Abstract
Invasive aspergillosis is among the most common human fungal infections and occurs in patients with severe and complex defects in immune responses. Natural killer cells have previously been found to be important in host defense against this infection, but the mechanism of this effect is not known. We hypothesized that NK cells mediate their protective effect in invasive aspergillosis by acting as the major source of IFN-γ during early infection. We found that, in the lungs of neutropenic mice with invasive aspergillosis, NK cells were the major population of cells capable of generating IFN-γ during early infection. Depletion of NK cells resulted in reduced lung IFN-γ levels and increased lung fungal load that was independent of T and B cell subsets. Depletion of NK cells and absence of IFN-γ resulted in a similar increase in susceptibility to the infection, but depletion of NK cells in IFN-γ-deficient hosts did not result in further increase in severity of the infection. NK cell-derived IFN-γ caused enhanced macrophage antimicrobial effects in vitro and also resulted in greater expression of IFN-inducible chemokines in the lungs. Finally, transfer of activated NK cells from wildtype, but not IFN-γ-deficient hosts, resulted in greater pathogen clearance from the lungs of both IFN-γ-deficient and wildtype recipients. Taken together, these data indicate that NK cells are the main source of early IFN-γ in the lungs in neutropenic invasive aspergillosis, and this is an important mechanism in the defense against this infection.
Keywords: Natural killer cells, fungal infections, cytokines, chemokines, lung
Introduction
Invasive aspergillosis is among the most common human fungal infections and is often characterized by a poor outcome, even with appropriate antimicrobial therapy (1). The causative microorganisms, Aspergillus species, are moulds that are extensively distributed in diverse environmental niches. The asexual reproductive spores of Aspergillus species, known as conidia, are released in large numbers into ambient air, remain air-borne for many hours, and are inhaled daily by all humans. Normal hosts uniformly eliminate these inhaled conidia without developing disease, but in immunocompromised hosts, the conidia can germinate to form multicellular filaments, known as hyphae, that penetrate the respiratory epithelium and produce a severe pneumonia, invasive pulmonary aspergillosis (2). Despite advances in antifungal therapy, invasive aspergillosis continues to carry a poor prognosis and there is a pressing need to better understand the host defense mechanisms in this infection.
Defects in neutrophil number or function have long been recognized as key risk factors that predispose the host to the development of invasive aspergillosis, but in the clinical setting, the immunological defects in patients with invasive aspergillosis are complex and extend beyond neutrophil deficiency. Indeed, the proportion of non-neutropenic immunocompromised patients succumbing to invasive aspergillosis has increased in recent clinical studies (3–5), underscoring the importance of understanding other cellular mechanisms of host defense in this infection. In the context of animal models, the importance of Th-1 acquired immunity is established as being critical to protective responses in invasive aspergillosis (6–8). We and others have previously observed the early expression of IFN-γ in the lungs of immunocompetent and neutropenic mice challenged with the microorganism (9, 10). Importantly, while the cellular source of IFN-γ during late infection and in immunized mice is CD4 T cells (6, 11, 12), the cellular source of IFN-γ early in the course of invasive aspergillosis is unknown.
We have previously found that, in the context of early innate immunity, NK cells are essential to defense against invasive aspergillosis in neutropenic mice (13). NK cell-derived IFN-γ has been shown to be required for successful elimination of many viral (14–16), bacterial (17, 18), and protozoal (19, 20) pathogens, as well as the yeast Cryptococcus (21–23). We therefore tested the hypothesis that NK cells mediate their protective effect in invasive aspergillosis by acting as the major source of IFN-γ during early infection.
Materials and methods
Animals
Wildtype C57BL/6 mice, mice with targeted deletion of IFN-γ or the RAG-1 genes (all on C57Bl/6 background) were purchased from Jackson Laboratories (Bar Harbor, Maine). Age-and gender-matched 6–14-week old animals were used in the experiments. All animals were maintained under pathogen-free conditions and in compliance with institutional animal care regulations.
In vivo procedures
We used a previously characterized model of invasive pulmonary aspergillosis in transiently neutropenic mice (10, 13, 24–26). Depletion of neutrophil was achieved using a single i.p. injection of a monoclonal Ab (anti-Gr-1; clone RB6-8C5) one day before intratracheal challenge with Aspergillus fumigatus conidia. As described previously, this resulted in peripheral blood neutropenia (absolute circulating neutrophil count less than 50 cells/µl) on days 1 and 3 after injection in both infected and uninfected mice, with a return of peripheral counts to pretreatment levels (>1000 cells/µl) by day 5 (27, 28). In uninfected animals, administration of the mAb did not influence the number of non-neutrophil peripheral blood leukocytes, nor lung or spleen lymphocyte or DC subsets, including plasmacytoid DCs (26). A. fumigatus (strain 13073, American Type Culture Collection, Manassas, VA) conidia were collected in 0.1% Tween-80 in PBS from 7–14 day old cultures on Sabouraud’s dextrose agar plates, filtered and counted under a hemacytometer, and administered in inocula ranging from 2–5×106 conidia per mouse in various experiments. At designated time points, animals were euthanized by CO2 asphyxiation, and whole lungs were removed and processed as described (10, 13, 26, 27). NK cells were depleted using a monoclonal antibody (PK136, anti-NK1.1) or anti-asialoGM1 (WAKO, Richmond, Virginia). Intraperitoneal administration of 200 µg of anti-NK1.1 or 25µl of anti-asialoGM1 2 days before A. fumigatus challenge resulted in depletion of NK cells for ϥ days, as previously described (13, 29, 30).
Identification of leukocyte subsets
Leukocyte-enriched whole lung single-cell suspensions were prepared as described (10, 13, 26, 27). The following reagents were used to label cells (from BD Biosciences, San Jose, California, unless otherwise noted): α-Gal-Cer–loaded or control FITC-labeled CD1d tetramers (National Institute of Allergy and Infectious Disease Tetramer Facility), anti-CD3-allophycocyanin (clone 17A2), anti-CD4-Pacific Blue (GK1.5), anti-CD45-peridinin chlorophyll A protein (30-F11), anti-IFN-γ-PE (XMG1.2) and anti-NK1.1-biotin (PK136) or respective isotype controls. To determine the lung cells capable of producing IFN-γ, lung cell suspensions were incubated with Brefeldin A (10 ng/ml), PMA (10 ng/ml) and ionomycin (100 ng/ml) in RPMI with 5% fetal calf serum for 4 hrs and intracellular staining was detected using a commercial kit (Cytofix/Cytoperm, BD Biosciences). Samples were analyzed on a FACS Canto II instrument using Diva software (all from BD Biosciences). The absolute number of each leukocyte subset was determined as the product of the percentage of the cell type and the total number of cells in the sample, as determined under a hemacytometer.
