Abstract
Both geniposide (Ge) and natural borneol (NB) are bioactive substances derived from traditional Chinese herbs. The effect of NB on the pharmacokinetics of Ge in rat via intranasal administration was investigated. The concentrations of Ge in plasma were determined by reversed-phase high-performance liquid chromatography (HPLC) after intranasal administration of Ge (4 mg/kg) alone and combined with different doses (0.08, 0.8, and 8 mg/kg) of NB. The intravenous administration was given as a reference (4 mg/kg of Ge and 8 mg/kg of NB). Compared with the intravenous administration, the absolute bioavailability of Ge was 76.14% through intranasal administration combined with NB. Compared with the intranasal administration of Ge alone, Ge could be absorbed rapidly in the nasal cavity combined with NB; the peak time of Ge in the plasma became shorter (3–5 min vs. 40 min); the peak concentration became higher (1.32–4.25 μg/ml vs. 0.67 μg/ml); and, the relative bioavailability of Ge combined with NB was 90.3%–237.8%. The enhancing effect was attenuated as the dose of NB decreased. The results indicated that NB can accelerate the absorption of Ge dose-dependently in the nasal cavity.
Keywords: Geniposide, Natural borneol, Intranasal administration, Intravenous administration, Pharmacokinetics
1. Introduction
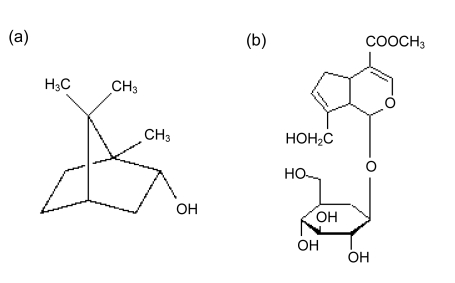
Geniposide (Ge), one of the biologically active ingredients of gardenia fruit (Gardenia jasminoides Ellis, Rubiaceae), is widely used in multi-component injection in the treatment of cerebrovascular and cardiovascular diseases in China for its anti-thrombotic and anti-inflammatory effects (Suzuki et al., 2001; Koo et al., 2006). Borneol is a monoterpenoid component in medicinal plants. It is widely used in traditional Chinese medicine (TCM) combined with gardenia for stroke treatment, such as ‘Xingnaojing’ injection. Some studies had shown that borneol can improve the nasal, oral, and gastrointestinal bioavailabilities of drugs, accelerate the opening of the blood-brain-barrier (BBB), and enhance the distribution of drugs in brain tissue (Chen et al., 2006; Li et al., 2007; Cai et al., 2008; Dai et al., 2009). The structures of geniposide and borneol are shown in Fig. 1. Both synthetic borneol (SB) and natural borneol (NB) are widely used in Chinese medicine. SB consists of d-borneol and isoborneol, while NB only contains d-borneol. It has been reported that isoborneol had more mucosa stimulus and hepatotoxicity (Hu et al., 2005). After a comprehensive evaluation, NB (d-borneol), the safer form of borneol, was used in the current research.
Fig. 1.
Structures of borneol (a) and geniposide (b)
As more and more adverse reactions have been reported on multi-component Chinese herbal medicine (MCHM) injection, it is urgent to find a new and safer way for drug administration. The intranasal route can be a hopeful substitution for MCHM injection, as drugs can be absorbed sufficiently and rapidly into blood for systemic administration (Albrecht et al., 2010; Zhou et al., 2010), then transported from the nasal cavity to the central nervous system (Illum, 2003). The intranasal route is safer due to the barricade effect of the nasal mucosa. According to the results of preliminary study (Zhang et al., 2009), the absorption percentage of Ge in intestinal tract of rats was only about 15.9% within 3 h, and the apparent partition coefficient (log P) of Ge between octanol and water was about 0.108.
The purpose of this study was to investigate the enhancing effect of NB on the absorption of Ge in rat via intranasal administration. The concentrations of Ge in rat plasma after intranasal administration combined with different amounts of NB were detected and the pharmacokinetic parameters of each group were compared.
2. Materials and methods
2.1. Materials
Ge was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China), and NB was obtained from Tongrentang Co. Ltd. (Beijing, China). Acetonitrile, methanol, and water were of high-performance liquid chromatography (HPLC) grade (Qualigens, China) and all other reagents were of analytical grade.
