Abstract
The hepatoprotective and antioxidant activities of the n-butanol extract of Rubus parvifolius L. (RPL), a widely used medicinal plant, were evaluated. Results demonstrated that RPL extract possessed pronounced hepatoprotective effects against carbon tetrachloride (CCl4)-induced hepatic injury in mice, which was at least partially attributed to its strong antioxidant capacity. Treatment with RPL extract markedly attenuated the increases in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels caused by CCl4 intoxication. It also significantly prevented the decrease in superoxide dismutase (SOD) activity and the increase in malondialdehyde (MDA) content of liver tissue. Meanwhile, histopathological changes of hepatic damage were also remarkably ameliorated. Phytochemical analysis based on high-performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS) revealed the presence of various phenolic compounds, including caffeic acid conjugates, ellagic acid glycosides, and flavonol glycosides, which might be responsible for the hepatoprotective and antioxidant activities of RPL.
Keywords: Rubus parvifolius L., High-performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS), Hepatoprotective activity, Antioxidant activity
1. Introduction
Hepatotoxicity has increased as a result of various environmental toxins and hepatotoxic drugs (Jin et al., 2005). Due to the absence of efficient liver-protective drugs in modern medicine, a large number of studies searching for hepatoprotective constituents from natural sources have been conducted in recent years (Adnyana et al., 2001; Huang et al., 2010).
Rubus parvifolius L. (RPL), a deciduous thorny shrub, is widely distributed in East and South Asia and is traditionally used as herbal medicine for the treatments of rheum, fever, angina, dysentery, enteritis, hepatitis, concretion, rheumatism, eczema, dermatitis, and so on (Compilation Group of Chinese Herbal Medicine Compilation, 1975; Wang et al., 2006). Attempts are being made to provide scientific evidence for pharmacological effects of the traditional herbal drug. It has been reported previously that RPL extract exhibited antioxidant capacity (Deighton et al., 2000; Shyur et al., 2005). However, to our knowledge, no published data documenting hepatoprotective effects of RPL are available. Furthermore, previous reports suggest that effective protection against chronic chemical-induced hepatic injury in vivo is usually associated with antioxidant properties (Hsiao et al., 2003; Gowri Shankar et al., 2008; Zeashan et al., 2009). These results motivate us to investigate the potential hepatoprotective and antioxidant activities of RPL extract.
In search of principal bioactive constituents responsible for these pharmacological activities, high-performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS) was utilized, providing an advantage over conventional methods by avoiding the drawbacks such as inefficiency and low sensitivity (Chen et al., 2008). Various natural constituents in crude plant extracts have been determined using HPLC-MS/MS (Carmona et al., 2007; Hollecker et al., 2009; Huang et al., 2009).
In this study, we evaluated the antioxidant activity of RPL by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay and assessed its hepatoprotective effects on chronic carbon tetrachloride (CCl4)-induced liver damage in mice by biochemical assays and histopathological observations. Major bioactive constituents of this extract were tentatively identified by HPLC-MS/MS rapidly. Offline Fourier transform ion cyclotron resonance mass spectrometry (FT-ICRMS) as an auxiliary method was performed to confirm the proposed structures further.
2. Materials and methods
2.1. Chemicals and reagents
HPLC-grade acetonitrile was obtained from Merck Co. (Darmstadt, Germany) and water was purified by a Milli-Q purification system (Millipore, Bedford, MA, USA). DPPH and resveratrol were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ethanol (95%, v/v) and n-butanol were obtained from Hangzhou Huipu Chemical and Apparatus Co., Ltd. (China). CCl4 was of analytical grade and was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (China). Formic acid (analytical grade) was obtained from Jiangsu Tongsheng Chemical Reagent Co., Ltd. (China). Bifendate pills were purchased from Zhejiang Medicine Co., Ltd. (China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), superoxide dismutase (SOD), and malondialdehyde (MDA) kits were obtained from Nanjing Jiancheng Bioengineering Institute (China).