NK cell culture and in vitro studies
Activated NK cells were prepared as described (13). Briefly, splenocytes were cultured with mIL-12 (mIL-12, 1 ng/ml) and mIL-18 (100 ng/ml) for 5 days and negatively selected by immunomagnetic depletion of CD5+, Ly-6G+, TER-119+, CD22+, and F4/80+ cells according to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC), resulting in ϩ5% purity of NK cells. The resulting cells were highly activated lymphoblasts, spontaneously produced large amounts of IFN-γ and perforin and exhibited potent natural cytotoxicity against NK-sensitive targets, as previously shown (31–34). In adoptive transfer experiments, 2 × 106 NK cells in 100 µl PBS were delivered intravenously.
For co-culture experiments, resident alveolar macrophages or thioglycollate-elicited inflammatory peritoneal macrophages were obtained from naive animals as described (35, 36) and phagocytosis and hyphal damage were assessed with minor modification of published protocols (26, 37–40). Macrophages and various numbers of NK cells were cultured in RPMI 1640 with 5% FCS in U-bottom 96-well plates at 37°C in 5% CO2 for 3 hrs before addition of fungal elements. To assess rates of phagocytosis, freshly harvested conidia were first conjugated to FITC, washed, and uniform staining verified by epifluorescent microscopy before addition to leukocytes. In different experiments, 2–4×105 macrophages were added per well and the ratio of macrophages: NK cells: conidia was 2:1:10. Phagocytosis was halted after 1 hour of incubation by addition of cytochalasin D (final concentration 4 µg/ml; Sigma) and extracellular fluorescence quenched in trypan blue (250 µg/ml in PBS) prior to flow cytometric analysis. In preliminary studies, no further increase in phagocytosis was noted after 1 hour (unpublished observation). In some experiments, hyphal viability was assessed after conidia were co-incubated with leukocytes for 16 hours. In other experiments, hyphae were prepared by incubating fresh A. fumigatus conidia in RPMI 1640 at 1.5×106 conidia/ml at 37°C for 18 hours; the resulting hyphae were then incubated with leukocytes (4×105 macrophages and various numbers of NK cells per well) for a further 2 hours before quantification of hyphal viability. To measure hyphal viability, leukocytes were lysed hypotonically and remaining hyphae were then incubated for 60 min in 100 µl of a solution containing 0.5 mg/ml 2,3-bis-2-methoxy-4-nitro-5-sulfophenyl-2H-tetrazolium-5-carboxanilide (XTT) and 40 µg/ml coenzyme Q (both from Sigma), and absorbence was determined at 450 nm. Hyphal killing was expressed as percent reduction in viability as compared to hyphae incubated without leukocytes.
Cytokine and chitin assays
Moulds, including Aspergillus species, grow as multicellular hyphae that do not form distinct reproductive structures in infected tissues. We therefore used a previously characterized assay for chitin, a carbohydrate component of hyphal wall absent from conidia and from mammalian tissues, to quantify the burden of hyphae in infected lungs, as detailed previously (10, 13, 24, 25). Briefly, homogenized organs were boiled in sodium lauryl sulfate and then heated in KOH (120% w/v) at 121° C for 60 minutes. Samples were then washed in ethanol, and NaNO2 (5% w/v), KHSO4 (5% w/v), ammonium sulfamate (12.5% w/v), and 3-methyl-2-benzothiazolinone hydrazone hydrochloride hydrate (MBTH, 5mg/ml) were added sequentially. Samples were boiled and OD650nm was measured after addition of FeCl3.6H2O (0.83% w/v). Chitin content was expressed as the ratio to glucosamine control. We have previously shown that lung chitin content on day 3 of infection, as measured by this protocol, correlates with mortality from the infection and histological severity of the infection in this model (24).
ELISA was performed on filtered supernatants of lung homogenates using complementary Ab pairs against murine IFN-γ, CXCL9, CXCL10 and CXCL11 (all from BD Biosciences) according to manufacturer’s instructions.
Statistical analysis
Data were analyzed on a Macintosh Powerbook G4 computer using Prism statistical package (v.4.0a, Graphpad Software, San Diego, CA). Survival data were compared using Fischer’s exact test. All other data were expressed as mean ± SEM. Values between 2 groups over multiple times were compared with 2-way ANOVA, comparisons between 2 groups at a single time were performed with unpaired two-tailed Mann-Whitney (non-parametric) test, and comparisons between multiple groups at a single time were compared using the Kruskal-Wallis test with Bonferroni post-test. Probability values were considered statistically significant if they were less than 0.05.
Results
NK cells as the major cellular source of lung IFN-γ in early invasive aspergillosis
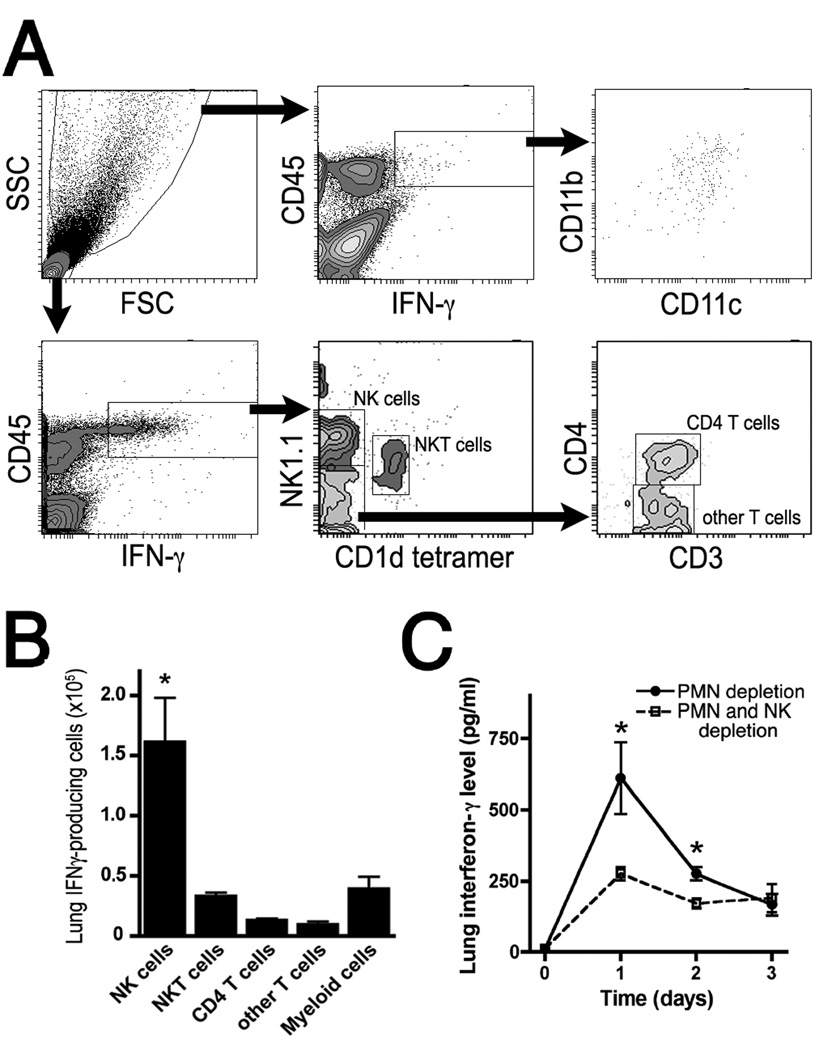
Given that early expression of IFN-γ in the lungs in response to Aspergillus has previously been documented (9, 10), we began by examining lungs of neutropenic mice with invasive aspergillosis for cells capable of IFN-γ production (Figures 1A–B). We found lung NK cells to be the largest population of IFN-γ-producing cells in the context of the infected lungs, with NKT cells, other T cell subsets, and myeloid cells making up much smaller numbers of IFN-γ-producing cells. We next examined the effect of depletion of NK cells on lung IFN-γ levels during the infection. In the context of neutropenia, depletion of NK cells with anti-NK1.1 mAb (clone pk136) resulted in reduction in lung levels of IFN-γ in the first 2 days of the infection (Figure 1C).