2.2. Animals
Male Sprague Dawley rats (250–280 g) were obtained from Weitong biotechnology Inc. (Beijing, China). All animals were clinically healthy and biochemically normal throughout the experimental period.
2.3. In vivo experiments
Twenty five male Sprague Dawley rats, weighing (265±16) g, were randomly assigned into five groups. All the animals were fasting for 12 h before experiment, but allowed to take water freely. The rats were anesthetized by intraperitoneal injection of urethane (1.2 g/kg body weight). An incision was made in the neck of rat, the distal end of left carotid artery was ligated, and the proximal end was clamped by a bulldog clamp. An intravenous tube was cannulated before administration. Blood samples were taken by controlling the bulldog clamp. One group was intravenously administered with about 0.5 ml of injection sample (a single dose of 4 mg/kg of Ge and 8 mg/kg NB) via tail vein. The other four groups were administered with 20 μl of nasal solution via nostril by a modified microinjector with a soft polyethylene (PE) tube, which contained the same amount of Ge (4 mg/kg) and 0, 0.08, 0.8, or 8 mg/kg of NB, respectively. The tube was inserted into the nostril about 4 mm and maintained at least 15 s in order to avoid drug loss. A total of 0.25 ml of blood was collected from the left carotid artery at 0.5, 1, 3, 5, 10, 20, 40, 60, 90, 120, 180, and 240 min after drug administration (Lu et al., 2010). The pharmacokinetic parameters associated with each animal were estimated by Kinetica 4.4 software. All the experiments on animals were performed according to the Guidelines for Animal Experimentation, University of Traditional Chinese Medicine of Beijing, China.
2.4. HPLC assay method
The concentration of Ge was quantitated by reversed phase (RP)-HPLC (Agilent1100, HP Inc., USA; Diamonsil® C18 column, 250 mm×4.6 mm, 5 µm, Dikma Technology Co., China), as described by Ye et al. (2006) with a slight modification. The mobile phase was acetonitrile and water (15:85, v/v) mixture, and the signal was monitored at 238 nm. The flow rate was maintained at 1.0 ml/min. All the samples were centrifuged at 12 000 r/min for 15 min before determination.
2.5. Plasma sample preparation and validity
Blood sample was collected via left carotid artery and placed into heparinized tubes, as described by Ueno et al. (2001) with a slight modification. After centrifugation, the obtained plasma was stored at −20 °C until determination. An aliquot of 100 µl plasma sample was placed into a centrifuge tube and 200 µl acetonitrile was added. After being vortexed for 1 min, the mixture was centrifuged at 12 000 r/min for 10 min. Then 250 µl of supernatant was placed into another centrifuge tube and evaporated under nitrogen gas. The residue was redissolved with methanol. The methanol solution was centrifuged at 12 000 r/min for 15 min. Then 20 µl of solution was injected into HPLC system.
Plasma standards (0.273–13.650 μg/ml) covering the expected sample concentration range were prepared by spiking various quantities of Ge into blank plasma. These calibration standards were used to validate the linearity, recovery, and precision of the analytical method.
2.6. Statistical evaluation
The in vivo pharmacokinetic parameters of the intranasal and intravenous groups were compared by one-way analysis of variance (ANOVA) at the 0.05 significance level, and the statistical differences were carried out by paired t-tests (Statistics Analysis System 8.0).
