Abstract
Regeneration of the liver is inhibited as a result of a sustained increase in S-adenosylmethionine levels in glycine N-methyltransferase (GNMT)−/− mice. This sets the stage for normally dormant stem cells/progenitor cells to replicate and differentiate to replenish the liver parenchyma with liver cells. With time the stem cells/progenitor cells may aggregate and ultimately form liver tumors. This transformation of stem cells persists within the tumors that form in order to maintain the growth of the tumors that have formed. To test this hypothesis, GNMT−/− mice were maintained for 18 months and their livers were studied at intervals, in order to document the process of tumors formation and the identification of stem cells/progenitor cells involved in the process. Progenitor cell (OV-6 positive cells) hyperplasia was already established at 8 months in the livers of the GNMT−/− mice. This process was expanded at 18 months when liver tumors had formed. Stem cells which stained positive in the livers at 8 months and within tumors at 18 months (Oct 4 and CK 19 positive cells) were found. Fat 10, a marker for progenitor liver cells, was uniformly expressed by all tumors that developed at 8 and 18 months in GNMT−/− mice.
Keywords: Progenitor, Stem cells, S-adenosylmethionine, Betaine, Epigenetics
Introduction
Glycine N-methyl transferase (GNMT) is considered a tumor suppressor gene because GNMT knockout (KO) mice develop liver hepatocellular carcinomas (Martinez-Chater et al., 2008; Lia 2009) as a result of sustained increased S-adenosylmethionine (SAMe) in their livers (Martinez-Chater et al., 2008). SAMe inhibits liver proliferation and regeneration by decreasing DNA synthesis in the liver (Varela-Ray et al., 2009). Liver regeneration is impaired in GNMT KO mice because increased SAMe levels are sustained, which inhibits liver cell proliferation (Varela-Ray et al., 2009). Increased SAMe levels lead to an increase in methylation of DNA and histones (Martinez-Chater et al., 2008). The net effect is on epigenetic modulation of gene expression, which promotes carcinogenesis. For instance, methylation of RASSF1 and SOCS2 promoters and binding of H3K27me3 to these two genes are present in the GNMT−/− mouse liver tumor (Martinez-Chater et al., 2008). The increase in SAMe content in the liver of GNMT−/− mice, therefore, sets the stage for hepatocellular carcinoma (HCC) development where liver regeneration is inhibited. Stem cell/progenitor activation then develops to compensate for the lack of regeneration capacity in response to liver damage caused by the lack of GNMT activity. The concept of stem cell/progenitor activation and liver cell HCC formation, when regeneration is suppressed, is well established (Lacconi, 2000). The concept of preneoplastic change which progresses to cancer is referred to as clonal adaptation during carcinogenesis (Farber, 1990) where progenitor cells which are resistant to liver injury selectively proliferate to form preneoplastic nodules in phenotypically altered adaptive response to liver (Lacconi, 2000; Farber, 1990; Duncan et al., 2009; Alison et al., 2009; Yoo and Mishra, 2009). These progenitor cells are known as oval cells, which have the potential to differentiate into bile ducts or liver cells and to transform into cancer stem cells. They are identified by an antibody to OV6.
Materials and methods
In the present study, stem cells/progenitor cells were identified in preneoplastic proliferation of oval cells. Cancer stem cells were identified in liver tumors by using immunofluorescent staining. Antibodies to stem cell/progenitor cell markers (SCPs) were used and fluorescent tagged secondary antibodies detected the primary antibodies to the stem cell/progenitor cell markers.
Blocks of liver tissue from GNMT−/− mice and wild type controls were fixed and embedded in paraffin. Sections from these livers were stained with hematoxylin and eosin (H&E) and examined. Immunostaining for stem cells/progenitor cells (SCPs) was performed. The mouse livers examined are listed in Table 1.
Table 1.
Mice livers examined.
| Controls | # | Tumors | GNMT−/− | # | Tumors |
|---|---|---|---|---|---|
| 8 months | 6 | 0 | 8 months | 2 | 2 |
| 10 months | 0 | 0 | 10 months | 3 | 3 |
| 12 months | 0 | 0 | 12 months | 3 | 3 |
| 18 months | 5 | 0 | 18 months | 2 | 2 |
| 11 | 0 | 10 | 10 |
The mice were fed a standard diet (Harlan Teklad irradiated mouse diet 2014, Madison, WI) and housed in a temperature-controlled animal facility with 12 h light/dark cycles. The mice were treated according to the Spanish Guide for the care and use of laboratory animals and the protocols were approved by the CIC bioGUNE Ethical Review Committee.
