Abstract
The deleterious effects of H2O2 on the electron transport chain of yeast mitochondria and on mitochondrial lipid peroxidation were evaluated. Exposure to H2O2 resulted in inhibition of the oxygen consumption in the uncoupled and phosphorylating states to 69% and 65%, respectively. The effect of H2O2 on the respiratory rate was associated with an inhibition of succinate-ubiquinone and succinate-DCIP oxidoreductase activities. Inhibitory effect of H2O2 on respiratory complexes was almost completely recovered by β-mercaptoethanol treatment. H2O2 treatment resulted in full resistance to QO site inhibitor myxothiazol and thus it is suggested that the quinol oxidase site (QO) of complex III is the target for H2O2. H2O2 did not modify basal levels of lipid peroxidation in yeast mitochondria. However, H2O2 addition to rat brain and liver mitochondria induced an increase in lipid peroxidation. These results are discussed in terms of the known physiological differences between mammalian and yeast mitochondria.
Keywords: Yeast, mitochondria, oxygen consumption, respiratory chain, H2O2, peroxidation
Introduction
In cells, the mitochondrial electron transport chain (ETC) is one of the most important sources of reactive oxygen species (ROS) [1]. ROS generation in ETC may be promoted in vitro through addition of certain inhibitors of respiratory complexes, whereas some pathological situations, such as cardiomyopathy [2,3], hyperglycaemia [4], calcium overload [5] or genetic defects in proteins that constitute respiratory complexes [6] may increase the in vivo rate of mitochondrial ROS production. Because ROS are short-lived species and react readily with molecules surrounding their generation site, it should be expected that lipids and proteins from mitochondrial membranes are their main target when mitochondrial ROS generation is augmented. Hence, the study of oxidative stress effects on ETC components has become important, because mitochondrial oxidative stress-related dysfunction could be implicated in a wide range of disorders, including liver damage by hepatitis B [7], heart ischemia-reperfusion [8] and ageing [9,10].
A number of studies have focused on lipid peroxidation as the main factor of ETC inhibition by ROS. Exposure of bovine heart submitochondrial particles to ascorbate plus ADP/Fe2+ diminished the ubiquinone content and succinate dehydrogenase and cytochrome c oxidase activities. These effects were parallelled by an increase in lipid peroxidation [11]. In rat synaptosomes, the activities of complexes II, III and V were partially inhibited by ascorbate-iron treatment. In this system, lipophilic antioxidants protected the activity of complex III, so it was suggested that lipid peroxidation was involved in inhibition. However, protein oxidation could also be involved in the effects on these complexes [12]. In mitochondria isolated from rat hearts subjected to an ischemia-reperfusion cycle, the rate of H2O2 production increased when using succinate as respiratory substrate, leading to lipid peroxidation-associated cardiolipin loss and diminution in state 3 respiration rate. This last effect was attributed to a partial inhibition in activities of complexes I and III [13,14].
Despite the above described, some authors have reported that ETC may be oxidatively damaged by mechanisms which do not necessarily involve peroxidative damage to lipids. Giulivi et al. [15] reported the preferential attack of oxygen singulet to proteic components of ETC over the attack to lipidic components of mitochondrial membranes. In beef heart submitochondrial particles exposed to increasing doses of hydroxyl radical (OH•) [16], lipophilic antioxidants that inhibit lipid peroxidation did not prevent the oxidative inactivation of NADH and succinate dehydrogenases. Similarly, the incubation of rat brain mitochondria with FeSO4/ascorbate mixtures (an OH• generating system) augmented lipid peroxidation levels and inhibit ETC function. However, the lipid peroxidation inhibitor, butylated hydroxytoluene, did not avoid inhibition in ETC function but did prevent the increase in lipid peroxides and the OH•-stimulated carbonylation of proteins [17]. Therefore, these reports suggest that lipid peroxidation-independent oxidative damage might be of critical importance in the mechanism of ETC inactivation.
Even when a more extensive characterization about the effects of OH• and superoxide radicals has been done, less attention has been put in the effects of H2O2 on ETC functionality. This aspect could be potentially important since H2O2 has been considered the most important ROS at cellular level [18], mitochondria are the principal source of H2O2 [18] and ETC components of mitochondria are enriched in cytochromes and Fe-S centres, which are important targets of oxidative attack by this ROS [19,20].