3. Results
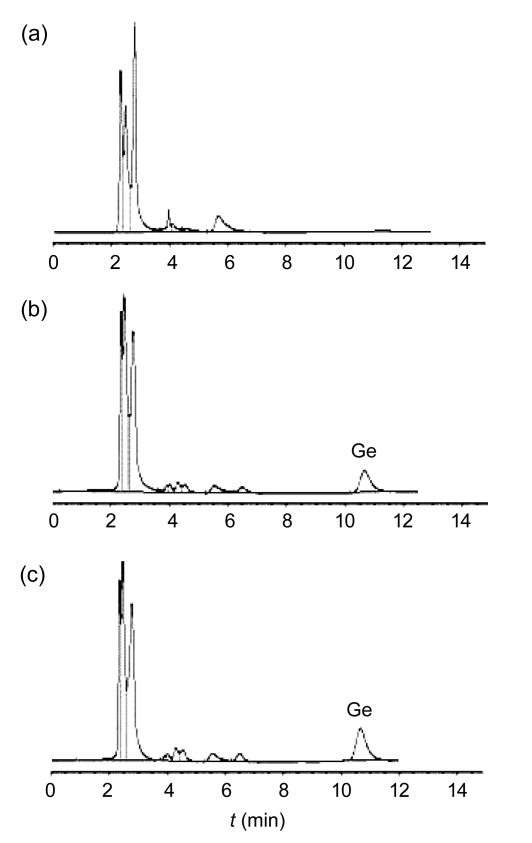
Under the chromatographic conditions described above, the retention time of Ge was found at 10.6 min (Fig. 2). The limit of detection of Ge was 20 ng/ml in plasma (signal-to-noise ratio (S/N) ≥3). The calibration curves of Ge were linear in the range of 0.273–13.650 μg/ml. There was a good linearity between concentration (C) and peak area (A) (C=0.0094A+0.2577, r=0.9997). The mean relative recoveries of Ge at low, middle, high concentrations were (97.14±3.78)%, (95.06±2.95)%, and (91.50±1.82)%, respectively. The relative standard deviations (RSDs) of intra-day precision of Ge at low, middle, and high concentrations were 1.68%, 3.87%, and 1.93%, respectively, while inter-day precisions were 4.09%, 0.33%, and 1.08%, respectively. The limit of quantification was 0.27 μg/ml. The validity of plasma sample assay method was satisfying.
Fig. 2.
Typical RP-HPLC chromatograms of blank rat plasma (a), blank rat plasma spiked with geniposide (Ge) (b), and plasma sample (c)
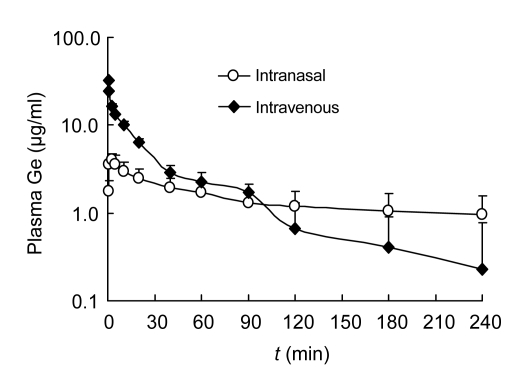
The concentration-time profiles of Ge (4 mg/kg) with NB (8 mg/kg) in the plasma after intravenous or intranasal administration are shown in Fig. 3. The data of intravenous and intranasal administrations best fitted a two-compartment open model. The main pharmacokinetic parameters of the compartment model were calculated using Kinetica 4.4 software as shown in Table 1. The results showed that the average value of calculated maximum concentration (c max (calc.)) was 4.696 μg/ml with the time corresponding to c max (calc.) (t max (calc.)) at about 2 min after intranasal administration of Ge with NB. The total partial areas under the curve (AUC0–∞) of injection and nasal solutions were 471.026 and 452.396 µg/(ml·min), respectively. The Ge in plasma of the intravenous group was eliminated faster than that of the intranasal group. The elimination rate constant from central compartment (K el) for injection solution was 0.053 min−1 while for nasal solution was 0.016 min−1. The total mean residence time (MRT0–∞) for injection and nasal solutions was 59.1 and 167.5 min, respectively.
Fig. 3.
Mean plasma concentration-time curve of geniposide (Ge) in rats after a single intravenous or intranasal administration of geniposide (4 mg/kg) combined with natural borneol (8 mg/kg)
Data are expressed as mean±SD (n=5)
Table 1.