Figure 1.
Cellular source of lung IFN-γ in neutropenic mice with invasive aspergillosis. A–B: Gating scheme and summary data of flow cytometry of lung single cell suspensions on day 1 of infection. Data are representative of 4 mice per group. C: Lung IFN-γ protein level in lung homogenates at various time points after onset of infection as measured by ELISA. Both groups were neutrophil-depleted; NK cell depletion was achieved with anti-NK1.1. Day 0 represents uninfected animals. n=10–12 mice/group in 2 separate experiments. *, p<0.05 compared to other groups.
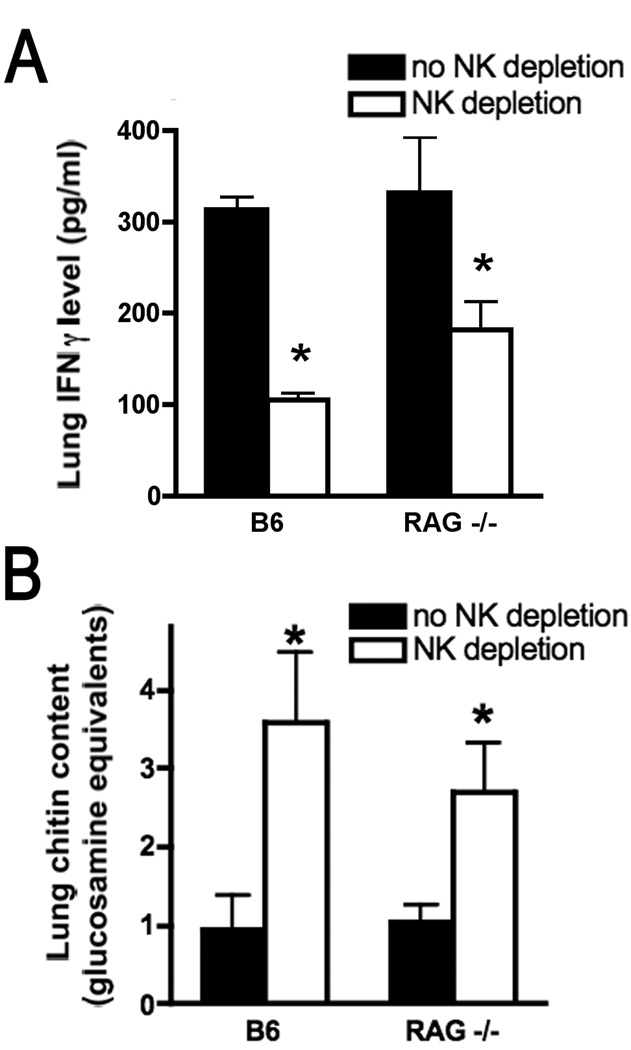
The NK1.1 antigen is also expressed, at a lower density, on a subset of NKT cells. We sought to ascertain that the observed effect of anti-NK1.1 was not related to an effect on lung NKT cells. Depletion of NK cells in wildtype mice with invasive aspergillosis using anti-asialo GM1 (which does not influence NKT cell populations) resulted in comparable reduction of lung IFN-γ levels to the effect achieved with anti-NK1.1 (Figure 2A). Similarly, depletion of classical NK cells in neutropenic RAG-deficient animals, which lack T cell subsets including NKT cells, also resulted in reduction in lung IFN-γ levels (Figure 2A). Interestingly, lung IFN-γ levels were markedly reduced but remained detectable in the lungs of RAG-deficient animals with NK cell depletion, suggesting a non-lymphocyte source for IFN-γ in this context. Depletion of NK cells resulted in a parallel increase in lung chitin content on day 3 of infection in both wildtype and RAG-1-deficient mice with neutropenia (Figure 2B), indicating that, during early infection, NK cells are necessary for host defense and their presence correlated with lung IFN-γ content. In addition, the lung fungal content did not differ significantly between neutropenic wildtype and RAG-1-deficient animals, suggesting that, at this early point in the infection, clearance of the microorganism is independent of acquired immunity.
Figure 2.
Effect of NK cell depletion on lung IFN-γ and fungal content in wildtype and RAG-1 deficient mice. Lung IFN-γ levels (panel A) and chitin content (panel B) were measured on days 1 and 3 of infection, respectively. All groups were neutrophil depleted; NK cell depletion was achieved with anti-asialo-GM1. n=6 mice/group; *, p<0.05 compared to mice of the same genetic background without NK depletion.