2.2. Sample preparation
The whole plants of RPL were collected from Longhua County, Hebei Province, China in October 2008 and were authenticated by Prof. Hai-tong WAN (Institute of Cardio-Cerebrovascular Disease, Zhejiang Chinese Medical University, China). The dried herbs (7 kg) were macerated with 95% ethanol (40 L) at room temperature for 14 d, and the process of extraction was repeated three times to yield a concentrated ethanol extract. The extract was partitioned with petroleum ether, ethyl acetate, and n-butanol (stirred for 2 h each time). The n-butanol extract was used for the subsequent studies.
2.3. Animals
Healthy Kunming mice of either sex (weighing 20–25 g) were obtained from Institute of Zoology, Zhejiang Chinese Medical University, Hangzhou, China. All animals were cared for according to local guidelines for the care of laboratory animals of Zhejiang Chinese Medical University, and were approved by Zhejiang Animal Care and Use Committee [Certificate No. SCXK (Zhe) 2008-0115]. The animals were housed in standard conditions with a 12-h light/12-h dark cycle and allowed free access to a standard dry pellet diet and water ad libitum.
2.4. HPLC-MS/MS
The HPLC system was comprised of an Agilent 1100 analytical HPLC equipped with a G1314A variable-wavelength detector (VWD) and an Agilent ChemStation for data analysis. A reversed-phase Akasil C18 column (4.6 mm×250 mm, 5 µm; Agela Technologies, Beijing, China) was used for separation, and the column temperature was set at 40 °C. A linear gradient elution of 0.01% (v/v) formic acid (A) and acetonitrile (B) was used with 8%–22% (v/v) B in 60 min. The flow rate was 1.0 ml/min, and the injection volume was 20 µl. The detection wavelength was 365 nm. HPLC-MS/MS analysis was performed using the Agilent HPLC system described above coupled to a Bruker Esquire 3000plus ion trap mass spectrometer (Bruker-Franzen Analytik GmbH, Bremen, Germany) equipped with an electrospray ionization (ESI) source. Instrument control and data acquisition were performed using Esquire 5.0 software. Samples were infused into the source chamber from the HPLC system though a T-junction with a splitting ratio of 2:1. The ESI-MS conditions were optimized as follows: ion source temperature, 250 °C; needle voltage, 4.0 kV; drying gas (N2), 10 L/min; nebulizing gas (N2), 2.1×105 Pa; collision gas (He), 6.3×10−7 Pa; scan range, m/z 50–1 200; and collision energy, 0.55–1.10 V.
2.5. Offline FT-ICRMS
The accurate mass spectrometric experiments were carried out using an Apex III Fourier transform ion cyclotron resonance mass spectrometer (Bruker Daltonics, Billerica, MA, USA) equipped with an Apollo electrospray ionization source operated in the negative ion mode. The solutions were infused at a rate of 3.0 µl/min using a Cole-Parmer syringe pump. The spray voltage was 4.5 kV and the temperature of the capillary was 250 °C. Nitrogen was used as nebulizing gas (2.4×105 Pa) and drying gas (30 units). Each spectrum was an average of eight transients, each composed of 512 K points and acquired using an SGI workstation operating XMASS Version 6.1.1.
2.6. DPPH free radical scavenging assay
Free radical scavenging activity was evaluated with DPPH assay, as described previously (Luo et al., 2002; He et al., 2008). The reaction mixtures containing 180 μl of 150 μmol/L DPPH in ethanol and 20 μl of sample solutions (2-fold serial dilutions) were added to a 96-well microplate and then maintained at 37 °C until the reaction plateau was reached (30 min). The absorbance was measured at 517 nm. Percent inhibition (I) was calculated by the following equation:
| I=(Acontrol−Asample)/Acontrol×100%, |
where A control is the absorbance of the ethanol-containing control, and A sample is the absorbance of the reaction mixture with the tested sample. IC50 values are determined to be the concentration at which DPPH radical is scavenged by 50%. Resveratrol was used as a positive control.