Main pharmacokinetic parameters of the compartment model after a single intravenous or intranasal administration of geniposide (4 mg/kg) combined with natural borneol (8 mg/kg) in rats
| Parameter | Value |
|
| Intravenous | Intranasal | |
| Ka (min−1) | − | 2.912±1.776 |
| A (μg/ml) | 22.231±2.668 | 0.917±0.266** |
| α | 0.106±0.016 | 0.314±0.443 |
| B (μg/ml) | 2.554±1.342 | −0.006±0.002 |
| β | 0.010±0.002 | 0.007±0.003 |
| AUC0–∞ (µg/(ml·min)) | 471.026±62.785 | 452.396±147.478 |
| MRT0–∞ (min) | 59.112±8.122 | 167.489±53.390** |
| cmax (calc.) (μg/ml) | 24.785±1.539 | 4.696±1.486** |
| tmax (calc.) (min) | − | 2.006±0.542 |
| Kel (min−1) | 0.053±0.009 | 0.016±0.012** |
| K12 (min−1) | 0.042±0.015 | 0.167±0.238* |
| K21 (min−1) | 0.020±0.009 | 0.138±0.204* |
| t1/2,Ka(min) | − | 0.327±0.206 |
| t1/2,α (min) | 6.666±0.985 | 8.802±7.894 |
| t1/2,β (min) | 74.073±14.781 | 127.867±56.484* |
K a: absorption rate constant; A: coefficient in the sum of exponentials; α, β: exponent; B: coefficient in the sum of exponentials; AUC0–∞: total partial area under the curve; MRT0–∞: total mean residence time; c max (calc.): calculated maximum concentration; t max (calc.): time corresponding to c max (calc.); K el: elimination rate constant from central compartment; K 12: transfer rate constant from central compartment to the superficial compartment; K 21: transfer rate constant from superficial compartment to the central compartment; t 1/2,Ka: half-life of the absorption phase; t 1/2,α: half-life of α; t 1/2,β: half-life of β. Data are expressed as mean±SD (n=5)
P<0.05 vs. the intravenous group
P<0.01 vs. the intravenous group
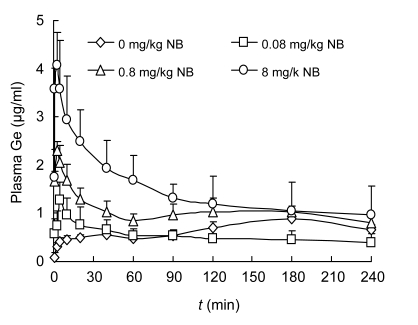
For the intranasal administrations of Ge (4 mg/kg) with various doses (0, 0.08, 0.8, and 8 mg/kg) of NB, the concentration-time profiles of Ge in the plasma of rats are shown in Fig. 4. Non-compartmental analysis of pharmacokinetic data was performed by Kinetica 4.4 software. The pharmacokinetic parameters are shown in Table 2. The results show that the average values of c max were 0.673, 1.322, 2.324, and 4.249 μg/ml after intranasal administration of 4 mg/kg Ge with 0, 0.08, 0.8, or 8 mg/kg NB, respectively. The t max was at about 40, 6, 3.4, and 3.8 min, respectively. The absolute bioavailability (F) of Ge in the plasma was calculated as the ratio of AUCintranasal/AUCintravenous, and the F was 30.54%, 27.59%, 56.89%, and 76.14% for Ge with 0, 0.08, 0.8, and 8 mg/kg NB, respectively. The relative bioavailability (F r) of Ge was calculated as the ratio of AUCGe+NB/AUCGe, and F r of Ge with 0.08, 0.8, and 8 mg/kg NB by intranasal administration was 90.33%, 186.31%, and 249.34%, respectively. Our present results demonstrate that nasal absorption of Ge is dose-dependent and can be enhanced effectively by NB.
Fig. 4.
Mean plasma concentration-time curve of geniposide (Ge) in rats after a single intranasal administration of geniposide (4 mg/kg) without or with natural borneol of 0.08, 0.8, and 8 mg/kg
Data are expressed as mean±SD (n=5)
Table 2.
Main pharmacokinetic parameters of the non-compartmental model after a single intravenous (4 mg/kg of geniposide (Ge) with 8 mg/kg natural borneol (NB)) or intranasal administration of Ge (4 mg/kg) combined with NB of 0, 0.08, 0.8, and 8 mg/kg in rats
| Group | Dose of NB (mg/kg) | cmax (μg/ml) | tmax (min) | AUC0–240 (µg/(ml·min)) | MRT0–240 (min) | F (%) | Fr (%) |
| 1 | 8 (i.v.) | 32.342±1.926 | 0.50 | 440.59±70.70 | 43.00±7.76 | 100.00 | − |
| 2 | 0 (i.n.) | 0.673±0.104 | 40.00Δ | 134.57±13.13 | 131.34±6.20 | 30.54 | 100.00 |
| 3 | 0.08 (i.n.) | 1.322±0.378** | 6.00±2.24** | 121.55±27.69 | 101.39±9.33* | 27.59 | 90.33 |
| 4 | 0.8 (i.n.) | 2.324±0.197** | 3.40±0.89** | 250.67±17.86** | 112.08±5.60* | 56.89 | 186.31 |
| 5 | 8 (i.n.) | 4.249±0.785** | 3.80±1.10** | 335.48±59.65** | 81.92±20.22** | 76.14 | 249.34 |
i.v.: intravenous; i.n.: intranasal; c max: experimental maximum concentration; t max: time corresponding to c max; AUC0–240: partial area under the curve from 0 to 240 min; MRT0–240: mean residence time from 0 to 240 min; F: absolute bioavailability; F r: relative bioavailability. Data are expressed as mean±SD (n=5)
P<0.05, vs. Group 2
P<0.01, vs. Group 2
The first peak of Group 2
4. Discussion
As TCM injections are used widely in China, more and more adverse effects have been reported. Thus, it is important to find a better route of administration. Intranasal administration may be a good choice. In our formulation, Ge is a water-soluble compound with low molecular weight (388.4). Its log P value in octanol/water system was −0.97, suggesting that it was hard for Ge to permeate mucous membrane. Its intestinal absorption coefficient (K) was only about 0.056 h−1 (Zhang et al., 2009). NB is a small lipophilic compound and can be rapidly absorbed via the mucous membrane because its liposolubility and low molecular weight (154.25). Based on our preliminary experiments, the half-life (t 1/2) of nasal absorption of NB was about 10 min, while the t max of borneol in mice plasma was 3 min after intragastric administration (Yu et al., 2006). When passing through the mucous membrane, NB might enhance epithelial junction permeability and promote paracellular drug absorption as SB (Xiao et al., 2007).
