0, 92.0) | 0.93§ |
SD = standard deviation; SF-36 = Short Form-36; IQR = interquartile range.
By Student’s t-test.
By Fisher’s exact test.
By Wilcoxon’s rank sum test.
Psychophysical Pain Measures
Compared to controls, OA patients had lower pressure pain thresholds at all sites (P < 0.005). Heat pain ratings were significantly higher among OA patients compared to controls (P < 0.05). Heat pain thresholds were lower among OA patients compared to controls, but this difference was not statistically significant (P = 0.15). Cold pain ratings and cold pain tolerance did not differ significantly between the two groups (P > 0.1) (Table 2).
Table 2.
Experimental pain measures among osteoarthritis patients (n = 26) compared to healthy controls (n = 33).*
| Experimental Pain Measure | Osteoarthritis | Controls | P value |
|---|---|---|---|
| Mean Leg Pressure PTh, kPa (SD) | 511.5 (221.1) | 732.3 (307.3) | 0.003† |
| Mean Thumb Pressure PTh, kPa (SD) | 260.7 (98.7) | 405.1 (159.4) | < 0.0001† |
| Mean Trapezius Pressure PTh, kPa (SD) | 342.3 (132.6) | 501.7 (220.5) | 0.001† |
| Median Heat PTh, °C (IQR) | 44.5 (40.4, 46.7) | 46.5 (42.8, 48.2) | 0.15‡ |
| Median Heat Pain Rating at 49°C (IQR) | 47.5 (40.0, 87.5) | 40 (22.5, 60.0) | 0.04‡ |
| Median Heat Pain Rating at 51°C (IQR) | 72.8 (42.5, 100) | 50.0 (25.0, 70.0) | 0.02‡ |
| Mean Cold Pain Rating (SD) | 73.7 (22.0) | 65.8 (22.4) | 0.18† |
| Median Cold Pain Tolerance, seconds (IQR) | 73.5 (36.0, 180.0) | 110.0 (35.0, 180.0) | 0.69‡ |
PTh = pain threshold; kPa = kilopascals; SD = standard deviation.
By Student’s t-test.
By Wilcoxon’s rank sum test.
Baseline Inflammatory Markers
At baseline, log CRP was significantly higher among OA patients compared to controls (P = 0.009). OA patients also had higher baseline log IL-6 levels than controls, though this was not statistically significant (P = 0.09). Baseline IL-1β and TNF-α levels did not differ between OA patients and controls (P > 0.1).
Inflammatory Markers after Psychophysical Pain Testing
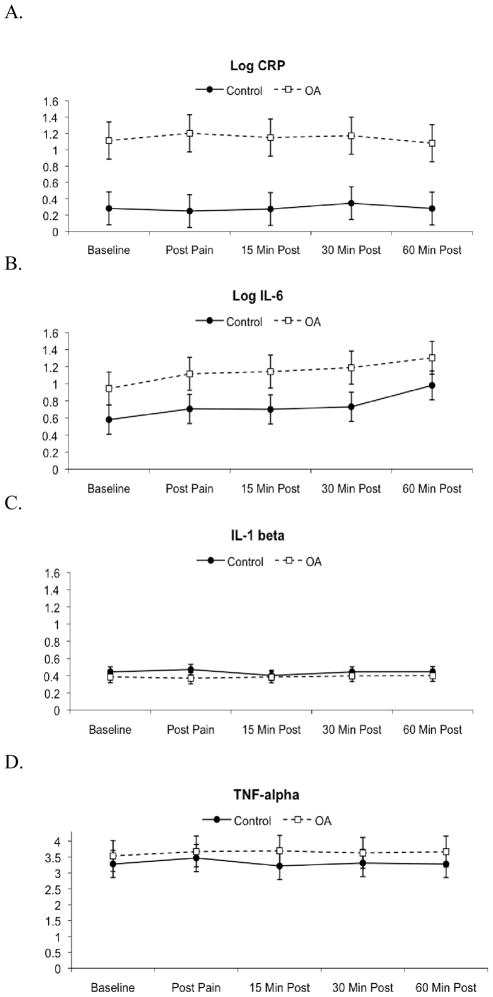
Throughout the duration of the study, log CRP values were higher among OA patients compared to controls (P = 0.007). There was no significant effect of time or group x time interaction (P > 0.1). Similarly, log IL-6 values were higher among OA patients compared to controls, throughout the study. However, this difference was not statistically significant (P = 0.12). There was a main effect of time (P < 0.0001), indicating increases in IL-6 from baseline to post-testing, but no interaction between group and time (P > 0.1). IL-1β and TNF-α levels were similar among OA patients and controls throughout the study. There was no significant effect of time or group x time interaction on IL-1β or TNF-α levels (P > 0.1) (Figure 1).