2.7. Hepatoprotective studies
Animals were divided into four groups of ten mice each: normal control group, CCl4 control group, RPL-treated group, and bifendate-treated group (bifendate as a positive control). Mice of RPL-treated group were fed with 0.5 ml RPL aqueous solution via gavage tube at a dose of 20 mg/kg twice daily for 7 d prior to the CCl4 treatment. The bifendate-treated group was given 200 mg/kg bifendate twice daily for 7 d, and the remaining two groups were given pure water only as controls. At 2 h following the last treatment, the normal control group received an equal volume of peanut oil and the other three groups were administered CCl4 (0.1% (v/v) CCl4 in peanut oil) by intraperitoneal injection at a dose of 10 ml/kg. After fasting for 18 h, blood samples were collected by removing the eyes, and the serum was separated for the measurements of ALT and AST. Liver tissue samples were taken and dissected into two pieces. One piece was utilized to prepare 10% (v/v) liver homogenates with physiological saline for the assay of SOD activity and MDA content. The other piece was fixed in 10% (v/v) formalin solution, dehydrated with ethanol solutions, and embedded in paraffin wax. Sections were prepared and then stained with hematoxylin-eosin (H&E) for histopathological observation. Quantitative data were expressed as mean±standard error (SE). Student’s t-test was used to evaluate the significance of the difference between groups. P<0.05 was considered statistically significant.
3. Results
3.1. Identification of principal components of RPL extract by HPLC-MS/MS
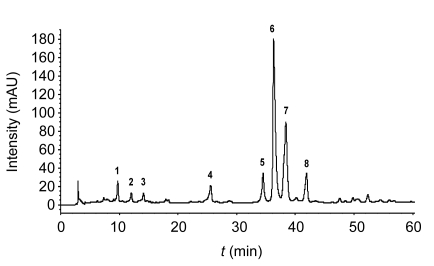
A suitable HPLC-MS/MS method for the n-butanol extract of RPL was developed by optimizing parameters. The HPLC chromatogram of the extract is shown in Fig. 1. Eight major bioactive components were investigated and designated as compounds 1 to 8 by retention time. Mass spectral data were obtained in the negative ion mode, which can give more structural information. ESI-MS analysis in combination with high-resolution FT-ICRMS data (Table 1) and relative literature resulted in the structural determination of these compounds. Details of these compounds are delineated as follows.
Fig. 1.
HPLC chromatogram of the n-butanol extract of RPL
Eight major bioactive components were investigated and designated as compounds 1 to 8
Table 1.
Accurate masses and assigned elemental compositions of eight compounds (1–8)
| Comp. | Composition | Measured m/z | Theoretical m/z | Error (×10−6) |
| 1 | C13H13O8− | 297.0609 | 297.0616 | −2.4 |
| 2 | C15H17O9− | 341.0872 | 341.0878 | −1.8 |
| 3 | C15H17O9− | 341.0871 | 341.0878 | −2.1 |
| 4 | C16H19O9− | 355.1024 | 355.1035 | −3.1 |
| 5 | C19H13O12− | 433.0405 | 433.0412 | −1.6 |
| 6 | C19H13O12− | 433.0408 | 433.0412 | −0.9 |
| 7 | C21H17O13− | 477.0658 | 477.0675 | −3.6 |
| 8 | C21H19O12− | 463.0877 | 463.0882 | −1.1 |
Compound 1 exhibited an ESI-MS spectrum with two abundant ions, [M-H]− at m/z 297 and [2M-H]− at m/z 595. In the MS2 spectrum, the deprotonated ion produced two key fragment ions at m/z 179 and 135, indicating the presence of a caffeoyl moiety. During MS3 experiment of the fragment ion at m/z 135, the product ions at m/z 117 and 89 were yielded by the loss of H2O and the consecutive loss of CO. On the bases of the mass spectra and previously published data (Lee et al., 2000; Parveen et al., 2008), compound 1 was tentatively identified as 2-caffeoyl-3,4-dihydroxybutanoic acid or 4-caffeoyl-2,3-dihydroxybutanoic acid.
