Abstract
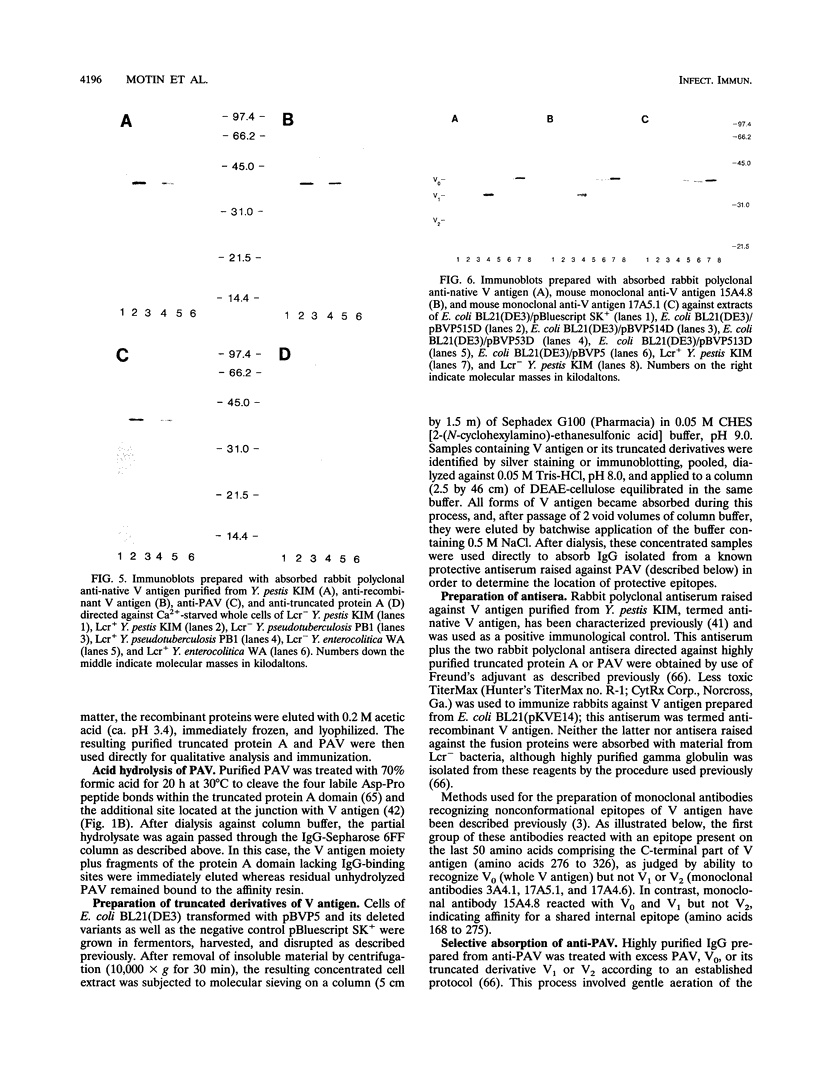
LcrV (V antigen), a known unstable 37.3-kDa monomeric peptide encoded on the ca. 70-kb Lcr plasmid of Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica, has been implicated as a regulator of the low-calcium response, virulence factor, and protective antigen. In this study, lcrV of Y. pestis was cloned into protease-deficient Escherichia coli BL21. The resulting recombinant V antigen underwent marked degradation from the C-terminal end during purification, yielding major peptides of 36, 35, 34, and 32 to 29 kDa. Rabbit gamma globulin raised against this mixture of cleavage products provided significant protection against 10 minimum lethal doses of Y. pestis (P < 0.01) and Y. pseudotuberculosis (P < 0.02). To both stabilize V antigen and facilitate its purification, plasmid pPAV13 was constructed so as to encode a fusion of lcrV and the structural gene for protein A (i.e., all but the first 67 N-terminal amino acids of V antigen plus the signal sequence and immunoglobulin G-binding domains but not the cell wall-associated region of protein A). The resulting fusion peptide, termed PAV, could be purified to homogeneity in one step by immunoglobulin G affinity chromatography and was stable thereafter. Rabbit polyclonal gamma globulin directed against PAV provided excellent passive immunity against 10 minimum lethal doses of Y. pestis (P < 0.005) and Y. pseudotuberculosis (P < 0.005) but was ineffective against Y. enterocolitica. Protection failed after absorption with excess PAV, cloned whole V antigen, or a large (31.5-kDa) truncated derivative of the latter but was retained (P < 0.005) upon similar absorption with a smaller (19.3-kDa) truncated variant, indicating that at least one protective epitope resides internally between amino acids 168 and 275.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. An antigen determining virulence in Pasteurella pestis. Nature. 1956 Mar 3;177(4505):426–427. doi: 10.1038/177426b0. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br J Exp Pathol. 1958 Jun;39(3):278–291. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. VIRULENCE OF PASTEURELLA PESTIS AND IMMUNITY TO PLAGUE. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1963;37:59–113. doi: 10.1007/978-3-662-36742-1_2. [DOI] [PubMed] [Google Scholar]
- Bergman T., Håkansson S., Forsberg A., Norlander L., Macellaro A., Bäckman A., Bölin I., Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991 Mar;173(5):1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991 Jul;4(3):309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Sample A. K., Yu D. Z., Zahorchak R. J., Hu P. C., Fowler J. M. Proteolysis of V antigen from Yersinia pestis. Microb Pathog. 1987 Jan;2(1):49–62. doi: 10.1016/0882-4010(87)90114-8. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. The V antigen of yersiniae: an overview. Contrib Microbiol Immunol. 1991;12:127–133. [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Brubaker R. R. RNA synthesis in Yersinia pestis during growth restriction in calcium-deficient medium. J Bacteriol. 1982 Mar;149(3):1089–1095. doi: 10.1128/jb.149.3.1089-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Vanootegem J. C., Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987 May;2(5):367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston J. D., Schuetze P., Perry R. D. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol Microbiol. 1992 Sep;6(18):2693–2704. doi: 10.1111/j.1365-2958.1992.tb01446.x. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Viitanen A. M., Skurnik M., Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991 Apr;5(4):977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Galyov E. E., Håkansson S., Forsberg A., Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993 Feb 25;361(6414):730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- Gandecha A. R., Owen M. R., Cockburn B., Whitelam G. C. Production and secretion of a bifunctional staphylococcal protein A::antiphytochrome single-chain Fv fusion protein in Escherichia coli. Gene. 1992 Dec 15;122(2):361–365. doi: 10.1016/0378-1119(92)90227-g. [DOI] [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Håkansson S., Bergman T., Vanooteghem J. C., Cornelis G., Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993 Jan;61(1):71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung K. Y., Reisner B. S., Straley S. C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990 Oct;58(10):3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier T. S., Brubaker R. R. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J Bacteriol. 1992 Apr;174(7):2078–2086. doi: 10.1128/jb.174.7.2078-2086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenadler B., Jansson B., Paleus S., Holmgren E., Nilsson B., Moks T., Palm G., Josephson S., Philipson L., Uhlén M. A gene fusion system for generating antibodies against short peptides. Gene. 1987;58(1):87–97. doi: 10.1016/0378-1119(87)90032-1. [DOI] [PubMed] [Google Scholar]