Immunohistochemistry
The liver sections were stained with antibodies to UbD (FAT10) (Biomol International, Plymouth Meeting, PA) ubiquitin (Chemicon, Temecula, CA), OCT 4 (Abcam, Cambridge M), CK19 (Abcam, Cambridge, MA), OV6 (R&D Systems, Inc., Minneapolis, MN), Nanog (Abcam, Cambridge, MA), GSTP (Santa Cruz, CA) in order to detect stem cells/progenitor cells (SCPs).
Results
Control mouse livers at 8 and 18 months appeared to be normal by H&E staining. (Figs. 1A and B) and were negative for SCP markers at 8 and 18 months (Fig. 2). GNMT−/− mice showed oval cell hyperplasia and early tumor formation separated by oval cell proliferation at 8 months (Figs. 1C and D). The oval cell proliferation expanded between liver tumor nodules, which were formed at 18 months (Figs. 1E and F).
Fig. 1.
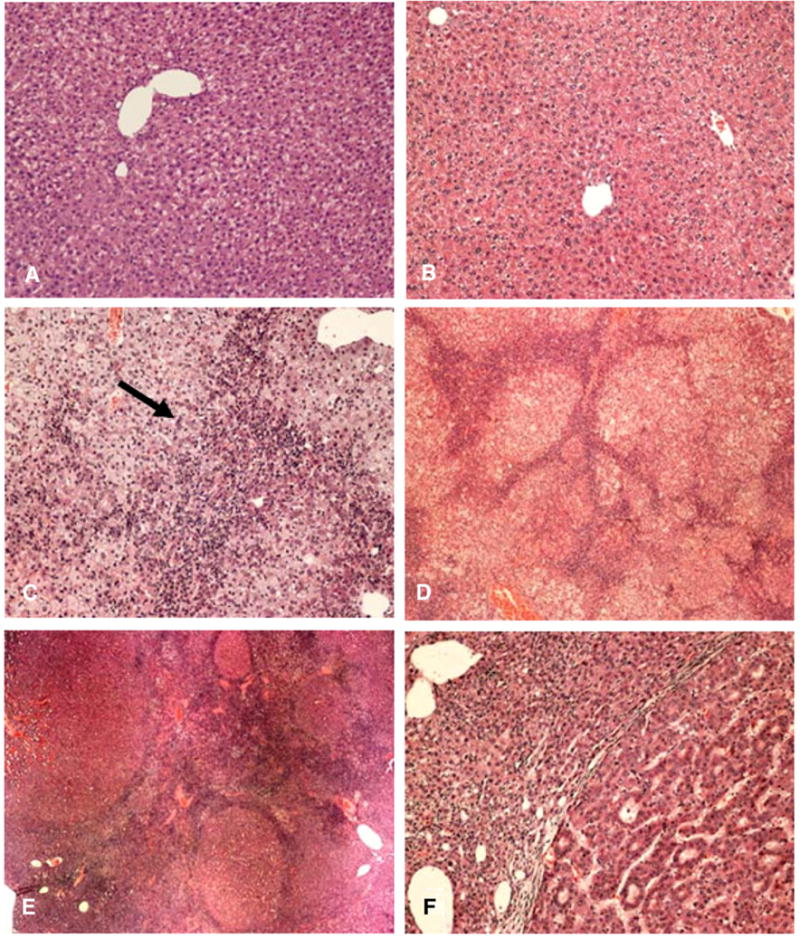
A) GNMT wild type mouse liver, 8 months control H&E stain ×130; B) GNMT wild type mouse 18 months control liver H&E stain ×130; C) GNMT−/− mouse liver at 8 months, showing extension of oval cells from the portal tract into the liver lobule (arrow) H&E ×130, D) GNMT−/− mouse liver at 8 months, showing multiple small tumor nodules separated by oval cell proliferation H&E ×52, E) GNMT−/− mouse liver at 18 months showing tumor nodules separated by proliferating oval cells H&E ×26. F) GNMT−/− mouse liver at 18 months showing a hepatocellular carcinoma on the right and small cell dysplasia on the left.
Fig. 2.