Compounds 2 and 3, a pair of isomers, gave the same [M-H]− at m/z 341 and showed similar MSn behaviors. In the MS2 spectra, the fragment ions at m/z 179 (M–162, loss of a hexose residue) and m/z 135 were also present, which was in accordance with the existence of a caffeoyl moiety. Furthermore, the precursor ion produced a sequence of fragments at m/z 281, 251, and 221, attributed to the different cleavages within the hexose residue. Compared with the previously published data (Moco et al., 2006), compounds 2 and 3 were proposed to be caffeoyl-hexoside.
The full scan mass spectrum of compound 4 presented the [M-H]− at m/z 355 and [2M-H]− at m/z 711, indicating the molecular weight of 356. Similarly, the product ions at m/z 179 and 135 were observed in MS2 spectra, suggesting that compound 4 also contained a caffeoyl moiety. Fragment ions at m/z 193 and 161 were derived from the neutral loss of 162 Da (a caffeoyl moiety) and the consecutive elimination of 32 Da (a molecule of CH3OH). Based on the above results and the literature (Ming et al., 2002), compound 4 was tentatively assigned as methyl-caffeoyl-hexoside.
The [M-H]− ion of compound 5 was obtained at m/z 433. In the MS2 experiment, the predominant ion at m/z 301 was generated by the loss of 132 Da, reasonably assigned as the elimination of pentose. The occurrence of the ion at m/z 300 was attributed to a homolytic rupture of the glycosidic bond (Hvattum and Ekeberg, 2003; Ferreres et al., 2005). In the MS3 spectrum of m/z 301, two characteristic fragment ions at m/z 257 and 229 were observed, which could be correlated with the presence of an ellagic acid moiety. Consequently compound 5 was proposed as ellagic acid-pentoside (Zafrilla et al., 2001; Mullen et al., 2003; Lee et al., 2005). Compound 6 had almost the same MS and MSn behaviors as compound 5, indicating the presence of a second ellagic acid-pentoside.
The mass spectra of compound 7 contained a significant [M-H]− at m/z 477. In the MS2 spectrum, the product ion at m/z 301 was generated by the loss of 176 Da, owing to the elimination of glucuronide residue. It should be noted that the further fragmentation of ion at m/z 301 was entirely different from that of compounds 5 and 6. MS3 of m/z 301 produced two major fragments at m/z 179 and 151, which matched the fragmentation pattern of quercetin (Fabre et al., 2001; Mullen et al., 2003). Thus, compound 7 was tentatively identified as quercetin-glucuronide.
Compound 8 presented an intense [M-H]− at m/z 463 and an MS2 spectrum with the predominant ion at m/z 301 (M–162, loss of a hexose residue). The product ion underwent further dissociation and also produced major ions at m/z 179 and 151 characteristics of quercetin. Compound 8 was therefore proposed to be quercetin-hexoside.
3.2. Antioxidant activity of RPL extract
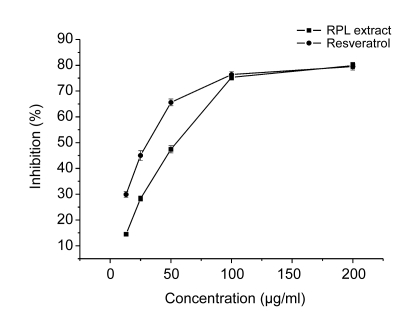
Fig. 2 illustrates that RPL extract has a significant concentration-dependent DPPH-free radical scavenging action, with an IC50 value of (52.2±0.9) μg/ml. Resveratrol as a positive control scavenged the DPPH radical with an IC50 value of (35.3±1.2) μg/ml.