Figure 1.
Changes in serum levels of a) log CRP, b) log IL-6, c) IL-1β and d) TNF-α over the course of the session. Data are presented as mean ± standard error. OA = osteoarthritis.
Associations between Psychophysical Pain Measures and Inflammatory Markers after Psychophysical Pain Testing among OA Patients
Log CRP was significantly associated with pressure pain threshold at the leg, pressure pain threshold at the trapezius and cold pain rating when these measures were modeled as quadratic predictors of log CRP over time (P < 0.05). Log CRP was linearly associated with pressure pain threshold at the thumb (P = 0.01). Log CRP was not significantly associated with other psychophysical pain measures (Table 3).
Table 3.
Association between baseline experimental pain sensitivity measures and log CRP and log IL-6 after experimental pain sensitivity testing among OA patients.* †
| Variable | Log CRP | Log IL-6 | ||
|---|---|---|---|---|
| F | P | F | P | |
| Leg Pressure PTh | 8.13 | 0.009 | 0.98 | 0.33 |
| Leg Pressure PTh * Leg Pressure PTh | 6.58 | 0.02 | − | − |
| Thumb Pressure PTh | 7.44 | 0.01 | 0.02 | 0.88 |
| Thumb Pressure PTh * Thumb Pressure PTh | − | − | − | − |
| Trapezius Pressure PTh | 9.99 | 0.004 | 0.97 | 0.33 |
| Trapezius Pressure PTh * Trapezius Pressure PTh | 9.36 | 0.006 | − | − |
| Heat PTh | 0.55 | 0.46 | 0.27 | 0.61 |
| Heat Pain Rating at 49°C | 1.17 | 0.29 | 4.51 | 0.04 |
| Heat Pain Rating at 49°C * Heat Pain Rating at 49°C | − | − | 4.27 | 0.05 |
| Heat Pain Rating at 51°C | 1.96 | 0.17 | 5.79 | 0.02 |
| Heat Pain Rating at 51°C * Heat Pain Rating at 51°C | − | − | 5.26 | 0.03 |
| Cold Pain Rating | 5.20 | 0.03 | 0.03 | 0.87 |
| Cold Pain Rating * Cold Pain Rating | 5.11 | 0.03 | − | − |
| Cold Pain Tolerance | 0.74 | 0.40 | 5.96 | 0.02 |
| Cold Pain Tolerance * Cold Pain Tolerance | − | − | 6.57 | 0.02 |
CRP = C-reactive protein; IL-6 = interleukin-6; PTh = pain threshold.
By repeated measures mixed models. Some rows do not have F and P values because the quadratic term was not significantly associated with log CRP or log IL-6. In these instances, the quadratic term was dropped, and only the linear relationship was modeled.
Log IL-6 was significantly associated with cold pain tolerance and heat pain ratings when these measures were modeled as quadratic predictors of log IL-6 over time (P < 0.05). Log IL-6 was not significantly associated with other psychophysical pain measures (Table 3). IL-1β and TNF-α were not significantly associated with any psychophysical pain measures.