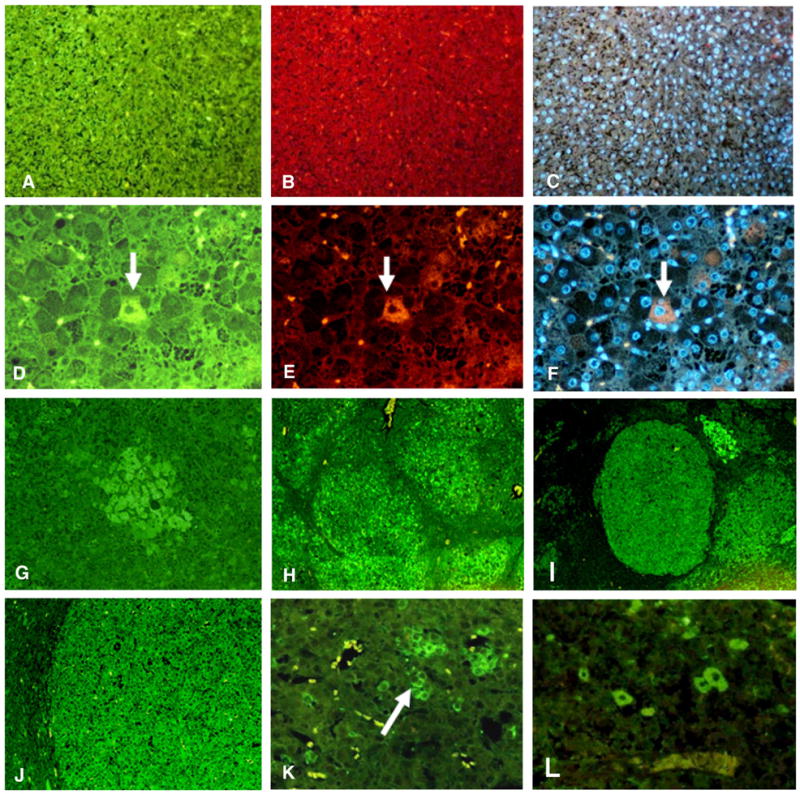
A–C) GNMT wild type mouse at 8 months control liver double stained with antibodies to Oct 4 (green) and Nanog (red) or DAPI (blue). There are no stem cell/progenitor cells ×218. D–F) GNMT−/− mouse liver at 8 months double stained with antibodies to Oct 4 (green), CK-19 (red) and DAPI. Note a single liver cell stains positive for both Oct 4 and CK-19 (arrow). This cell shows colocalization of both antibodies when viewed with the tricolor filter (arrow) ×436. G–J) GNMT−/− mouse livers stained with an antibody to FAT10. G) FAT10 stain shows an early phase of liver tumor formation at 18 months ×109; H) FAT10 stain shows multiple tumors at 8 months liver of FAT10 positive cells that formed ×44. I) FAT10 stain shows multiple FAT10 positive tumors formed at 18 months ×44; J) Higher power of a FAT10 positive tumor at 18 months ×109. K) GNMT−/− mouse liver stained with an antibody to OV6. Note the cluster of OV6 positive cells (arrow) ×436; L) GNMT−/− mouse liver at 18 months. A tumor was stained with an antibody to Oct 4. Note several tumor cells stained positive for Oct 4.
Scattered SPCs were identified among liver cells at 8 months (Figs. 2D, E, and F). Small groups of cells (Fig. G) and larger tumors (Figs. 2H–J) stained positive for FAT10. Oval cells stained positive focally for OV6 (Fig. 2K). Scattered tumor stem cells stained positive for Oct 4, shown in Fig. 2L.
Discussion
The GNMT−/− mice developed oval cell proliferation and liver tumors associated with liver cell expression of stem cell/progenitor cell (SCP) markers. This phenomenon is consistent with a cancer stem cell driven neoplastic process (Lacconi, 2000; Farber, 1990; Duncan et al., 2009; Alison et al., 2009; Yoo and Mishra, 2009).
The deficiency of GNMT increases hepatic SAMe levels (Martinez-Chater et al., 2008; Lia 2009). The increase in SAMe that results in this GNMT−/− model inhibits liver cell proliferation because SAMe inhibits DNA synthesis (Varela-Ray et al., 2009). SAMe treatment in rat models of carcinogenesis reduced tumor establishment and growth (Lu et al., 2009; Pascale et al., 2002). Absence of GNMT results in aberrant DNA and histone hypermethylation, which can lead to a major epigenetic alteration in gene expression regulation.