Fig. 2.
DPPH free radical scavenging activity of the n-butanol extract of RPL
Data are expressed as mean±SE calculated from three separate experiments
3.3. Effects of RPL extract on serum ALT and AST in CCl4-induced hepatic injury
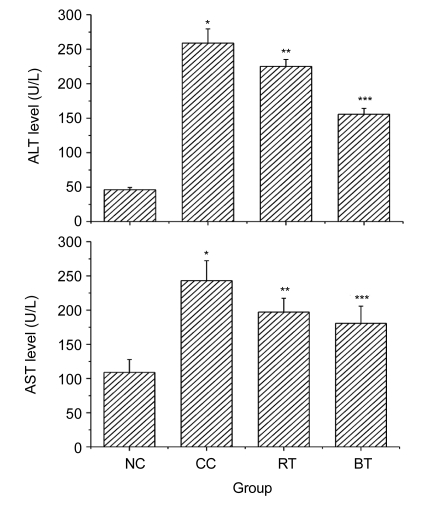
The effects of the n-butanol extract of RPL on serum enzymes ALT and AST were studied, and the results were summarized in Fig. 3. The administration of CCl4 to mice produced hepatotoxicity, which was demonstrated by a significant increase in the serum levels of ALT and AST in comparison with the normal control group (P<0.01). RPL extract and bifendate attenuated significantly (P<0.05 and P<0.01, respectively) the increased levels of the serum enzymes ALT and AST. The results show that RPL extract had significant hepatoprotective effects on liver injury induced by CCl4 in mice.
Fig. 3.
Effects of the n-butanol extract of RPL on serum ALT and AST in CCl4-intoxicated mice
NC: normal control group; CC: CCl4 control group; RT: RPL-treated group; BT: bifendate-treated group. Data are expressed as mean±SE calculated from three separate experiments. * P<0.01, compared to the NC group; ** P<0.05 and *** P<0.01, compared to the CC group
3.4. Effects of RPL extract on hepatic SOD and MDA in CCl4-induced hepatic injury
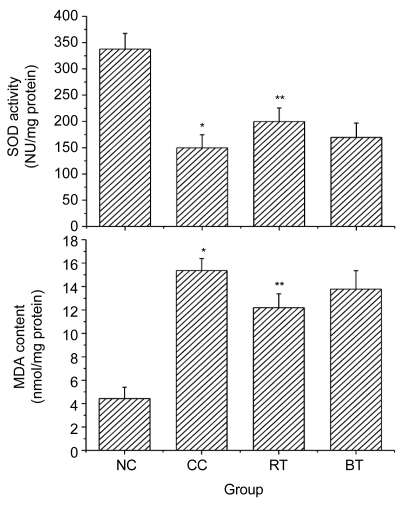
Results shown in Fig. 4 clearly reveal the effects of the n-butanol extract of RPL on hepatic SOD and MDA in CCl4-induced hepatic injury. Hepatic injury induced by CCl4 caused a significant decrease in hepatic antioxidant enzyme SOD activity (P<0.01) and a significant increase in MDA content of liver tissues (P<0.01). Treatment with RPL extract significantly increased SOD activity towards the normal value (P<0.05) and markedly prevented the rise in MDA content (P<0.05), thus demonstrating the significant protective effects of RPL extract on CCl4-induced hepatotoxicity in mice.
Fig. 4.
