Abstract
An extracellular elastinolytic metalloproteinase, purified from Aspergillus fumigatus isolated from an aspergillosis and patient/and an internal peptide derived from it were subjected to N-terminal sequencing. Oligonucleotide primers based on these sequences were used to PCR amplify a segment of the metalloproteinase cDNA, which was used as a probe to isolate the cDNA and gene for this enzyme. The gene sequence matched exactly with the cDNA sequence except for the four introns that interrupted the open reading frame. According to the deduced amino acid sequence, the metalloproteinase has a signal sequence and 227 additional amino acids preceding the sequence for the mature protein of 389 amino acids with a calculated molecular mass of 42 kDa, which is close to the size of the purified mature fungal proteinase. This sequence contains segments that matched both the N terminus of the mature protein and the internal peptide. A. fumigatus metalloproteinase contains some of the conserved zinc-binding and active-site motifs characteristic of metalloproteinases but shows no overall homology with known metalloproteinases. The cDNA of the mature protein when introduced into Escherichia coli directed the expression of a protein with a size, N-terminal sequence, and immunological cross-reactivity identical to those of the native fungal enzyme. Although the enzyme in the inclusion bodies could not be renatured, expression at 30 degrees C yielded soluble enzyme that showed chromatographic behavior identical to that of the native fungal enzyme and catalyzed hydrolysis of elastin. The metalloproteinase gene described here was not found in Aspergillus flavus.
Full text
PDF





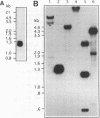
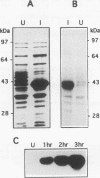
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D., Silen J. L., Agard D. A. Protease pro region required for folding is a potent inhibitor of the mature enzyme. Proteins. 1992 Apr;12(4):339–344. doi: 10.1002/prot.340120406. [DOI] [PubMed] [Google Scholar]
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Quinn F. D., Tompkins L. S. Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase. J Bacteriol. 1990 May;172(5):2608–2613. doi: 10.1128/jb.172.5.2608-2613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Chang P. C., Lee Y. H. The roles of propeptide in maturation and secretion of Npr protease from Streptomyces. J Biol Chem. 1994 Feb 4;269(5):3548–3554. [PubMed] [Google Scholar]
- David V. A., Deutch A. H., Sloma A., Pawlyk D., Ally A., Durham D. R. Cloning, sequencing and expression of the gene encoding the extracellular neutral protease, vibriolysin, of Vibrio proteolyticus. Gene. 1992 Mar 1;112(1):107–112. doi: 10.1016/0378-1119(92)90310-l. [DOI] [PubMed] [Google Scholar]
- Denning D. W., Stevens D. A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990 Nov-Dec;12(6):1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993 Dec;57(4):823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaton-Ogay K., Suter M., Crameri R., Falchetto R., Fatih A., Monod M. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992 Apr 15;71(2):163–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- Jiang W., Bond J. S. Families of metalloendopeptidases and their relationships. FEBS Lett. 1992 Nov 9;312(2-3):110–114. doi: 10.1016/0014-5793(92)80916-5. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Lee J. D., Rogers L. M., Zimmerman P., Ceselski S., Fox B., Stein B., Copelan E. A. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993 Jun;61(6):2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Chase T., Jr, Macmillan J. D. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun. 1984 Jan;43(1):320–325. doi: 10.1128/iai.43.1.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Imanaka T. Cloning and nucleotide sequence of the highly thermostable neutral protease gene from Bacillus stearothermophilus. J Gen Microbiol. 1988 Jul;134(7):1883–1892. doi: 10.1099/00221287-134-7-1883. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kämper J. T., Kämper U., Rogers L. M., Kolattukudy P. E. Identification of regulatory elements in the cutinase promoter from Fusarium solani f. sp. pisi (Nectria haematococca). J Biol Chem. 1994 Mar 25;269(12):9195–9204. [PubMed] [Google Scholar]