The GNMT deficiency mouse model described here is somewhat analogous to the DDC fed mouse model of liver carcinogenesis (Oliva et al., 2008). In the DDC model, GNMT levels are down regulated (Bardag-Gorce et al., 2008; Oliva et al., 2009), trimethylation of histone 3 lysine 9 and 4 is decreased and acetylation of histone 3 lysine 9 is increased (Bardag-Gorce et al., 2008) (in GNMT KO mice liver H3K27me3 is increased, H3K9 and H3K4 methylation was not determined; and histone acetylation was also not tested). In this model SAMe levels do not increase but S-adenosylhomocysteine (SAH) levels are decreased because of the reduced GNMT levels (Oliva et al., 2009). The SCP marker positive cells proliferate in response to DDC and 8 months after DDC withdrawal tumors form. These tumors are composed of the SCP marker positive cells (FAT10 positive cells) (Oliva et al., 2008) as was the case in the present study of the tumors formed by the GNMT−/− mice. Other SCP markers are also up regulated by DDC feeding including alpha fetoprotein, Kruppel 6, and glutathione S transferase as indicated by microarrays (Li et al., 2008).
Acknowledgments
This work was supported by NIH/NIAAA 8116 and by Alcohol Center Grant on Liver and Pancreas P50-011999, Morphology Core.
Abbreviations
- CK-19
cytokeratin 19
- H3K4me3, H3K9me3, H3K27me3
histone 3 lysine 4, 9, and 27 trimethylated
References
- Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. S Pathol. 2009;217:282–298. doi: 10.1002/path.2453. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Villegas J, Fraley S, Amidi F, Li J, Dedes J, French B, French SW. Epigenetic mechanisms regulate Mallory–Denk body formation in the livers of drug-primed mice. Exp Mol Pathol. 2008;84:113–121. doi: 10.1016/j.yexmp.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Cloncal adaption during carcinogenesis. Biochem Pharmacol. 1990;39:1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Lacconi E. Differential growth: from carcinogenesis to liver repopulation. Am J Pathol. 2000;156:389–392. doi: 10.1016/S0002-9440(10)64741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, Dedes J, French BA, Oliva J, Amidi F, French SW. S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting epigenetic memory. Hepatology. 2008;47:613–624. doi: 10.1002/hep.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia YJ. Characterization of a glycine N-methyltransferase gene knockout mouse model for hepatocellular carcinoma: implications of the gender disparity in liver cancer susceptibility. Int J Cancer. 2009;124:816–826. doi: 10.1002/ijc.23979. [DOI] [PubMed] [Google Scholar]
- Lu SC, Romani K, Ou X, Lin M, Yu V, Kwangsuk KO, Park R, Bottiglieri T, Tsukamoto H, Kanel G, French SW, Mato JM, Moats R, Grant E. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinomas in a rat model. Hepatology. 2009;50:462–471. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Chater ML, Vazquez-Chantada M, Ariz U, Martinez A, Varela M, Loka Z, Capdevia A, Rodriguez J, Aransay AM, Matthiesen R, Yang H, Calvisim DF, Estellerm M, Fragam M, Lum SC, Wagner C, Mato JM. Loss of glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, French SW, Li J, McPhaul L, Amidi F, Dedes J, Habibi A, Nguyen S, French SW. FAT10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp Mol Pathol. 2008;84:102–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, Li J, French BA, Nguyen SK, Lu SC, French SW. Betaine prevents Mallory–Denk body formation in drug-primed mice by epigenetic mechanisms. Exp Mol Pathol. 2009;86:77–86. doi: 10.1016/j.yexmp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale RM, Simile MM, DeMiglio MR, Feo F. Chemoprevention of hepatocarcinogenesis: S-adenosyl-L-methionine. Alcohol. 2002;27:193–198. doi: 10.1016/s0741-8329(02)00227-6. [DOI] [PubMed] [Google Scholar]
- Varela-Ray M, Fernandez-Ramos SD, Martinez-Lopez A, Embade A, Gomez-Santos L, Beraza A, Vazquez-Chantada M, Rodriguez J, Luka Z, Wagner C, Lu SC, Martinez-Chantar ML, Mato JM. Impaired liver regeneration in mice lacking glycine N methyltransferase. Hepatology. 2009;50:443–452. doi: 10.1002/hep.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo Z, Mishra C. Cancer stem cells and hepatocellular carcinoma. Cancer Biol Therap. 2009;8:1691–1698. doi: 10.4161/cbt.8.18.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]