NK cell-derived IFN-γ exerts a protective role in invasive aspergillosis
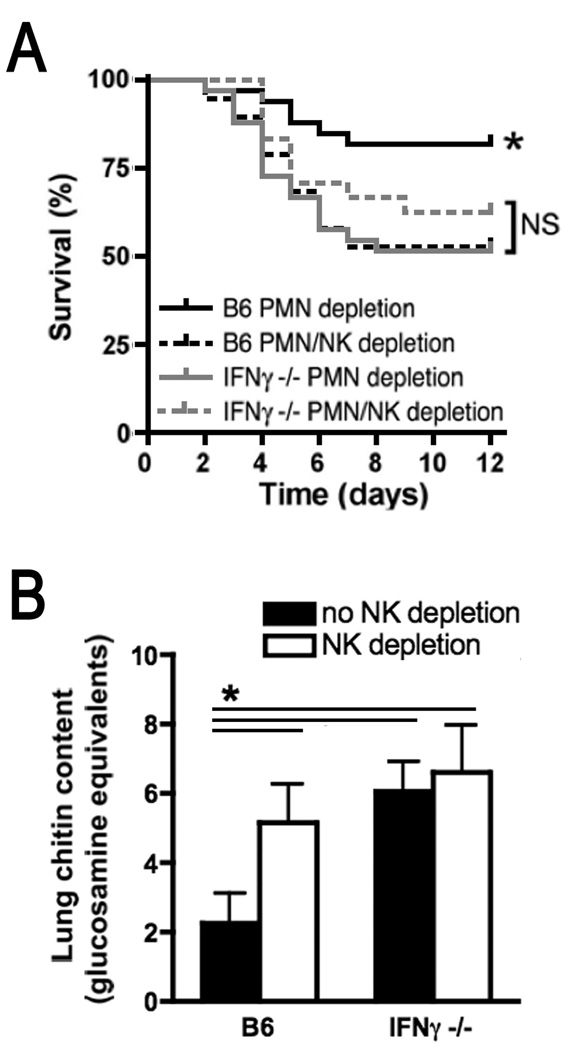
In order to specifically link NK cell-derived IFN-γ to host defense during invasive aspergillosis, we next compared the effect of NK cell depletion on the severity of infection in wildtype and IFN-γ-deficient mice. As expected, an LD20 inoculum of Aspergillus conidia for neutropenic wildtype animals resulted in higher mortality in wildtype mice with depletion of both neutrophils and NK cells. Mortality from the infection in wildtype mice with NK cell depetion was comparable to the increased mortality observed in neutropenic IFN-γ-deficient animals. Furthermore, depletion of NK cells in neutropenic IFN-γ-deficient mice did not result in further increase in mortality (Figure 3A). Similar results were found when lung fungal content of these groups were compared: On day 3 of infection, depletion of NK cells, absence of IFN-γ, or depletion of NK cells in IFN-γ-deficient hosts each resulted in a comparable 2-to 3-fold increase in lung fungal content as compared to wildtype neutropenic mice (Figure 3B). These data indicate that, in the setting of IFN-γ deficiency, NK cells do not contribute measurably to host defense against this infection, and that, in the absence of NK cells, generation of IFN-γ by other cellular sources does not measurably affect host responses in this model. These observations support the hypothesis that NK cell-derived IFN-γ mediates host defense in this infection.
Figure 3.
Role of NK cell-derived IFN-γ in outcome of neutropenic invasive aspergillosis. A: Survival study comparing neutropenic wildtype and IFN-γ-deficient mice with invasive aspergillosis with or without NK cell depletion after infection with an LD20 inoculum for neutropenic wildtype mice. n=24–35 mice/group, pooled from 3 experiments. B: Lung chitin content on day 3 on infection. n=14–18 mice/group, pooled from 3 experiments. *, p<0.05 compared to each of the other experimental groups.
Anti-microbial mechanisms of NK cell-derived IFN-γ
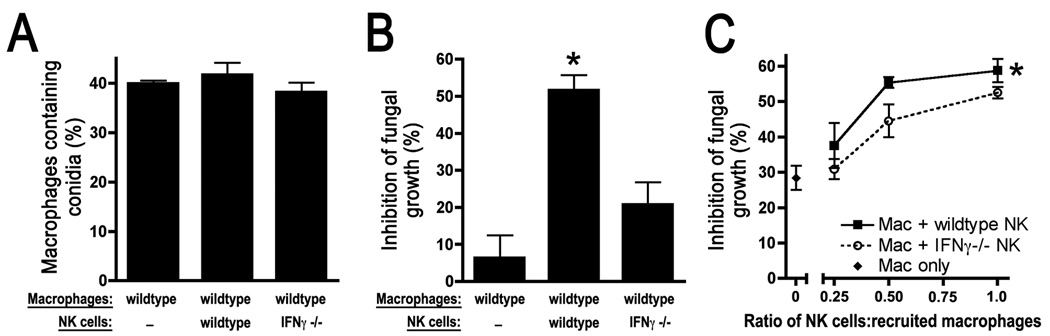
To assess the mechanism by which NK cell-derived IFN-γ mediates antimicrobial effects, we examined the in vitro interaction of NK cells, macrophages, and Aspergillus elements. Resident alveolar macrophages are critical to early defense of the lung against many micro-organisms, including Aspergillus (41–43). We therefore first examined the effect of NK cells on the efficiency of alveolar macrophage phagocytosis of conidia. Co-incubation of alveolar macrophages with either wildtype or IFN-γ-deficient NK cells was found to have little effect on the ability of alveolar macrophages to ingest conidia (Figure 4A). When incubation periods were extended to 16 hours to allow conidial germination and formation of hyphae, however, alveolar macrophages incubated with wildtype NK cells induced 2–8-fold greater fungal killing as compared to macrophages alone or macrophages incubated with IFN-γ-deficient NK cells (Figure 4B). These data indicate that, in the confines of this in vitro co-culture system, NK cell-derived IFN-γ results in inhibition of hyphal growth from resting conidia.
Figure 4.
Effect of NK cell-derived IFN-γ on macrophage antimicrobial functions in vitro. A–B: Effect of NK cells on alveolar macrophage phagocytosis and killing of Aspergillus elements. Macrophages with or without NK cells from various donors were co-cultured for 3 hours prior to addition of conidia. Phagocytosis was determined after 1 hour and fungal growth was measured after 16 hours. *, p<0.05 compared to each of the other experimental groups. C: Effect of NK cells on inflammatory macrophage killing of Aspergillus hyphae. Macrophages from wildtype mice and activated NK cells from various donors were incubated at the indicated ratios with pre-formed hyphae. *, p<0.05 compared to macrophages alone and macrophages incubated with IFN-γ-deficient NK cells.
