Abstract
Background
We examined the correlation of angiographic collaterals in acute stroke with the presence, extent, and distribution of white matter changes, so called Leukoaraiosis, in an effort to determine if Leukoaraiosis indicates chronic cerebral hypoperfusion and/or is associated with the development of cerebral collateral circulation.
Methods
Consecutive acute strokes due to large vessel occlusion on angiography had pre-procedure CT or MRI white matter changes graded utilizing the Fazekas scale incorporating deep and periventricular components. Angiographic collaterals evaluated with a 5-point scale were correlated with leukoaraiosis.
Results
Collaterals were evaluated in 102 cases (51 men, 51 women; mean age 66 (SD 18) years with acute occlusions of the proximal MCA (47%), distal ICA (28%), distal MCA (9%), basilar (7%), proximal ICA (7%), vertebral (1%), PCA (1%), and CCA (1%). Collateral grade was well distributed across the scale. Periventricular and deep white matter changes were evident in 34% and 51% of cases, respectively. Collateral grade exhibited no relationship with either the presence or extent of periventricular disease (p=.772, r=.029) or deep white matter changes (p=.559, r=−.059).
Conclusions
Leukoaraisosis exhibits no overt relationship with the extent of collaterals measured at angiography in acute ischemic stroke. Chronic small vessel disease may be a distinct pathophysiologic entity unrelated to arteriogenesis and compensatory aspects of collateral flow.
Indexing Terms: Stroke, Leukoaraiosis, Collateral
INTRODUCTION
Leukoaraiosis was originally described in 1987 as an abnormality of the subcortical white matter visualized on CT and was subsequently evaluated in greater detail using MRI.[1] Leukoaraiosis is the imaging analogue to subcortical ischemic vascular disease and is thought to be due to ischemia secondary to small-vessel disease, reflecting chronic covert brain injury. Although the exact prevalence of leukoaraiosis is unknown, the incidence of white matter lesions on neuroimaging increases with age and vascular risk factors, particularly hypertension.[2, 3] Leukoaraiosis is often asymptomatic in its early stages, yet its progression to more advanced stages may lead to substantial neurological dysfunction including cognitive impairment, depression and dementia.[2, 4]
Chronic cerebral hypoperfusion may be an underlying mechanism for development of leukoaraiosis. There is a predilection of white matter changes to subcortical regions with relative vulnerability to ischemia, such as periventricular and deep white matter areas perfused with long penetrating arteries. This hypoperfusion and its associated ischemic preconditioning may induce development of collateral circulation, thereby offering protection in the setting of acute stroke. The mechanisms and impetus for collateral formation or collateralization are not well characterized and the relationship in the extent of collaterals with parenchymal changes has never been studied.
We examined the correlation of angiographic collaterals in acute ischemic stroke with the presence, extent, and distribution of white matter changes on CT and MRI studies obtained less than 24 hours before angiography.
METHODS
Data on 632 consecutive patients evaluated by the stroke team at a single academic institution from 5/2001 to 7/2005 was retrospectively analyzed. All cases with admission CT or MRI studies and cerebral angiography performed within 24 hours were included in our analyses. Patients were excluded if there was no evidence of large-vessel occlusion on angiography or if their MRI/CT or angiographic digital subtraction images were not available for review.
Independent review of pre-procedure CT or MRI white matter changes was performed by two readers utilizing the Fazekas scale incorporating deep and periventricular components (Table 1).[5, 6]
Table 1.
Characteristics of study patients
| Number of patients | 102 |
|---|---|
| Age (years) | Mean 66 (SD 18) |
| Gender | 51 women (50%) |
| Pre-existing Risk Factors | |
| Coronary Artery Disease | 34% |
| Tobacco | 10% |
| Dyslipidemia | 30% |
| Atrial Fibrillation | 32% |
| Prior Stroke | 9% |
| Hypertension | 72% |
| Diabetes | 20% |
| Final TOAST stroke mechanism | |
| Cardioembolism | 55% |
| Large-Vessel Atherothrombosis | 23% |
| Small Vessel Disease | 0% |
| Other | 8% |
| Multiple Competing Mechanisms | 4% |
| Unknown | 10% |
TOAST- Trial of ORG-19 in Acute Stroke
Angiographic images obtained up to 24 hours after CT/MRI were reviewed. Location of occlusions and stenosis within vessel segments, direction of flow in blood vessels and routes of collateral blood flow were noted. Angiographic collaterals in acute ischemic stroke due to large artery occlusion were evaluated with the 5-point ASITN/SIR scale,[7] blinded to the noninvasive imaging results, with data abstraction using a standardized form.
Spearman correlations were performed for the association between collateral grade and leukoaraiosis.
RESULTS
There were 102 cases (51 women) with a mean age of 66 (SD18) years included in this study (Table 1). Angiography revealed 47 middle cerebral artery (MCA) M1, 28 distal internal carotid artery (ICA), 9 distal MCA (M2 or M3), 7 basilar artery, 1 vertebral, 1 posterior cerebral, and 1 common carotid artery occlusions. Angiographic collateral scores were documented across the entire ASITN/SIR scale: 10% grade 0, 19% grade 1, 16% grade 2, 27% grade 3, 28% grade 4, with collaterals extending throughout the ischemic territory in 57%.