Effects of the n-butanol extract of RPL on hepatic SOD and MDA in CCl4-intoxicated mice
NC: normal control group; CC: CCl4 control group; RT: RPL-treated group; BT: bifendate-treated group; NU: nitrite unit. Data are expressed as mean±SE calculated from three separate experiments. * P<0.01, compared to the NC group; ** P<0.05, compared to the CC group
3.5. Histopathological observation
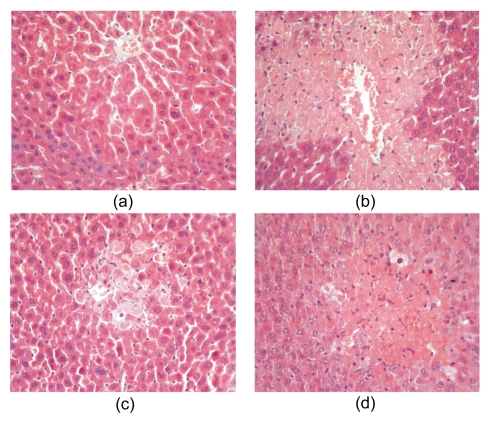
The histology of the liver sections of normal control animals exhibited normal liver architecture with well-preserved cytoplasm, prominent nucleus, and radiately arranged hepatocytes around the central vein (Fig. 5a). The injection of CCl4 induced ballooning degeneration, karyopyknosis, piecemeal necrosis, bridging necrosis, and loss of cellular boundaries, as shown in Fig. 5b. The hepatohistological changes induced by CCl4 were markedly ameliorated by the treatment with RPL extract, which significantly reduced ballooning-degenerated hepatocytes and markedly decreased the necrosis area. Only spotty and focal necrosis was observed in the central area of hepatic lobule (Fig. 5c). Results indicate that RPL extract affords much stronger protection than the reference drug bifendate (Fig. 5d) in CCl4-induced acute hepatic injury.
Fig. 5.
Sections of the livers of CCl4-treated mice showing the central vein (CV) and hepatocytes (H&E staining; original magnification, 40×)
(a) Normal control group; (b) CCl4 control group; (c) RPL-treated group; (d) Bifendate-treated group
4. Discussion
As a hepatotoxic agent, CCl4 has been extensively used in animal models to investigate chemical-induced hepatic injury. The CCl4-induced experimental damage resembles viral hepatitis histologically due to the formation of free radicals and the occurrence of lipid peroxidation in cellular and organelle membranes (Wu et al., 2007). Toxicity begins with a highly reactive trichloromethyl free radical (·CCl3), which is further converted to trichloromethyl peroxyl radical (CCl3OO·), a classical form of reactive oxygen species (ROS), as the initiator of lipid peroxidation (Hsiao et al., 2003; Jain et al., 2008; Ranawat et al., 2010). That is, excessive production of free radicals accelerates the peroxidative degradation of cellular membrane, leading to the breakdown of cell integrity and the leakages of ALT and AST. Consequently, the levels of serum ALT and AST are elevated. SOD and other antioxidant enzymes automatically form a mutually supportive team of defense against ROS (Gowri Shankar et al., 2008). SOD activity being easily inhibited by lipid peroxides or ROS has been suggested (Hsiao et al., 2003). Moreover, the increase of MDA content, as evident in our study, is suggestive of enhanced lipid peroxidation.
In our present work, treatment with the n-butanol extract of RPL significantly decreased the levels of ALT, AST, and MDA and increased the activity of SOD in mice intoxicated with CCl4, demonstrating that RPL extract has hepatoprotective effects on CCl4-induced liver injury. On the other hand, its potent scavenging activity on DPPH indicated that RPL extract perhaps acted as an efficient radical scavenger to ameliorate oxidative stress and inhibit the chain reaction of lipid peroxidation. Therefore, it appears that the free radical scavenging activity of RPL extract is considered to be responsible for its hepatoprotective effects on liver injury induced by CCl4 in vivo.
Phytochemical analysis of the n-butanol extract of RPL by HPLC-MS/MS concluded that phenolic compounds could be the principal components of this extract. Previous studies have claimed that phenolic compounds have diverse pharmacological activities such as antioxidant, anti-inflammatory, and hepatoprotective activities (Oh et al., 2004; Wu et al., 2007; Ranawat et al., 2010). RPL extract exhibited prominent antioxidant and hepatoprotective potential against CCl4-induced damage, in accordance with the description on the pharmacological properties of phenolic compounds in the aforementioned reports. Hence, phenolic compounds such as caffeic acid conjugates, ellagic acid glycosides, and flavonol glycosides may be the major bioactive constituents responsible for antioxidant effects and hepatoprotection of RPL.