Discussion
Our results confirm previous studies reporting enhanced pain sensitivity at multiple sites among OA patients (16–18, 22). While localized pain sensitivity is associated with alterations in peripheral pain processing, widespread pain sensitivity is associated with dysfunction in central pain processing. These observations strengthen arguments that physicians should “think outside the joint” when examining the relationship between pain and inflammation (23–25). OA patients had lower pain thresholds and higher suprathreshold pain ratings than controls for all experimental stimuli, but the only statistically significant results were the differences in pressure pain thresholds and heat pain ratings between OA patients and controls. This may indicate that different experimental pain measures are influenced by diverse underlying pain mechanisms. The literature is divided regarding whether sensitivity to specific painful stimuli represents distinct pain pathways or whether multiple pain mechanisms overlap to create a general sensitivity to pain. In previous studies, pain thresholds for different experimental pain stimuli have been moderately associated (r = 0.33–0.46) (26, 27). These modest correlations may reflect peripheral differences in sensation. For example, pressure nociceptors, which lie within the muscle, likely respond differently than heat nociceptors, which lie in the skin (28). Similarly, the difference in OA and control participants in heat pain ratings, but not heat pain thresholds, may be due to variations in the pain mechanisms assessed by pain ratings compared to pain thresholds. In this study, suprathreshold heat pain ratings and heat pain thresholds were moderately correlated (r = −0.38 – −0.43), suggesting that these measures reflect overlapping but not identical mechanisms. While pain thresholds assess the initial sensation of pain, transmitted by fast-conducting Aδ fibers, suprathreshold pain ratings measure intense pain stimuli, mediated by slower-conducting C-fibers. Although these mechanisms are peripheral, they do not negate the importance of central pain mechanisms, as both likely contribute to overall pain severity. As in previous studies (29, 30), serum CRP levels were elevated in OA patients compared to controls. These observations are consistent with the hypothesis that OA may have a systemic inflammatory component. Previous longitudinal studies have also indicated that high CRP levels predict incident OA (30), supporting the contention that inflammation plays a role in the etiopathogenesis of disease.
In case-only analyses, widespread hyperalgesia to pressure stimuli was associated with elevated CRP levels during the course of the study. This is the first report of a relationship between widespread pressure hyperalgesia and serum CRP levels. Our prior study in RA patients showed that serum CRP levels were associated with pain threshold at joints but not at non-joint sites, suggesting a peripheral, rather than a central, mechanism linking CRP to pain sensitivity (31). However, the median CRP concentration among RA patients in that study was 2.6 mg/L, which is lower than the median CRP concentration among OA patients in this study (3.6 mg/L). The low CRP concentrations in our earlier study likely reflect good disease control, as most RA patients were treated with DMARDs. Given the role of pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α in pain modulation (32), it is possible that effective DMARD treatment may prevent the development and/or maintenance of widespread hyperalgesia.
Alternatively, hyperalgesia may lead to CRP elevations by increasing the aversive impact of pain. Individuals who verbally express greater sensitivity to experimental pain may also have greater inflammatory responses to pain. In the short course of this study, we did not observe an increase in CRP after acute pain stimulation, but the effects of chronic pain may be different. Although the relationship between pressure hyperalgesia and CRP concentration is intriguing, these results need to be replicated in larger, longitudinal studies before conclusions can be made. We also observed a trend towards higher log IL-6 levels among OA patients compared to controls. This observation is consistent with the Chingford study, which showed that log IL-6 levels were significantly higher in patients diagnosed with knee OA than those who did not develop knee OA (33).
The Chingford study, however, reported statistically significant results whereas our study only showed a trend towards higher log IL-6 levels among OA patients. This discrepancy is likely due to statistical power. Although the difference in mean log IL-6 levels was similar in both studies, the Chingford study included 908 individuals, whereas this study only included 59 participants (33). IL-6 increased after experimental pain stimuli in OA patients and controls, complementing previous studies showing IL-6 reactivity to painful stimuli in healthy individuals (34, 35), low back pain patients (36) and RA patients (10). Previous studies have suggested that increases in IL-6 occur due to de novo synthesis and/or stress-induced reductions in plasma volume (21). While initial increases in IL-6 concentration may be due to changes in plasma volume, IL-6 elevations at 60 minutes likely reflect increased IL-6 production because plasma volume returns to baseline levels within 30 minutes (21).