A large body of experimental evidence has demonstrated a direct correlation between susceptibility to peroxidation of membrane lipids and its degree of unsaturation [21-24]. In fact, this correlation has also been shown at mitochondrial level. In rat mitochondria the three main membrane phospholipids (cardiolipin, phosphatidylcholine, phosphatidylethanolamine) are composed of the highly unsaturated docosahexaenoic acid (22:6) and arachidonic acid (20:4) and they are significantly more sensitive to lipid peroxidation, whereas, in membranes from pigeon mitochondria, which are more resistant to lipid peroxidation than rat mitochondria, these highly unsaturated fatty acids are substituted by linoleic acid (18:2) [23,24].
Saccharomyces cerevisiae mitochondria present some important differences in comparison to rat mitochondria, including (a) the lack of a rotenone-sensible complex I [25,26] and (b) when grown using lactate as a carbon source, the membrane fatty acid composition of mitochondria consists mainly of C16:1 and C18:1 fatty acids [27]. The last feature of yeast mitochondria probably results in higher resistance to oxidative stress. Hence, the way in which ROS inhibit yeast ETC could be different from their action on rat mitochondria, because yeast mitochondria might be less sensitive to lipid peroxidation due to their lipid composition. For the same reason, yeast mitochondria may be a more suitable system than lipophilic antioxidants to discriminate the direct effects of ROS on components of ETC from those attributable to lipid peroxidation.
The toxicity of H2O2 on yeast mitochondria was studied. It was found that yeast mitochondria are highly resistant to ROS and, in addition, the mechanism of damage seems to be circumscribed to thiol groups of proteins since thiol reductanct β-mercaptoethanol was able to revert the damaging effect of H2O2 on dehydrogenase activities of ETC, phospholipids in the membrane were not damaged, cytochrome content was not altered and hydroxyl radical scavenger mannitol or iron quelant EDTA were not able to prevent the inhibitory effect exerted by H2O2 on ETC reactions. It was observed that the Q0 site in complex-III was the most affected function on the ETC.
Materials and methods
Materials
Zymolyase 20T was obtained from ICN Biomedicals, Inc. (Aurora, OH). H2O2 (38% v/v), succinic acid, β-mercaptoethanol, 2,6-dichlorophenolindophenol (DCIP), potassium cyanide, tetramethyl-p-phenyle-nediamine (TMPD), cytochrome c, flavone, antimycin A and myxothiazol were obtained from Sigma Chemical. Co. (St. Louis, MO). Stigmatellin was obtained from Fluka Chemie GmbH (Buchs, Switzerland). All other reagents were of the highest purity commercially available.
Biologicals
An industrial wild type diploid strain of Saccharomyces cerevisiae, Yeast Foam was used (kind gift from Professor Michel Rigoulet, IBGC, U. of Bordeaux-2, France). Three-month old, male Wistar rats from our local colony were used for isolation of liver and brain mitochondria.
Isolation of yeast mitochondria
Cells of Yeast Foam were grown aerobically at 28°C in a medium containing 0.12% (NH4)2SO4, 0.1% KH2PO4, 1% yeast extract, 2% dl-lactate (pH 5.0 with NaOH) and harvested in mid-exponential growth phase (OD550 = 03.5–4.0). Mitochondria were isolated from spheroplasts as described previously [28,29], except that zymoliase was used instead of cytohelicase.
Isolation of rat brain mitochondria
Rat brain mitochondria were isolated by differential centrifugation in a Percoll gradient as described [30,31], with some modifications. Briefly, rats were decapitated and the brain was extracted and placed in cold medium containing 210 mm mannitol, 70 mm sucrose, 1 mm EGTA, 0.5% bovine serum albumin and 10 mm MOPS (pH 7.4). The brain was homogenized manually in a glass homogenizer and centrifuged at 400 g; the supernatant was centrifuged at 9 000 g. Centrifugations were carried out for 10 min at 4°C. The pellet was resuspended in 15% Percoll and placed in a discontinuous Percoll (23% and 40%) gradient. The gradient was centrifuged at 30 700 g, during 6 min, band 3 was extracted, diluted 1:4, centrifuged and washed at 16 700 g in the isolation medium supplemented with 0.5% bovine serum albumin for 10 min. The last centrifugation was done at 6900 g for 10 min.