Leukoaraiosis was divided into periventricular hyperintensity (PVH) and deep white matter hyperintensity (DWMH) sub-scores according to the method described by Fazekas.[6] Overall 51% of cases demonstrate some DWMH, with 49% grade 0, 44% grade 1, 6% grade 2, and 1% grade 3. PVH was much less common and present in about 1/3rd of cases. The PVH sub-scores were 66% grade 0, 26% grade 1, 8% grade 2 and 1% grade 3.
Either PVH or DWMH were present in 62% of patients with poorer grade collaterals (defined as 0–2) and 49% with good collaterals (defined as 3–4) (p=.231). Finer collateral gradations in scale measures were not related to the presence or extent of PVH (p=.772, r=.029) or DWMH (p=.559, r=.059). There was also no relationship between collateral grade and either PVH or DWMH score when evaluating just the 47 cases of MCA M1 occlusion. Collateral grade was not associated with a higher composite Fazekas (PVH + DWMH) score (p=0.232).
DISCUSSION
Cerebral white matter ischemic changes (leukoaraiosis) were not associated with collateral development at time of acute arterial occlusion in this study.
Collaterals may be one of the most potent predictors of clinical outcome in the setting of acute stroke.[8] Although the extent of collateral circulation at the time of acute ischemic stroke varies across individuals, the factors leading to this variability have not been characterized.[9] When collaterals are well-developed, they are able to sustain ischemic regions where the primary source of arterial inflow is blocked.[10] In conditions where arterial blockage occurs gradually, such as atherosclerosis, there is the opportunity to gradually develop more extensive collaterals which prove beneficial during acute cerebral ischemia. Development of collaterals is a multi-factorial process and may be promoted by statin use, higher serum HDL cholesterol, and lower blood pressure at time of angiography.[11] There are likely to be a host of yet-unidentified factors which promote collateral formation and further account for collateral variability. It is important to recognize that collaterals can only truly be quantitatively measured in the setting of acute arterial occlusion using one of several scales,[12] such as the ASITN/SIR (Table 2) used in this study.
Table 2.
Description of Rating Scales used for grading collaterals and leukoaraiosis
| American Society of Interventional & Therapeutic Neuroradiology (ASITN) Angiographic Collateral Grading Scale | |
| Grade 0 | No Collaterals visible to ischemic site |
| Grade 1 | Slow collaterals to the periphery of the ischemic site with persistence of some of the defect |
| Grade 2 | Rapid collaterals to the periphery of the ischemic site with persistence of some of the defect and to only a portion of the ischemic territory |
| Grade 3 | Collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase |
| Grade 4 | Rapid and completer collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion |
| Fazekas Leukoaraiosis Scale: Periventricular Hyperintensities (PVH) Sub-score | |
| Grade 0 | Absence |
| Grade 1 | Capping or pencil thin lining |
| Grade 2 | Smooth halo |
| Grade 3 | Irregular PVH extending into the deep wite matter |
| Fazekas Leukoaraiosis Scale: Deep White Matter Hyperintense Signal (DWMH) Sub-score | |
| Grade 0 | Absence |
| Grade 1 | Punctate foci |
| Grade 2 | Beginning confluence of foci |
| Grade 3 | Large confluent areas |
Leukoaraiosis has been associated with both large-vessel atherosclerosis and small-vessel lacunar stroke.[5][13][14] All three result from chronic exposure to risk factors such as hypertension, and may cause physiological stress in the brain. Our initial hypothesis was that the presence of leukoaraiosis may indicate chronic hypoperfusion, a brain stressor which may potentially drive recruitment of collaterals leading to cerebral protection from ischemia. The lack of any such association in this study may indicate that the chronic process reflected in leukoaraiosis may be a distinct pathophysiologic entity unrelated to superficial territory arteriogenesis, development of leptomeningeal/pial vascular networks and compensatory aspects of collateral flow.
This study has limitations that may obscure any potential pathophysiologic link between leukoaraiosis and collaterals. Although the Fazekas scale, a visual rating tool, is widely used, the absolute volume of white matter disease may better measure leukoariaosis. Furthermore, most of our subjects had low Fazekas scores reflecting a population with minimal leukoaraiosis. Patients are unlikely to undergo angiography unless being considered for acute interventional therapy, thus biasing the studied group toward better collaterals. Collateral flow scales may also require further refinement to discern critical features of perfusion, including microcirculatory and downstream factors that preclude effective cerebral perfusion.
In summary, the presence of leukoaraiosis is an independent predictor of stroke risk, [16] but does not seem to be related to standard measures of angiographic collaterals in acute stroke. Leukoaraiosis and the process leading to formation of arterial collaterals appear to be distinct and unrelated pathophysiological entities.
Figure 1.