In case-only analyses, heat pain ratings and cold pain tolerance were significantly associated with IL-6 during the course of the study. IL-6 is a pro-inflammatory cytokine that affects both peripheral and central pain processing (32). In rats, peripheral and central application of IL-6 leads to thermal and mechanical hyperalgesia and allodynia (12–14). In humans, IL-6 is associated with clinical pain severity in RA patients (10), chronic pain patients (37), and patients with post-operative pain (38–40). Combined with our data showing increases in IL-6 after psychophysical pain testing, these studies suggest that increases in IL-6 may sensitize nociceptors to further noxious inputs, leading to enhanced clinical pain severity. We hypothesize that the heightened pain sensitivity may also reflect lower thresholds for immunological responses to pain, leading to further increases in IL-6, creating a cycle of inflammation, hyperalgesia and enhanced clinical pain severity. However, larger longitudinal studies are needed to specifically delineate the relationships between pain mechanisms and the inflammatory response. It is not clear whether similar relationships exist with respect to CRP, TNF-α and IL-1β, though this study did not demonstrate reactivity of these biomarkers to acute experimental pain.
The lack of association between pressure pain thresholds and IL-6 contrasts with animal data as well as our data showing significant associations between pressure pain thresholds and CRP. The discrepancy in these results may be due to the different mechanisms by which specific molecules modulate pain (32). CRP is an acute phase reactant regulated by cytokines, including IL-6, IL-1 and TNF-α. A recent study showed that IL-6 alters inhibitory dorsal horn synaptic transmission, while TNF-α controls excitatory synaptic transmission. IL-1β exerted both excitatory and inhibitory influences on synaptic transmission (32). It is not clear whether CRP exhibits an independent effect on pain processing or whether it is a marker of these cytokines’ effects.
Neither TNF-α nor IL-1β differed between OA patients and controls at baseline or in response to painful stimuli. In contrast to our findings, a previous study reported higher IL-1β and TNF-α levels among OA patients compared to controls (41). It is possible that comparisons between OA patients and controls in our study were biased towards the null because our inclusion criteria did not require radiographic evidence of OA for patients in the OA group nor the absence of radiographic evidence of OA for controls. Thus, some patients classified as OA may not have had radiographic OA changes, and some controls may have had undiagnosed radiographic OA. Our results also contrast with our previous data in RA, which showed enhanced TNF-α reactivity in response to pain (10). The differences in TNF-α reactivity between OA and RA patients may reflect differences in inflammatory pathways. Compared to OA patients, RA patients have higher serum TNF-α concentrations (42). RA patients also have significantly elevated synovial fluid TNF-α levels compared to OA patients with similar levels of synovitis (43). These baseline differences in TNF-α concentration may reflect underlying differences in the capacity to synthesize TNF-α, which may impact TNF-α reactivity in response to pain.
This study is limited by its small sample size, short visit duration and cross-sectional design. Due to the small sample size and high intra-group variability in pain thresholds and ratings, clinically significant differences in pain responses, as well as associations between psychophysical pain measures and pro-inflammatory biomarkers, may not have been detected. The small sample size also precluded subgroup analyses to determine whether specific groups (e.g. OA patients with longer disease duration) demonstrate enhanced pain reactivity. In addition, all study measures were performed at one study visit, during which blood samples were obtained up to 60 minutes after pain testing. Thus, possible changes in biomarker concentrations occurring after 60 minutes were not documented. Furthermore, the cross-sectional design made it impossible to determine whether inflammation led to heightened pain sensitivity or whether heightened pain sensitivity led to chronic pain, which stimulated the production and/or release of pro-inflammatory biomarkers.
In future studies, it will be important to collect data regarding OA severity via radiographs. In addition, measurements of synovial fluid inflammatory markers may provide insight about joint inflammation. Currently, data regarding the association between serum and synovial fluid levels of cytokines is lacking, and it is unclear whether serum cytokine levels accurately reflect joint inflammation in OA (44).
In summary, this study highlights the relationship between pain and the inflammatory response in OA by identifying associations between psychophysical pain measures and pro-inflammatory cytokine levels. Compared to controls, OA patients are more sensitive to pressure stimuli at multiple sites, suggesting defects in central pain processing. In response to painful stimuli, IL-6 levels increased in OA patients and controls, while CRP, TNF-α and IL-1β levels did not change. Among OA patients, high CRP levels were associated with low pressure pain thresholds, and high IL-6 levels were associated with high heat pain ratings. These data are hypothesis-generating and suggest that pain and inflammation may play reciprocally interactive roles in OA, with inflammation contributing to enhanced pain sensitivity and clinical pain severity, which, in turn, may stimulate inflammatory responses. These data must be replicated in larger, longitudinal studies before firm conclusions can be made.