Several methods have been used to measure the concentration of Ge in plasma (Hou et al., 2008; Lv et al., 2008). We have developed a simple and sensitive RP-HPLC method to measure the concentration of Ge in rat plasma. The pharmacokinetic profiles of Ge (intravenous or intranasal) and the enhancement of absorption of Ge with different amounts of NB in rat plasma after intranasal administration were investigated. The MRT results show that the absorption of Ge combined with NB after intranasal administration was faster, but eliminated slower than that of intravenous administration. The absolute bioavailability of Ge by intranasal administration combined with NB (8 mg/kg) was high, reaching 76.14%.
NB did increase the nasal absorption of Ge. With the increase of NB, nasal bioavailability of Ge was increased. After intranasal coadministration of 0.08, 0.8, and 8 mg/kg of NB, nasal bioavailability of Ge in plasma was 0.90, 1.86, and 2.49 times, respectively, higher than that without NB, based on the data of a non-compartment model. There was no significant difference in the bioavailability between groups of 0 and 0.08 mg/kg of NB. The results showed the enhancing effect of NB was in proportion with the log m NB (m NB: amount of NB), with the equation of AUCGe=106.97×log m NB+246.27 (r=0.9929). From Fig. 4, the results showed that the absorption of Ge combined with NB (low or high) after intranasal administration was very rapid and t max was less than 10 min in plasma. The presumption was that the nasal absorption of Ge combined with NB could be divided into two consecutive processes, the rapid absorption phase (with NB) and the slow absorption phase (without NB). With the existence of a high concentration of NB, Ge was absorbed rapidly at the very beginning. Along with the absorption, the concentration of NB reduced rapidly, and its enhancement of absorption weakened greatly. The absorption of Ge got into the slow absorption phase when most of NB had been absorbed by rats. The nasal absorption enhancement of Ge by NB was reversible and dose-dependent. Many researchers have used one of the following methods to study the mechanisms of nasal absorption enhancer: increasing membrane fluidity, inhibiting enzyme activity, reducing mucus viscosity or elasticity, opening up tight junctions, or solubilizing the drug (Merkus et al., 1996). However, it is more essential to study whether absorption enhancer can be absorbed and to determine the speed and extent of enhancer absorption. The absorption characteristics of enhancers themselves are closely related to their effects on promoting drug absorption.