- Mehigh R. J., Braubaker R. R. Major stable peptides of Yersinia pestis synthesized during the low-calcium response. Infect Immun. 1993 Jan;61(1):13–22. doi: 10.1128/iai.61.1.13-22.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehigh R. J., Sample A. K., Brubaker R. R. Expression of the low calcium response in Yersinia pestis. Microb Pathog. 1989 Mar;6(3):203–217. doi: 10.1016/0882-4010(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991 Mar;173(5):1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988 Dec;5(6):449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Michiels T., Vanooteghem J. C., Lambert de Rouvroit C., China B., Gustin A., Boudry P., Cornelis G. R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991 Aug;173(16):4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Motin V. L., Pokrovskaya M. S., Telepnev M. V., Kutyrev V. V., Vidyaeva N. A., Filippov A. A., Smirnov G. B. The difference in the lcrV sequences between Y. pestis and Y. pseudotuberculosis and its application for characterization of Y. pseudotuberculosis strains. Microb Pathog. 1992 Mar;12(3):165–175. doi: 10.1016/0882-4010(92)90050-x. [DOI] [PubMed] [Google Scholar]
- Mulder B., Michiels T., Simonet M., Sory M. P., Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989 Aug;57(8):2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Brubaker R. R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993 Jan;61(1):23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Holmgren E., Josephson S., Gatenbeck S., Philipson L., Uhlen M. Efficient secretion and purification of human insulin-like growth factor I with a gene fusion vector in Staphylococci. Nucleic Acids Res. 1985 Feb 25;13(4):1151–1162. doi: 10.1093/nar/13.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Harmon P. A., Bowmer W. S., Straley S. C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986 Nov;54(2):428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Cowan C., Perry R. D., Straley S. C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2(+)-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991 Apr;173(8):2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Leung K. Y., Barve S. S., Straley S. C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989 Oct;171(10):5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner B. S., Straley S. C. Yersinia pestis YopM: thrombin binding and overexpression. Infect Immun. 1992 Dec;60(12):5242–5252. doi: 10.1128/iai.60.12.5242-5252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Rimpiläinen M., Bergman T., Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990 Apr;4(4):657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991 Dec;59(12):4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample A. K., Brubaker R. R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987 Oct;3(4):239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- Sample A. K., Fowler J. M., Brubaker R. R. Modulation of the low-calcium response in Yersinia pestis via plasmid-plasmid interaction. Microb Pathog. 1987 Jun;2(6):443–453. doi: 10.1016/0882-4010(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Sato K., Nakajima R., Hara F., Une T., Osada Y. Preparation of monoclonal antibody to V antigen from Yersinia pestis. Contrib Microbiol Immunol. 1991;12:225–229. [PubMed] [Google Scholar]
- Sodeinde O. A., Sample A. K., Brubaker R. R., Goguen J. D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988 Oct;56(10):2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Subrahmanyam Y. V., Stark K., Quan T., Bao Y., Goguen J. D. A surface protease and the invasive character of plague. Science. 1992 Nov 6;258(5084):1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982 Apr;36(1):129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Cibull M. L. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989 Apr;57(4):1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Une T., Brubaker R. R. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984 Oct;133(4):2226–2230. [PubMed] [Google Scholar]
- Une T., Nakajima R., Brubaker R. R. Roles of V antigen in promoting virulence in Yersiniae. Contrib Microbiol Immunol. 1987;9:179–185. [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]