Isolation of rat liver mitochondria
Rat liver mitochondria were prepared from the same rats used for brain mitochondria isolation. Once the liver was extracted, it was cut into small pieces and homogenized. The homogenate was subjected to differential centrifugation as described previously [32]. In all cases, mitochondrial protein concentration was assayed by Biuret assay [33].
Treatments with H2O2
Treatments were performed before each assay at 4°C and mitochondrial protein at a concentration of 0.3 mg/ml. Mitochondria were suspended in 50 mm KH2PO4 buffer (pH 7.6 with NaOH), except for oxygen consumption measurements, where mitochondrial protein (0.3 mg/ml) was suspended in a medium with 0.6 m sucrose, 2 mm EGTA, 4 mm KH2PO4, 10 mm Tris-maleate, pH 6.8. This was done because incubation of mitochondria in isotonic KH2PO4 buffer inhibited oxygen consumption in intact mitochondria due to swelling. H2O2 was added at the beginning of incubations at the concentrations indicated in the legend to each figure. For experiments of protection with EDTA or mannitol, mitochondria were incubated with 50 μm EDTA or 10 mm mannitol during 15 min previous to treatment with H2O2. For experiments to determinate reversion of the H2O2 effects by β-mercaptoethanol, mitochondria were incubated during 30 min with β-mercaptoethanol after treatments with H2O2. To avoid interferences due to cytochromes release in cytochrome spectra experiments and unspecific reduction of DCIP by β-mercaptoethanol during measurements of succinate-DCIP oxidoreductase activity, H2O2 was removed to carry out both determinations at the end of the treatment by centrifugating mitochondrial solution at 8700 g during 15 min and mitochondrial pellet were re-suspended in 50 mm KH2PO4 buffer (pH 7.6 with NaOH) at adequate concentration and volume for each determination. Controls were treated in the same way except for H2O2 addition.
Oxygen consumption
The rate of cyanide-sensitive oxygen consumption in resting state (state 4), phosphorylating state (state 3) and uncoupled state (state U) was monitored at room temperature using an YSI 5300 biological oxygen monitor (Yellow Springs Instrument, Yellow Springs, OH), equipped with a Clark-type electrode. Pre-treated mitochondria (0.3 mg/ml) were placed in a glass chamber with constant stirring, containing 3.0 ml of a medium with 0.6 m sucrose, 2 mm EGTA, 4 mm KH2PO4, 10 mm Tris-maleate, pH 6.8. Respiration in states 3 and U was initiated with 300 μm ADP and 2 μm FCCP, respectively.
Succinate dehydrogenase activity
This enzymatic activity was measured at room temperature in permeabilized mitochondria, following the TTFA sensitive secondary reduction of DCIP [34]. Mitochondria were permeabilized with Triton X-100 as reported by Hallberg et al. [35] and treated with H2O2 as indicated above. H2O2 was removed at the end of the treatment to eliminate unspecific reduction of DCIP by β-mercaptoethanol by centrifugating mitochondrial solution at 8700 g during 15 min and mitochondrial pellet was resuspended at a concentration of 0.3 mg in a final 1 ml volume of reaction mixture contained 50 mm KH2PO4 phosphate buffer (pH 7.6), 10 mm sodium succinate (pH 7.6), 50 μm flavone, 1 μg antimycin A and 1 mm KCN. After incubating for 3 min with inhibitors, the reaction was initiated with 80 μm DCIP. Reaction was stopped by the addition of 1 mm TTFA. Absorbance changes were recorded in a SLM Aminco DW 2000 spectrophotometer, in the dual wavelength mode at 600–590 nm. The activity was calculated from the slope of the absorbance plot using the molar extinction coefficient of 21 mm−1 cm−1 for DCIP. The TTFA-sensitive activity was calculated by subtracting the activity in the presence of TTFA plus succinate to the activity stimulated only with succinate.