In addition to resident alveolar macrophages, there is an influx of inflammatory monocyte/macrophages into the lungs during invasive aspergillosis. Based on our prior observations regarding the timing of influx of these cells to the lungs (26), we reasoned that recruited macrophages are likely to encounter pre-formed hyphae rather than conidia upon their arrival to the lungs. While neutrophils constitute the primary defense against the hyphal form of Aspergillus, monocytic phagocytes are capable of hyphal killing and constitute an important line of defense in neutropenic hosts (44, 45). Since the design of the experiments depicted in Figure 4B did not differentiate between activity against resting conidia, swollen conidia, or hyphae, we next examined the capacity of NK cell-derived IFN-γ to mediate fungicidal activity against pre-formed hyphae in the presence of inflammatory monocyte/macrophages. Incubation of increasing numbers of both wildtype and IFN-γ-deficient NK cells with inflammatory macrophages resulted in measurable increases in hyphal killing, but this effect was significantly greater with wildtype NK cells (Figure 4C). In the absence of macrophages, however, activated NK cells only induced detectable hyphal killing when the number of NK cells exceeded the number of fungal cells (data not shown), a circumstance that is likely not relevant to the in vivo setting of infection. Thus, these data indicate that NK cell-derived IFN-γ is required for optimal macrophage-mediated anti-fungal effects. In addition, IFN-γ-deficient NK cells were found to augment macrophage antifungal effects to a lesser degree; this effect did not result in improved fungal clearance in vivo (Figure 3).
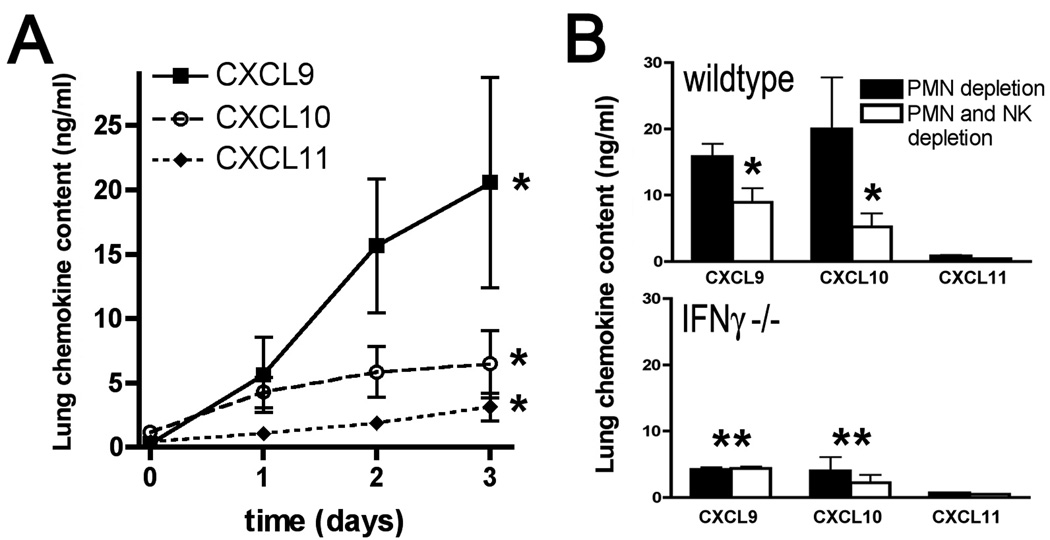
In addition to direct immunostimulation of phagocytes, we sought to determine if NK cell-derived IFN-γ could affect local expression of mediators during the infection. We therefore examined role of NK cells in mediating expression of interferon-inducible CXC chemokines, CXCL9, CXCL10 and CXCL11. There was a marked induction of CXCL9, CXCL10 and CXCL11 in the lungs during early invasive aspergillosis, with greater induction of CXCL9 and CXCL10 as compared to CXCL11 (Figure 5A). Depletion of NK cells in this context resulted in significant reduction in expression of CXCL9 and CXCL10 in wildtype mice, indicating that NK cells are necessary for optimal expression of these CXC chemokines in the lungs during invasive aspergillosis (Figure 5B). As expected, IFN-γ deficient mice had lower lung levels of CXCL9, CXCL10 and CXCL11 compared to wildtype animals; but lung levels of these chemokines in IFN-γ deficient mice was not substantially affected by the depletion of NK cells (Figure 5B). This indicates that the effect of NK cells on the lung expression of CXCL9 and CXCL10 is dependent on IFN-γ, which is consistent with the hypothesis that this effect is attributable to NK cell-derived IFN-γ. Interestingly, lung levels of CXCL9 and CXCL10 were somewhat higher in wildtype NK cell-depleted mice as compared to IFN-γ deficient mice with NK depletion; this effect may be attributable to non-NK cells sources of IFN-γ in lungs of wildtype mice, as noted in Figure 1. Taken together, Figures 4 and 5 suggest that NK cell-derived IFN-γ influences host defense during invasive aspergillosis by several mechanisms, including stimulation of phagocyte anti-fungal effects as well as induction of interferon-inducible CXC chemokines.
Figure 5.
Protein levels of CXCL9, CXCL10 and CXCL11 in lung homogenates in neutropenic mice with invasive aspergillosis. A: Lung chemokine levels at various time points after inoculation. Day 0 represents uninfected animals. Data represent mean ± SEM; n = 5–6 mice per time point; *, p<0.05 for increase over time for each chemokine. B: Lung chemokine levels in neutropenic C57BL/6 and IFN-γ gene knockout mice on C57BL/6 background with or without NK depletion on day 3 of infection. Data represent mean ± SEM; n = 5–6 mice per group; *, p<0.05 compared to no NK depletion; **, p<0.05 compared to wildtypes.