In conclusion, the hepatoprotective and antioxidant effects of RPL were evaluated for the first time. Our results demonstrated that RPL possessed significant protection against CCl4-induced hepatotoxicity, which was at least partly due to its antioxidant properties through scavenging free radicals to ameliorate oxidative stress and inhibit lipid peroxidation. Phytochemical analysis based on HPLC-MS/MS and offline FT-ICRMS indicated the presence of various phenolic compounds, which may contribute to the pharmacological activities of RPL.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 21072174, 30873430, and 30973933) and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talent Fellowship, China
References
- 1.Adnyana IK, Tezuka Y, Banskota AH, Tran KQ, Kadota S. Three new triterpenes from the seeds of Combretum quadrangulare and their hepatoprotective activity. J Nat Prod. 2001;64(3):360–363. doi: 10.1021/np000486x. [DOI] [PubMed] [Google Scholar]
- 2.Carmona M, Sánchez AM, Ferreres F, Zalacain A, Tomás-Barberán F, Alonso GL. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: comparative study of samples from different geographical origins. Food Chem. 2007;100(2):445–450. doi: 10.1016/j.foodchem.2005.09.065. [DOI] [Google Scholar]
- 3.Chen JJ, Li XY, Sun CR, Pan YJ, Schlunegger UP. Identification of polyoxypregnane glycosides from the stems of Marsdenia tenacissima by high-performance liquid chromatography/tandem mass spectrometry. Talanta. 2008;77(1):152–159. doi: 10.1016/j.talanta.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 4.Compilation Group of Chinese Herbal Medicine Compilation. Chinese Herbal Medicine Compilation. Beijing, China: People’s Medical Publishing House; 1975. p. 512. (in Chinese) [Google Scholar]
- 5.Deighton N, Brennan R, Finn C, Davies HV. Antioxidant properties of domesticated and wild Rubus species. J Sci Food Agric. 2000;80(9):1307–1313. doi: 10.1002/1097-0010(200007)80:9<1307::AID-JSFA638>3.0.CO;2-P. [DOI] [Google Scholar]
- 6.Fabre N, Rustan I, de Hoffmann E, Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J Am Soc Mass Spectrom. 2001;12(6):707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 7.Ferreres F, Sousa C, Justin M, Valentão P, Andrade PB, Llorach R, Rodrigues A, Seabra RM, Leitão A. Characterisation of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem Anal. 2005;16(6):451–458. doi: 10.1002/pca.869. [DOI] [PubMed] [Google Scholar]
- 8.Gowri Shankar NL, Manavalan R, Venkappayya D, David Raj C. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2008;46(9):3182–3185. doi: 10.1016/j.fct.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 9.He S, Wu B, Pan YJ, Jiang LY. Stilbene oligomers from Parthenocissus laetevirens: isolation, biomimetic synthesis, absolute configuration, and implication of antioxidative defense system in the plant. J Org Chem. 2008;73(14):5233–5241. doi: 10.1021/jo8001112. [DOI] [PubMed] [Google Scholar]
- 10.Hollecker L, Pinna M, Filippino G, Scrugli S, Pinna B, Argiolas F, Murru M. Simultaneous determination of polyphenolic compounds in red and white grapes grown in Sardinia by high performance liquid chromatography-electron spray ionisation-mass spectrometry. J Chromatogr A. 2009;1216(15):3402–3408. doi: 10.1016/j.chroma.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, Lin CH, Su CH, Sheu JR. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51(11):3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, Ban XQ, He JS, Tong J, Tian J, Wang YW. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010;120(3):873–878. doi: 10.1016/j.foodchem.2009.11.020. [DOI] [Google Scholar]