Succinate cytochrome c oxidoreductase activity
The antimycin A sensitive succinate-mediated reduction of cytochrome c was followed by measuring the reduction of cytochrome c in permeabilized mitochondria by mild detergent treatment [35]. The reaction mixture contained 50 mm KH2PO4 (pH 7.6), 1.5 mg cytochrome c, 50 μm flavone and 1 mm KCN in a final 1 ml volume. Pre-treated mitochondrial protein (0.1 mg) was added and, after 3 min, the reaction was initiated by adding 10 mm sodium succinate (pH 7.6). The reaction was stopped by the addition of 1 μg antimycin A. Absorbance changes at 550–540 nm were recorded as described above. The rate of cytochrome c reduction was calculated from the slopes of the absorbance plots using a molar extinction coefficient of 19.1 mm−1 cm−1 mm for cytochrome c. The antimycin-sensitive reduction of cytochrome c was calculated by subtracting the activity in the presence of antimycin plus succinate to the activity stimulated only with succinate.
Cytochrome oxidase activity
This activity was evaluated by measuring the cyanide-sensitive oxygen consumption at room temperature using an YSI 5300 biological oxygen monitor, equipped with a Clark-type electrode. The reaction mixture contained 50 mm KH2PO4 buffer (pH 7.6), 1 μg antimycin A, 50 μm TMPD and 0.9 mg detergent-solubilized mitochondrial protein in a final concentration of 0.3 mg/ml. The assay was started by the addition of 5 mm sodium ascorbate or 0.6 mg oxidized cytochrome c (pH 7.6) and stopped by the addition of 100 μm KCN.
Cytochrome spectra
Cytochrome spectra were obtained by measuring at room temperature the reduced minus oxidized spectra with a SLM Aminco DW 2000 spectrophotometer fitted in the split mode. To eliminate the possibility of cytochromes released by H2O2 treatment interfering with the results, pre-treated mitochondrial samples (2 mg) were centrifuged at 8700 g during 15 min and re-suspended in a medium with 0.6 m sucrose, 10 mm Tris maleate (pH 6.8), 4 mm KH2PO4, 2 mm EGTA and 1 mm KCN. bc1 complex inhibitors were added 5 min before spectra recording. Reference and sample cuvettes were treated in the same way and the baseline was recorded. Then a small amount of KFeCN was added to the reference cuvette while 10 mm succinate or, when indicated, a small quantity of sodium dithionite, was added to the sample cuvette. These cuvettes were used to record the differential spectra. Spectra were scanned between 500–580 nm. Cytochrome contents were calculated using the following wavelengths and molar extinction coefficients: cytochrome c+c1, ΔA=552 nm minus 540 nm and ε=19.1 mm−1 cm−1; cytochrome b, ΔA=562 minus 575 nm and ε=20 mm−1 cm−1 [11].
Lipid peroxidation measurements
The extent of lipid peroxidation was determined with the thiobarbituric acid (TBA) assay [36]. Mitochondria were treated with H2O2 as indicated above and later combined with 2 ml of an acid mixture containing 0.25 N HCl, 15% w/v trichloroacetic acid and 0.375% w/v thiobarbituric acid. This combination was heated for 15 min in a boiling water bath, cooled on ice and centrifuged at 1 000 g for 5 min. The absorbance of the supernatant was measured at 535 nm against a blank containing all the reagents minus mitochondrial protein. A Perkin Elmer Lambda 18 UV/vis spectrometer was used. The results were calculated using the molar extinction coefficient for malondialdehyde and expressed in terms of nanomoles of TBA reactants (TBARS) per milligram of protein.
Results
Isolated yeast mitochondria were incubated for 30 min in the presence of increasing concentrations of H2O2 and the effects on the rate of oxygen consumption were tested. Measurements were conducted in both the resting state (state 4) and the phosphorylating state (state 3). In state 4 (Figure 1A) the addition of increasing concentrations of H2O2 resulted in a progressive inhibition of respiration, reaching a significative decrease in 51% and 60%, with 0.5 mm and 1.0 mm, respectively. A more pronounced effect was mediated by H2O2 in state 3 (Figure 1B), resulting in a decrease in respiration from the lowest concentration tested (0.125 mm). Wide variations in the concentration of H2O2 (0.25–1.0 mm) exhibited a stronger inhibitory effect (60–75%) (Figure 1B).
Figure 1.
Effect of H2O2 on the rate of O2 uptake in state 4 (A), state 3 (B) or uncoupled state (C). Mitochondria were incubated during 30 min at 4°C in respiration medium (0.6 m sucrose, 2 mm EGTA, 4 mm KH2PO4, 10 mm Tris-maleate), with indicated amounts of H2O2. After pre-incubation, oxygen uptake was measured as described in Materials and methods. Data are presented as mean±SE of >3 independent experiments. Signifintly different when compared to control (0 mm H2O2) (*p<0.05, **p<0.01).