NK cell transfer mediates a protective effect in hosts with invasive aspergillosis
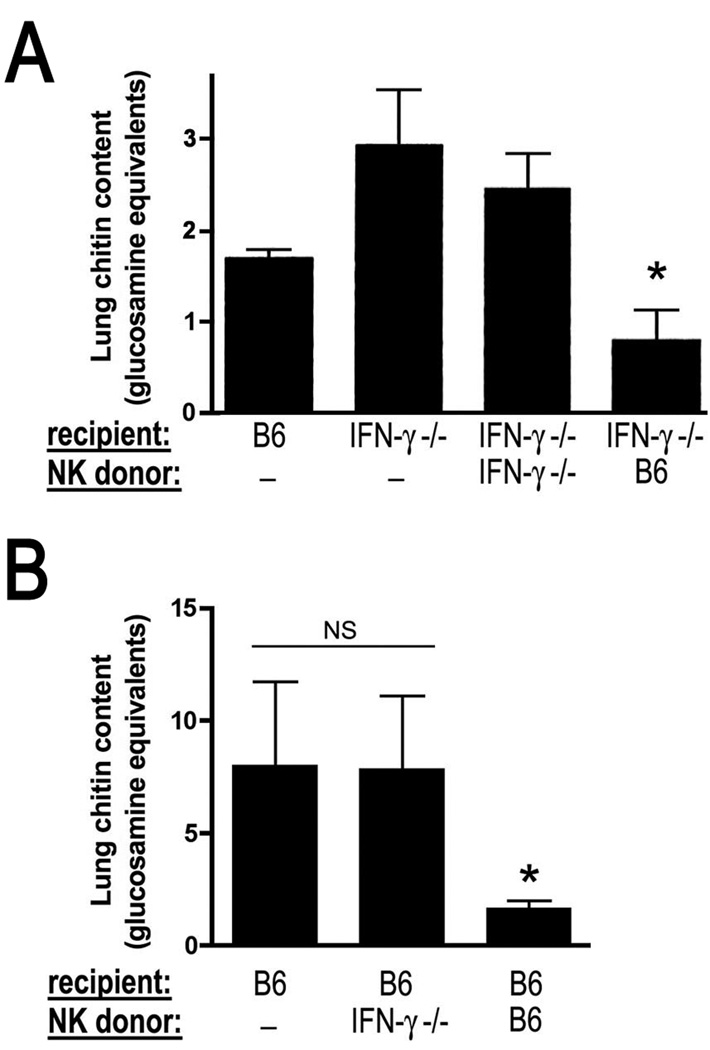
We next sought to determine whether the transfer of exogenous NK cells could improve the outcome of experimental invasive aspergillosis. The transfer of activated NK cells from wildtype, but not IFN-γ-deficient, donors reversed the susceptibility of IFN-γ-deficient recipients to invasive aspergillosis (Figure 6A). Interestingly, the transfer of these highly activated wildtype NK cells to IFN-γ-deficient hosts resulted in lower lung fungal content as compared to infected wildtype mice that did not receive cell transfer (Figure 6A). This finding led us to examine the utility of NK cell transfer as a therapeutic strategy in wildtype mice inoculated with lethal (LD100) infectious inocula. We found that the transfer of wildtype, but not IFN-γ-deficient, NK cells to neutropenic recipients resulted in lower lung fungal burden in wildtype hosts (Figure 7B). Taken together, these data indicate that NK cells are the main source of early IFN-γ in neutropenic invasive aspergillosis, and this mechanism is an important mechanism in the early defense against this infection.
Figure 6.
Effect of NK cell transfer on lung fungal clearance in neutropenic invasive aspergillosis. Lung chitin content on day 3 of invasive aspergillosis after infection with an LD20 (panel A) or LD100 (panel B) inocula for neutropenic mice after adoptive transfer of activated NK cells. Animals were given PBS, or activated NK cells from various donors on days 1 and 2 of infection. *, p<0.05 compared to each of the other groups. Data shown represent mean ± SEM of n=5–12 mice per group.
Discussion
The innate defenses against inhaled Aspergillus conidia include the physical barriers of the respiratory tract, recognition of the pathogen via cell-bound and soluble pattern-recognition receptors, and a complex array of cellular and soluble effector mechanisms. The cellular effector mechanisms have long been recognized to include myeloid cells, specifically neutrophils, recruited and resident monocyte/macrophages and dendritic cells; but less in known about the role of innate lymphocytes in this infection. NK cells have been strongly implicated in early defenses against many infections (46), including defense against several viral and bacterial pathogens that cause pneumonia (47–50). The role of NK cells in pneumonia is compatible with the large numbers of resident NK cells found in normal lungs, which is only exceeded by the number of NK cells found in the spleen (51–54). In addition, NK cells are capable of homing to inflamed tissues via several chemokine receptor-ligand interactions (51). We have previously found NK cells to be essential to early innate immunity in invasive aspergillosis in a neutropenic mouse model (13), but the mechanism of their contribution to host defense in this infection has not been defined.
NK cells were first described for their ability to mediate direct cytotoxicity against tumor cells, a finding that was subsequently extended to virally-infected cells and transplanted cells. In addition to this cytotoxic effector function, NK cells contribute to innate immunity and to the development of Th-1 acquired immunity by mediating a potent regulatory effect via elaboration of several key cytokines, notably TNF-α and IFN-γ. In this context, the importance of Th1 immunity to defense against Aspergillus infection was initially noted when susceptibility of different inbred mouse strains to aspergillosis was found to correlate with their production of Th-1 cytokines (6, 7). In mouse models that use cytotoxic drugs to mimic human immunosuppression, IFN-γ knockout animals are more susceptible to invasive aspergillosis (8). In contrast, vaccination with Aspergillus proteins with Th-1-priming adjuvant (55), exogenous administration of IFN-γ (56, 57), or administration of Th-1 T cells (58) or antigen-pulsed dendritic cells (59) result in improved outcome of experimental infection. In contrast, Th2-mediated immunity to Aspergillus results in a lesser degree of protection from infection as compared to a Th1 response (6, 8) and early evidence suggests that Th17 responses may also be less effective than the Th1 responses (60). Importantly, while the cellular source of IFN-γ in late infection and in immunized mice is recognized as Aspergillus-specific clones of CD4 T cells (6, 11, 12), the cellular source of early IFN-γ in invasive aspergillosis is unknown. In the context of early infection, IFN-γ, IL12, and IL18 are induced in the lung of immunocompetent animals in the first 48 hours after microbial challenge (7, 9). Similarly, human mononuclear cells express Th1 cytokines when exposed to Aspergillus conidia in vitro (61–64).
IFN-γ mediates diverse and complex effects in the context of antimicrobial immunity, including activation of oxidative and non-oxidative microbicidal mechanisms in phagocytes, enhancement of antigen presentation, and reciprocal induction of IL12 leading to the development of Th-1 acquired immunity (65). In addition, IFN-γ mediates influx of effector leukocytes to sites of infection via induction of adhesion molecules and expression of several local mediators. Notable among these are the IFN-inducible CXC chemokines, CXCL9, CXCL10 and CXCL11, which mediate the influx of Th-1 effector cells via their interaction with their common receptor CXCR3 (66, 67). These ligands are potently induced by both type-1 and type-2 interferons in diverse biological settings and their induction is further potentiated by exposure to TNF (68–70). We found that NK cell-derived IFN-γ both augments the anti-microbial effects of macrophages against Aspergillus. NK cell-derived IFN-γ also had a marked effect on lung expression of CXCL9 and CXCL10; we speculate that the induction of these chemokines is further promoted by high levels of lung TNF in this infection (71). Interestingly, these chemokine ligands are capable of inducing the influx of activated NK cells and Th-1 CD4 T cells (72, 73), and may therefore be a component of a beneficial positive feedback cycle in this infection.