- Lövgren A., Zhang M., Engström A., Dalhammar G., Landén R. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol Microbiol. 1990 Dec;4(12):2137–2146. doi: 10.1111/j.1365-2958.1990.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Mach L., Mort J. S., Glössl J. Maturation of human procathepsin B. Proenzyme activation and proteolytic processing of the precursor to the mature proteinase, in vitro, are primarily unimolecular processes. J Biol Chem. 1994 Apr 29;269(17):13030–13035. [PubMed] [Google Scholar]
- Markaryan A., Morozova I., Yu H., Kolattukudy P. E. Purification and characterization of an elastinolytic metalloprotease from Aspergillus fumigatus and immunoelectron microscopic evidence of secretion of this enzyme by the fungus invading the murine lung. Infect Immun. 1994 Jun;62(6):2149–2157. doi: 10.1128/iai.62.6.2149-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D. Fungal infections in bone marrow transplant patients. Semin Oncol. 1990 Jun;17(3 Suppl 6):10–13. [PubMed] [Google Scholar]
- Monod M., Paris S., Sanglard D., Jaton-Ogay K., Bille J., Latgé J. P. Isolation and characterization of a secreted metalloprotease of Aspergillus fumigatus. Infect Immun. 1993 Oct;61(10):4099–4104. doi: 10.1128/iai.61.10.4099-4104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M., Paris S., Sarfati J., Jaton-Ogay K., Ave P., Latgé J. P. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol Lett. 1993 Jan 1;106(1):39–46. doi: 10.1111/j.1574-6968.1993.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Monod M., Togni G., Rahalison L., Frenk E. Isolation and characterisation of an extracellular alkaline protease of Aspergillus fumigatus. J Med Microbiol. 1991 Jul;35(1):23–28. doi: 10.1099/00222615-35-1-23. [DOI] [PubMed] [Google Scholar]
- Ramesh M. V., Sirakova T., Kolattukudy P. E. Isolation, characterization, and cloning of cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect Immun. 1994 Jan;62(1):79–85. doi: 10.1128/iai.62.1.79-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard U., Büttner S., Eiffert H., Staib F., Rüchel R. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J Med Microbiol. 1990 Dec;33(4):243–251. doi: 10.1099/00222615-33-4-243. [DOI] [PubMed] [Google Scholar]
- Rhodes J. C., Amlung T. W., Miller M. S. Isolation and characterization of an elastinolytic proteinase from Aspergillus flavus. Infect Immun. 1990 Aug;58(8):2529–2534. doi: 10.1128/iai.58.8.2529-2534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Tarrand J. J., Eyerman M. C., Nahm M. H. A simple in situ cyanogen bromide cleavage method to obtain internal amino acid sequence of proteins electroblotted to polyvinyldifluoride membranes. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1353–1359. doi: 10.1016/s0006-291x(88)81290-7. [DOI] [PubMed] [Google Scholar]
- Shinde U., Li Y., Chatterjee S., Inouye M. Folding pathway mediated by an intramolecular chaperone. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6924–6928. doi: 10.1073/pnas.90.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcher B. C. Elastin and the lung. Thorax. 1986 Aug;41(8):577–585. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Imanaka T., Aiba S. Nucleotide sequence and promoter region for the neutral protease gene from Bacillus stearothermophilus. J Bacteriol. 1985 Sep;163(3):824–831. doi: 10.1128/jb.163.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa S., Uozumi N., Tsukagoshi N., Udaka S. Proteases involved in generation of beta- and alpha-amylases from a large amylase precursor in Bacillus polymyxa. J Bacteriol. 1991 Nov;173(21):6820–6825. doi: 10.1128/jb.173.21.6820-6825.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Cohen J., Krausz T., Van Noorden S., Holden D. W. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect Immun. 1993 May;61(5):1650–1656. doi: 10.1128/iai.61.5.1650-1656.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Murakami S., Tsuji R. F., Ishida Y., Murakami K., Masaki A., Kawabe H., Arimura H., Nakano E., Motai H. Cloning and expression in yeast of a cDNA clone encoding Aspergillus oryzae neutral protease II, a unique metalloprotease. Mol Gen Genet. 1991 Aug;228(1-2):97–103. doi: 10.1007/BF00282453. [DOI] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J., Gadek J. E. Alveolar fluid neutrophil elastase activity in the adult respiratory distress syndrome is complexed to alpha-2-macroglobulin. J Clin Invest. 1988 Oct;82(4):1260–1267. doi: 10.1172/JCI113724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]