Acknowledgments
Dr. Lee’s work was supported by the NIH (K23 AR057578). Dr. Smith’s work was supported by the NIH (R01 AR054871). Dr. Haythornthwaite’s work was supported by the NIH (R21 NS048593). Dr. Edwards’s work was supported by the NIH (K23 AR051315) and awards from the American College of Rheumatology and Arthritis Foundation.
Footnotes
Disclosures: Dr. Lee has stock ownership in Merck and Company, Novartis and Elan.
Contributor Information
Yvonne C. Lee, Email: ylee9@partners.org.
Bing Lu, Email: blu1@partners.org.
Joan M. Bathon, Email: jbathon1@jhmi.edu.
Jennifer A. Haythornthwaite, Email: jhaytho1@jhmi.edu.
Michael T. Smith, Email: msmith62@jhmi.edu.
Gayle G. Page, Email: gpage@son.jhmi.edu.
Robert R. Edwards, Email: rredwards@partners.org.
References
- 1.Straub RH, Kalden JR. Stress of different types increases the proinflammatory load in rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):114. doi: 10.1186/ar2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straub RH, Dhabhar FS, Bijlsma JW, Cutolo M. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum. 2005;52(1):16–26. doi: 10.1002/art.20747. [DOI] [PubMed] [Google Scholar]
- 3.Motivala SJ, Khanna D, FitzGerald J, Irwin MR. Stress activation of cellular markers of inflammation in rheumatoid arthritis: protective effects of tumor necrosis factor alpha antagonists. Arthritis Rheum. 2008;58(2):376–83. doi: 10.1002/art.23165. [DOI] [PubMed] [Google Scholar]
- 4.Hirano D, Nagashima M, Ogawa R, Yoshino S. Serum levels of interleukin 6 and stress related substances indicate mental stress condition in patients with rheumatoid arthritis. J Rheumatol. 2001;28(3):490–5. [PubMed] [Google Scholar]
- 5.Veldhuijzen van Zanten JJ, Ring C, Carroll D, Kitas GD. Increased C reactive protein in response to acute stress in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(9):1299–304. doi: 10.1136/ard.2004.032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullinger M, Naber D, Pickar D, Cohen RM, Kalin NH, Pert A, et al. Endocrine effects of the cold pressor test: relationships to subjective pain appraisal and coping. Psychiatry Res. 1984;12(3):227–33. doi: 10.1016/0165-1781(84)90028-3. [DOI] [PubMed] [Google Scholar]
- 7.Edelson JT, Robertson GL. The effect of the cold pressor test on vasopressin secretion in man. Psychoneuroendocrinology. 1986;11(3):307–16. doi: 10.1016/0306-4530(86)90016-8. [DOI] [PubMed] [Google Scholar]
- 8.Griffis CA, Irwin MR, Martinez-Maza O, Doering L, Nyamathi A, Kaufman R, et al. Pain-related activation of leukocyte cellular adhesion molecules: preliminary findings. Neuroimmunomodulation. 2007;14(5):224–8. doi: 10.1159/000112046. [DOI] [PubMed] [Google Scholar]
- 9.Roupe van der Voort C, Heijnen CJ, Wulffraat N, Kuis W, Kavelaars A. Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. J Neuroimmunol. 2000;110(1–2):223–9. doi: 10.1016/s0165-5728(00)00328-3. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Wasan AD, Bingham CO, 3rd, Bathon J, Haythornthwaite JA, Smith MT, et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swellam M, Mahmoud MS, Samy N, Gamal AA. Potential influence of interleukin-1 receptor antagonist gene polymorphism on knee osteoarthritis risk. Dis Markers. 2010;28(5):299–305. doi: 10.3233/DMA-2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20(16):6289–93. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96(1–2):57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 14.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152(2):521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka Y, Ibuki T, Matsumura K, Namba M, Yamazaki Y, Poole S, et al. Interleukin-6 is a candidate molecule that transmits inflammatory information to the CNS. Neuroscience. 2007;145(2):530–8. doi: 10.1016/j.neuroscience.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88(1):69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 17.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4(3):229–38. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- 18.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59(10):1424–31. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 19.Brooks P. Use and benefits of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):9S–13S. doi: 10.1016/s0002-9343(97)00204-0. discussion 21S–22S. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 21.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Wessel J. The reliability and validity of pain threshold measurements in osteoarthritis of the knee. Scand J Rheumatol. 1995;24(4):238–42. doi: 10.3109/03009749509100881. [DOI] [PubMed] [Google Scholar]