In summary, our data provide evidence for a protective role of NK cell-derived IFN-γ in the early phase of infection in neutropenic hosts with invasive aspergillosis. This mechanism appears to boost the antimicrobial responses of resident and recruited myeloid cells against the pathogen and augment the expression of IFN-inducible chemokines that may, in turn, mediate further recruitment of effector leukocytes. The recognition of NK cells as the early cellular source of IFN-γ is likely to be relevant to human invasive aspergillosis for several reasons. First, human invasive aspergillosis typically occurs in patients with impaired T cell responses and can follow a rapid course (74, 75); cellular sources of IFN-γ other than clonally-expanded CD4 T cells may therefore be particularly relevant in host defense in this setting. Second, NK cell-derived IFN-γ during the effector phase of innate immunity has been shown to promote the development of Th-1 immunity by priming CD4 T helper cells in other experimental systems (76, 77); this effect may be relevant in the development of protective immunity in invasive aspergillosis. Finally, transfusion of cultured autologous NK cells has been utilized therapeutically in human clinical trials for the treatment of several cancers, in some of which the transfer of NK cells resulted in enhanced expression of IFN-γ in the plasma (78). Administration of exogenous IFN-γ has been used successfully as adjunctive therapy in human invasive aspergillosis in case-reports (79–81); therapeutic infusion of NK cells has the potential to allow for targeting the IFN-γ response to the tissue micro-niche of infection.
Acknowledgments
Supported by NIH grant HL73848 and an American Lung Association Career Investigator Award (Mehrad).
References
- 1.Patterson TF. Advances and challenges in management of invasive mycoses. Lancet. 2005;366:1013–1025. doi: 10.1016/S0140-6736(05)67381-3. [DOI] [PubMed] [Google Scholar]
- 2.Phadke AP, Mehrad B. Cytokines in host defense against Aspergillus: recent advances. Med Mycol. 2005;43 Suppl 1:S173–S176. doi: 10.1080/13693780500052099. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21:161–172. doi: 10.1007/s10096-002-0699-z. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 6.Cenci E, Perito S, Enssle KH, Mosci P, Latge JP, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci E, Mencacci A, Fe d'Ostiani C, Del Sero G, Mosci P, Montagnoli C, Bacci A, Romani L. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J Infect Dis. 1998;178:1750–1760. doi: 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 8.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, d'Ostiani CF, Mosci P, Bachmann M, Bistoni F, Kopf M, Romani L. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180:1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 9.Brieland JK, Jackson C, Menzel F, Loebenberg D, Cacciapuoti A, Halpern J, Hurst S, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O'Garra A. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect Immun. 2001;69:1554–1560. doi: 10.1128/IAI.69.3.1554-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrad B, Wiekowski M, Morrison BE, Chen SC, Coronel EC, Manfra DJ, Lira SA. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am J Respir Crit Care Med. 2002;166:1263–1268. doi: 10.1164/rccm.200204-367OC. [DOI] [PubMed] [Google Scholar]
- 11.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant'Angelo DB, Pamer EG. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665–675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Rivera A, Van Epps HL, Hohl TM, Rizzuto G, Pamer EG. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect Immun. 2005;73:7170–7179. doi: 10.1128/IAI.73.11.7170-7179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–1870. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biron CA, Welsh RM. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129:2788–2795. [PubMed] [Google Scholar]
- 15.Lucin P, Jonjic S, Messerle M, Polic B, Hengel H, Koszinowski UH. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 16.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 20.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami K, Koguchi Y, Qureshi MH, Yara S, Kinjo Y, Uezu K, Saito A. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol Immunol. 2000;44:1043–1050. doi: 10.1111/j.1348-0421.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 23.Salkowski CA, Balish E. Role of natural killer cells in resistance to systemic cryptococcosis. J Leukoc Biol. 1991;50:151–159. doi: 10.1002/jlb.50.2.151. [DOI] [PubMed] [Google Scholar]
- 24.Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol. 1999;163:6086–6094. [PubMed] [Google Scholar]
- 25.Mehrad B, Moore TA, Standiford TJ. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J Immunol. 2000;165:962–968. doi: 10.4049/jimmunol.165.2.962. [DOI] [PubMed] [Google Scholar]
- 26.Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med. 2007;175:1165–1172. doi: 10.1164/rccm.200602-256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SJ, Wiekowski MT, Lira SA, Mehrad B. Neutrophils regulate airway responses in a model of fungal allergic airways disease. J Immunol. 2006;176:2538–2545. doi: 10.4049/jimmunol.176.4.2538. [DOI] [PubMed] [Google Scholar]
- 28.Mehrad B, Park SJ, Akangire G, Standiford TJ, Wu T, Zhu J, Mohan C. The lupus-susceptibility locus, Sle3, mediates enhanced resistance to bacterial infections. J Immunol. 2006;176:3233–3239. doi: 10.4049/jimmunol.176.5.3233. [DOI] [PubMed] [Google Scholar]
- 29.Morris MA, Liu J, Arora V, George TC, Klem J, Schatzle JD, Kumar V, Bennett M. B6 strain Ly49I inhibitory gene expression on T cells in FVB.Ly49IB6 transgenic mice fails to prevent normal T cell functions. J Immunol. 2002;169:3661–3666. doi: 10.4049/jimmunol.169.7.3661. [DOI] [PubMed] [Google Scholar]
- 30.Taylor MA, Chaudhary PM, Klem J, Kumar V, Schatzle JD, Bennett M. Inhibition of the death receptor pathway by cFLIP confers partial engraftment of MHC class I-deficient stem cells and reduces tumor clearance in perforin-deficient mice. J Immunol. 2001;167:4230–4237. doi: 10.4049/jimmunol.167.8.4230. [DOI] [PubMed] [Google Scholar]
- 31.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–1853. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 32.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 33.Ortaldo JR, Young HA. Expression of IFN-gamma upon triggering of activating Ly49D NK receptors in vitro and in vivo: costimulation with IL-12 or IL-18 overrides inhibitory receptors. J Immunol. 2003;170:1763–1769. doi: 10.4049/jimmunol.170.4.1763. [DOI] [PubMed] [Google Scholar]
- 34.Tomura M, Zhou XY, Maruo S, Ahn HJ, Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M, Fujiwara H. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3- cells. J Immunol. 1998;160:4738–4746. [PubMed] [Google Scholar]
- 35.Standiford TJ, Wilkowski JM, Sisson TH, Hattori N, Mehrad B, Bucknell KA, Moore TA. Intrapulmonary tumor necrosis factor gene therapy increases bacterial clearance and survival in murine gram-negative pneumonia. Hum Gene Ther. 1999;10:899–909. doi: 10.1089/10430349950018300. [DOI] [PubMed] [Google Scholar]