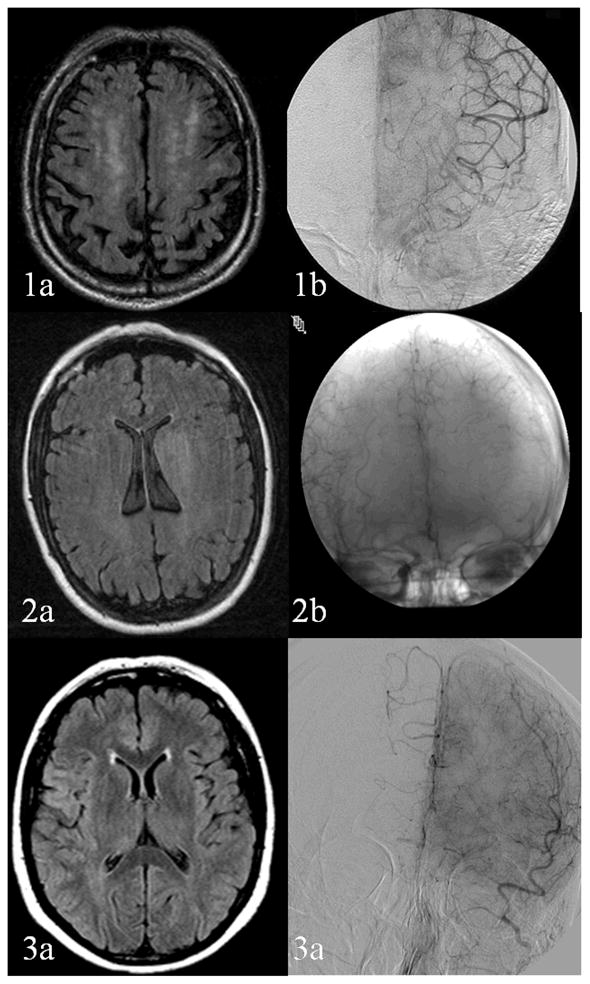
Illustrative Cases Demonstrating Variable Association of Leukoaraiosis with Collateral Flow. 1a, b: Extensive leukoaraiosis with exuberant collaterals. 2 a, b: No leukoaraiosis with extensive collaterals. 3a, b: No leukoaraiosis with minimal collateral flow
Acknowledgments
This research was supported by grant K23NS054084 (DSL) from the National Institute of Neurological Disorders and Stroke and 0765127Y (NS) from the American Heart Association.
References
- 1.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–3. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MMB, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, Hofman A. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology. 1994;44:1246. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 3.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke Risk Profile Predicts White Matter Hyperintensity Volume: The Framingham Study. Stroke. 2004;35:1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt R, Fazekas F, Offenbacher H, Dusek T, Zach E, Reinhart B, Grieshofer P, Freidl W, Eber B, Schumacher M, Koch M, Lechner H. Neuropsychologic correlates of MRI white matter hyperintensities: A study of 150 normal volunteers. Neurology. 1993;43:2490. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Niederkorn K, Schmidt R, Offenbacher H, Horner S, Bertha G, Lechner H. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–8. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol. 1987;8:421–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 7.Higashida RT, Furlan AJ. Trial Design and Reporting Standards for Intra-Arterial Cerebral Thrombolysis for Acute Ischemic Stroke. Stroke. 2003;34:109e–37. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 8.Jo KD, Saver JL, Starkman S, Kim D, Ali LK, Ovbiagele B, Bang OY, Yun Susan, Towfighi A, Shah SH, Vespa PM, Miller C, Tateshima S, Jahan Reza, Vinuela F, Duckwiler GR, Liebeskind DS. Predictors of Recanalization with Mechanical Thrombectomy for Acute Ischemic Stroke (Abs.) Stroke. 2008;39:599. [Google Scholar]
- 9.Liebeskind DS. Collaterals in acute ischemic stroke: beyond the clot. Neuroimaging Clinics of North America. 2005;15:553–73. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–65. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
- 11.Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, Duckwiler GR, Vinuela F, Pineda S, Liebeskind DS. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–31. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- 12.Liebeskind DS, Nogueira RG. Angiographic Scales in Acute Ischemic Stroke: The MERCI/Multi MERCI Experience (Abs.) Stroke. 2008;39:602. [Google Scholar]
- 13.Wiszniewska M, Devuyst G, Bogousslavsky J, Ghika J, van Melle G. What Is the Significance of Leukoaraiosis in Patients With Acute Ischemic Stroke? Arch Neurol. 2000;57:967–73. doi: 10.1001/archneur.57.7.967. [DOI] [PubMed] [Google Scholar]
- 14.Streifler JY, Eliasziw M, Benavente OR, Alamowitch S, Fox AJ, Hachinski V, Barnett HJM. Development and Progression of Leukoaraiosis in Patients With Brain Ischemia and Carotid Artery Disease. Stroke. 2003;34:1913–6. doi: 10.1161/01.STR.0000080939.39414.83. [DOI] [PubMed] [Google Scholar]
- 15.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–9. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Nick Bryan R, Beauchamp NJ, Jr, for the Cardiovascular Health Study Collaborative Research G White Matter Hyperintensity on Cranial Magnetic Resonance Imaging: A Predictor of Stroke. Stroke. 2004;35:1821–5. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]