5. Conclusions
In conclusion, the present study demonstrates that NB can accelerate the transnasal absorption of Ge, which is consistent with our previous findings (Lu et al., 2010). The enhancing effect is temporary and closely related to the dose of NB. Pharmacokinetic results provide valuable information for studying the dosage of NB in the transnasal preparations containing Ge, and also indicate the potential effect of NB on other drugs. After all, it is possible for intranasal administration, with high bioavailability and immediate effect, as a hopeful route to substitute the TCM injections containing Ge and NB.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81073057), the Key New Drug Creation and Development Programme of China (Nos. 2009ZX09502-008 and 2009ZX09308-003), and the Doctoral Fund of the Ministry of Education of China (No. 20090013110007)
References
- 1.Albrecht J, Kopietz R, Frasnelli J, Wiesmann M, Hummel T, Lundstrom JN. The neuronal correlates of intranasal trigeminal function—an ALE meta-analysis of human functional brain imaging data. Brain Res Rev. 2010;62(2):183–196. doi: 10.1016/j.brainresrev.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S, Pu J. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16(2):178–184. doi: 10.1080/10611860701794395. [DOI] [PubMed] [Google Scholar]
- 3.Chen XM, Zhu JB, Sun WD, Zhang LJ. Effect of absorption enhancers on nasal ginsenoside Rg1 delivery and its nasal ciliotoxicity. Acta Pharm Sin. 2006;41(2):149–155. (in Chinese) [PubMed] [Google Scholar]
- 4.Dai JP, Chen J, Bei YF, Han BX, Wang S. Influence of borneol on primary mice oral fibroblasts: a penetration enhancer may be used in oral submucous fibrosis. J Oral Pathol Med. 2009;38(3):276–281. doi: 10.1111/j.1600-0714.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Hou YC, Tsai SY, Lai PY, Chen YS, Chao PD. Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem Toxicol. 2008;46(8):2764–2769. doi: 10.1016/j.fct.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Hu LM, Fan GW, Gao XM, Zhang BL. Comparison of influence of natural borneol and synthetic borneol on gastric mucosal barrier in rats. J Tianjin Univ Tradition Chin Med. 2005;24(3):123–125. (in Chinese) [Google Scholar]
- 7.Illum L. Nasal drug delivery–possibilities, problems and solutions. J Control Release. 2003;87(1-3):187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 8.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103(3):496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Feng J, Cheng Q, Zhu W, Jin Y. Delivery of 125I-cobrotoxin after intranasal administration to the brain: a microdialysis study in freely moving rats. Int J Pharm. 2007;328(2):161–167. doi: 10.1016/j.ijpharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Chen X, Du S, Wu Q, Yao Z, Zhai Y. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of geniposide in rats. Arch Pharm Res. 2010;33(5):691–696. doi: 10.1007/s12272-010-0507-8. [DOI] [PubMed] [Google Scholar]
- 11.Lv H, Sun H, Sun W, Liu L, Wang P, Wang X, Cao H. Pharmacokinetic studies of a Chinese triple herbal drug formula. Phytomedicine. 2008;15(11):993–1001. doi: 10.1016/j.phymed.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Merkus FWHM, Schipper NGM, Verhoef JC. The influence of absorption enhancers on intranasal insulin absorption in normal and diabetic subjects. J Control Release. 1996;41(1-2):69–75. doi: 10.1016/0168-3659(96)01357-0. [DOI] [Google Scholar]
- 13.Suzuki Y, Kondo K, Ikeda Y, Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001;67(9):807–810. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 14.Ueno K, Takeda Y, Iwasaki Y, Yoshizaki F. Simultaneous estimation of geniposide and genipin in mouse plasma using high-performance liquid chromatography. Anal Sci. 2001;17(10):1237–1239. doi: 10.2116/analsci.17.1237. [DOI] [PubMed] [Google Scholar]
- 15.Xiao YY, Ping QN, Chen ZP. The enhancing effect of synthetical borneol on the absorption of tetramethyl-pyrazine phosphate in mouse. Int J Pharm. 2007;337(1-2):74–79. doi: 10.1016/j.ijpharm.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Ye G, Zhu HY, Zhao HL, Xu B, Huang CG. HPLC method for the determination and pharmacokinetic studies on geniposide in rat serum after oral administration of traditional Chinese medicinal preparation Yin-Zhi-Ku decoction. Biomed Chromatogr. 2006;20(8):743–747. doi: 10.1002/bmc.590. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Wang W, Li SM. Simultaneous determination of borneol and nimodipine in mice plasma by GC-MSn . Chin Pharm J. 2006;41(16):1259–1262. (in Chinese) [Google Scholar]
- 18.Zhang Q, Du S, Lu Y, Rao X. Studies on O/W partition coefficient and absorption kinetics of geniposide in fructus gardeniae extract in rat intestine. China J Chin Mat Med. 2009;34(14):1840–1844. (in Chinese) [PubMed] [Google Scholar]
- 19.Zhou S, Kawakami S, Yamashita F, Hashida M. Intranasal administration of CpG DNA lipoplex prevents pulmonary metastasis in mice. Cancer Lett. 2010;287(1):75–81. doi: 10.1016/j.canlet.2009.05.037. [DOI] [PubMed] [Google Scholar]