- 23.Clauw DJ, Witter J. Pain and rheumatology: thinking outside the joint. Arthritis Rheum. 2009;60(2):321–4. doi: 10.1002/art.24326. [DOI] [PubMed] [Google Scholar]
- 24.Bradley LA, Kersh BC, DeBerry JJ, Deutsch G, Alarcon GA, McLain DA. Lessons from fibromyalgia: abnormal pain sensitivity in knee osteoarthritis. Novartis Found Symp. 2004;260:258–70. discussion 270–9. [PubMed] [Google Scholar]
- 25.Gwilym SE, Pollard TC, Carr AJ. Understanding pain in osteoarthritis. J Bone Joint Surg Br. 2008;90(3):280–7. doi: 10.1302/0301-620X.90B3.20167. [DOI] [PubMed] [Google Scholar]
- 26.Neddermeyer TJ, Fluhr K, Lotsch J. Principle components analysis of pain thresholds to thermal, electrical, and mechanical stimuli suggests a predominant common source of variance. Pain. 2008;138(2):286–91. doi: 10.1016/j.pain.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Hastie BA, Riley JL, 3rd, Robinson ME, Glover T, Campbell CM, Staud R, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116(3):227–37. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59(1):45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 29.Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40(4):723–7. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 30.Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10(8):595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 31.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11(5):R160. doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60(7):2037–45. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutgendorf SK, Logan H, Costanzo E, Lubaroff D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav Immun. 2004;18(1):55–64. doi: 10.1016/s0889-1591(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140(1):135–44. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114(1–2):104–17. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Koch A, Zacharowski K, Boehm O, Stevens M, Lipfert P, von Giesen HJ, et al. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm Res. 2007;56(1):32–7. doi: 10.1007/s00011-007-6088-4. [DOI] [PubMed] [Google Scholar]
- 38.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237(2–3):65–8. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 39.Lisowska B, Maldyk P, Kontny E, Michalak C, Jung L, Cwiek R. Postoperative evaluation of plasma interleukin-6 concentration in patients after total hip arthroplasty. Ortop Traumatol Rehabil. 2006;8(5):547–54. [PubMed] [Google Scholar]
- 40.Lisowska B, Maslinski W, Maldyk P, Zabek J, Baranowska E. The role of cytokines in inflammatory response after total knee arthroplasty in patients with rheumatoid arthritis. Rheumatol Int. 2008;28(7):667–71. doi: 10.1007/s00296-007-0508-1. [DOI] [PubMed] [Google Scholar]
- 41.Hussein MR, Fathi NA, El-Din AM, Hassan HI, Abdullah F, Al-Hakeem E, et al. Alterations of the CD4(+), CD8 (+) T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: preliminary observations. Pathol Oncol Res. 2008;14(3):321–8. doi: 10.1007/s12253-008-9016-1. [DOI] [PubMed] [Google Scholar]
- 42.Steiner G, Studnicka-Benke A, Witzmann G, Hofler E, Smolen J. Soluble receptors for tumor necrosis factor and interleukin-2 in serum and synovial fluid of patients with rheumatoid arthritis, reactive arthritis and osteoarthritis. J Rheumatol. 1995;22(3):406–12. [PubMed] [Google Scholar]
- 43.Manicourt DH, Poilvache P, Van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000;43(2):281–8. doi: 10.1002/1529-0131(200002)43:2<281::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Rowe IF, Sheldon J, Riches PG, Keat AC. Comparative studies of serum and synovial fluid C reactive protein concentrations. Ann Rheum Dis. 1987;46(10):721–6. doi: 10.1136/ard.46.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]