- 36.Fortier AH, Falk LA. Isolation of Murine Macrophages. In: Coico R, editor. Current Protocols in Immunology. John Wiley & Sons; 2007. 14.11.11-19. [DOI] [PubMed] [Google Scholar]
- 37.Levitz SM, Diamond RD. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 38.Ahlin A, Elinder G, Palmblad J. Dose-dependent enhancements by interferon-gamma on functional responses of neutrophils from chronic granulomatous disease patients. Blood. 1997;89:3396–3401. [PubMed] [Google Scholar]
- 39.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)- tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 41.Cornish EJ, Hurtgen BJ, McInnerney K, Burritt NL, Taylor RM, Jarvis JN, Wang SY, Burritt JB. Reduced nicotinamide adenine dinucleotide phosphate oxidase-independent resistance to Aspergillus fumigatus in alveolar macrophages. J Immunol. 2008;180:6854–6867. doi: 10.4049/jimmunol.180.10.6854. [DOI] [PubMed] [Google Scholar]
- 42.Dubourdeau M, Athman R, Balloy V, Huerre M, Chignard M, Philpott DJ, Latge JP, Ibrahim-Granet O. Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J Immunol. 2006;177:3994–4001. doi: 10.4049/jimmunol.177.6.3994. [DOI] [PubMed] [Google Scholar]
- 43.Philippe B, Ibrahim-Granet O, Prevost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latge JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roilides E, Sein T, Holmes A, Chanock S, Blake C, Pizzo PA, Walsh TJ. Effects of macrophage colony-stimulating factor on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Infect Dis. 1995;172:1028–1034. doi: 10.1093/infdis/172.4.1028. [DOI] [PubMed] [Google Scholar]
- 45.Simitsopoulou M, Roilides E, Paliogianni F, Likartsis C, Ioannidis J, Kanellou K, Walsh TJ. Immunomodulatory effects of voriconazole on monocytes challenged with Aspergillus fumigatus: differential role of Toll-like receptors. Antimicrob Agents Chemother. 2008;52:3301–3306. doi: 10.1128/AAC.01018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyazaki S, Ishikawa F, Shimizu K, Ubagai T, Edelstein PH, Yamaguchi K. Gr-1high polymorphonuclear leukocytes and NK cells act via IL-15 to clear intracellular Haemophilus influenzae in experimental murine peritonitis and pneumonia. J Immunol. 2007;179:5407–5414. doi: 10.4049/jimmunol.179.8.5407. [DOI] [PubMed] [Google Scholar]
- 48.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 49.Reading PC, Whitney PG, Barr DP, Wojtasiak M, Mintern JD, Waithman J, Brooks AG. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol. 2007;179:3214–3221. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- 50.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol. 2008;180:5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 51.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein-Streilein J, Bennett M, Mann D, Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983;131:2699–2704. [PubMed] [Google Scholar]
- 53.Reynolds CW, Timonen T, Herberman RB. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981;127:282–287. [PubMed] [Google Scholar]
- 54.Weissler JC, Nicod LP, Lipscomb MF, Toews GB. Natural killer cell function in human lung is compartmentalized. Am Rev Respir Dis. 1987;135:941–949. doi: 10.1164/arrd.1987.135.4.941. [DOI] [PubMed] [Google Scholar]
- 55.Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- 56.Nagai H, Guo J, Choi H, Kurup V. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J Infect Dis. 1995;172:1554–1560. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 57.Shao C, Qu J, He L, Zhang Y, Wang J, Wang Y, Zhou H, Liu X. Transient overexpression of gamma interferon promotes Aspergillus clearance in invasive pulmonary aspergillosis. Clin Exp Immunol. 2005;142:233–241. doi: 10.1111/j.1365-2249.2005.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol. 2000;165:381–388. doi: 10.4049/jimmunol.165.1.381. [DOI] [PubMed] [Google Scholar]
- 59.Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, Burchielli E, Nkwanyuo G, Pitzurra L, Velardi A, Romani L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–3814. doi: 10.1182/blood-2003-03-0748. Epub 2003 Jun 3805. [DOI] [PubMed] [Google Scholar]
- 60.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 61.Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, Lehrnbecher T. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–2569. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 62.Grazziutti ML, Rex JH, Cowart RE, Anaissie EJ, Ford A, Savary CA. Aspergillus fumigatus conidia induce a Th1-type cytokine response. J Infect Dis. 1997;176:1579–1583. doi: 10.1086/514157. [DOI] [PubMed] [Google Scholar]
- 63.Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latge JP, Einsele H. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–4528. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 64.Warris A, Netea MG, Verweij PE, Gaustad P, Kullberg BJ, Weemaes CM, Abrahamsen TG. Cytokine responses and regulation of interferon-gamma release by human mononuclear cells to Aspergillus fumigatus and other filamentous fungi. Med Mycol. 2005;43:613–621. doi: 10.1080/13693780500088333. [DOI] [PubMed] [Google Scholar]
- 65.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 66.Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97:755–762. [PMC free article] [PubMed] [Google Scholar]
- 67.Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176:1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 68.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol. 1998;161:4736–4744. [PubMed] [Google Scholar]
- 69.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677–6688. [PubMed] [Google Scholar]
- 70.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 71.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162:1633–1640. [PubMed] [Google Scholar]
- 72.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 73.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 75.Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006;173:707–717. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- 76.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 77.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36:2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 78.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 79.Bernhisel-Broadbent J, Camargo EE, Jaffe HS, Lederman HM. Recombinant human interferon-gamma as adjunct therapy for Aspergillus infection in a patient with chronic granulomatous disease. J Infect Dis. 1991;163:908–911. doi: 10.1093/infdis/163.4.908. [DOI] [PubMed] [Google Scholar]
- 80.Kelleher P, Goodsall A, Mulgirigama A, Kunst H, Henderson DC, Wilson R, Newman-Taylor A, Levin Mn. Interferon-gamma therapy in two patients with progressive chronic pulmonary aspergillosis. Eur Respir J. 2006;27:1307–1310. doi: 10.1183/09031936.06.00021705. [DOI] [PubMed] [Google Scholar]
- 81.Saulsbury FT. Successful treatment of aspergillus brain abscess with itraconazole and interferon-gamma in a patient with chronic granulomatous disease. Clin Infect Dis. 2001;32:E137–E139. doi: 10.1086/320158. [DOI] [PubMed] [Google Scholar]