- 13.Huang X, Liu Y, Song FR, Liu ZQ, Liu SY. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta. 2009;78(3):1090–1101. doi: 10.1016/j.talanta.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Hvattum E, Ekeberg D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J Mass Spectrom. 2003;38(1):43–49. doi: 10.1002/jms.398. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Soni M, Deb L, Jain A, Rout SP, Gupta VB, Krishna KL. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol. 2008;115(1):61–66. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Jin YS, Sa JH, Shim TH, Rhee HI, Wang MH. Hepatoprotective and antioxidant effects of Morus bombycis Koidzumi on CCl4-induced liver damage. Biochem Biophys Res Commun. 2005;329(3):991–995. doi: 10.1016/j.bbrc.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Kang SJ, Lee SH, Ro J, Lee K, Kinghorn AD. Phenolic compounds from the leaves of Cornus controversa . Phytochemistry. 2000;53(3):405–407. doi: 10.1016/S0031-9422(99)00502-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Johnson JV, Talcott ST. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J Agric Food Chem. 2005;53(15):6003–6010. doi: 10.1021/jf050468r. [DOI] [PubMed] [Google Scholar]
- 19.Luo XD, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (Star Apple) J Agric Food Chem. 2002;50(6):1379–1382. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- 20.Ming DS, Jiang RW, But PPH, Towers GHN, Yu DQ. A new compound from Geum Rivale L. J Asian Nat Prod Res. 2002;4(3):217–220. doi: 10.1080/10286020290024022. [DOI] [PubMed] [Google Scholar]
- 21.Moco S, Bino RJ, Vorst O, Verhoeven HA, de Groot J, van Beek TA, Vervoort J, de Vos CHR. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol. 2006;141(4):1205–1218. doi: 10.1104/pp.106.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen W, Yokota T, Lean MEJ, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn . Phytochemistry. 2003;64(2):617–624. doi: 10.1016/S0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 23.Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense . J Ethnopharmacol. 2004;95(2-3):421–424. doi: 10.1016/j.jep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Parveen I, Winters A, Threadgill MD, Hauck B, Morris P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochemistry. 2008;69(16):2799–2806. doi: 10.1016/j.phytochem.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Ranawat L, Bhatt J, Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J Ethnopharmacol. 2010;127(3):777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Shyur LF, Tsung JH, Chen JH, Chiu CY, Lo CP. Antioxidant properties of extracts from medicinal plants popularly used in Taiwan. Int J Appl Sci Eng. 2005;3(3):195–202. [Google Scholar]
- 27.Wang Y, He JM, Chen H, Zhang DS, Cai H, Shao HB. Analysis of flavones in Rubus parvifolius Linn by high performance liquid chromatography combined with electrospray ionization-mass spectrometry and thin-layer chromatography combined with Fourier transform surface enhanced Raman spectroscopy. Chin J Anal Chem. 2006;34(8):1073–1077. doi: 10.1016/S1872-2040(06)60050-9. [DOI] [Google Scholar]
- 28.Wu YH, Yang LX, Wang F, Wu XM, Zhou CX, Shi SY, Mo JX, Zhao Y. Hepatoprotective and antioxidative effects of total phenolics from Laggera pterodonta on chemical-induced injury in primary cultured neonatal rat hepatocytes. Food Chem Toxicol. 2007;45(8):1349–1355. doi: 10.1016/j.fct.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Zafrilla P, Ferreres F, Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem. 2001;49(8):3651–3655. doi: 10.1021/jf010192x. [DOI] [PubMed] [Google Scholar]
- 30.Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective and antioxidant activity of Amaranthus spinosus against CCl4 induced toxicity. J Ethnopharmacol. 2009;125(2):364–366. doi: 10.1016/j.jep.2009.05.010. [DOI] [PubMed] [Google Scholar]