Addition of the protonophore FCCP to mitochondria dissipated membrane potential; thus, respiratory chain activity is stimulated (state U respiration) to compensate membrane potential dissipation. In this way, it is possible to study the maximal activity of respiratory chain. On this basis, respiration in the presence of FCCP was measured to discard that impairment on ATP synthesis was responsible for the inhibition observed in state 3 respiration. In Figure 1C it can be observed that 0.5 mm H2O2 inhibited respiration to a similar degree than observed in state 3 at the same concentration of H2O2 (65% and 69% in state 3 and state U, respectively). This result and the partial inhibitory effect on respiration even to the highest concentration of H2O2 suggest that damage was exerted at the level of at least one of the respiratory complexes. As well, impairment in ATP synthesis probably is not participating in the observed effects.
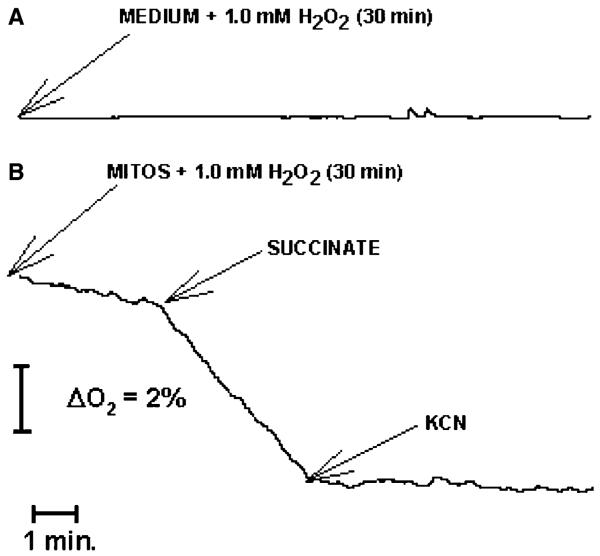
Control experiments were carried out to eliminate the possibility that oxygen produced by spontaneous decomposition of H2O2 may produce an artificial inhibition on respiration. As Figure 2A shows, oxygen consumption was not detected when this parameter was evaluated in respiratory medium plus 1.0 mm H2O2 for 30 min at 4°C. Also, oxygen consumption was not detected in mitochondria treated in the same way and inhibited with 0.1 mm KCN (Figure 2B). Together, these results validate the inhibition on respiration induced by H2O2.
Figure 2.
Influence of H2O2 decomposition on oxygen consumption determinations. Respiration medium (A) or respiration medium plus 0.9 mg mitochondria (B) were incubated during 30 min at 4°C with 1.0 mm H2O2. Then, oxygen production was evaluated with a Clark-type oxygen electrode as described in Materials and methods. (A). Experiment was repeated at least three times. Data are from a representative experiment.
Even at the highest concentrations (1.0 mm), H2O2 addition resulted in only a partial inhibition, suggesting that electron flow through ETC was compromised at some point. To locate the site of inhibition, the activities of some partial reactions occurring during electron transport in ETC were measured. Succinate-DCIP oxidoreductase activity (complex II) was diminished by 23% and 30% with low (0.125 mm) and intermediate (0.250 mm) concentrations of H2O2 (Figure 3A), respectively. Higher quantities of H2O2 (0.5–1.0 mm) did not produce an expected inhibitory effect, reaching just 40–45%. A higher sensitivity to H2O2 challenge was detected in succinate-cytochrome c oxidoreductase activity (complex II–III segment). In this case, a more clear correlation between concentration of H2O2 and activity was found; half of the activity was lost when mitochondria was incubated with 0.5 mm, while 1.0 mm H2O2 fully inhibited this activity (Figure 3B).
Figure 3.
Effect of H2O2 on partial reactions of ETC. Triton-solubilized mitochondria were incubated during 30 min at 4°C in 50 mm KH2PO4 buffer with indicated concentrations of H2O2 under conditions described in Materials and methods. Succinate-DCIP oxidoreductase (A), succinate-cytochrome c oxidoreductase (B), cytochrome oxidase (C) or cytochrome c oxidase (D) activities were measured as described in Materials and methods. Results are expressed as the mean±SE of >3 independent experiments. Significantly different when compared to control (0 mm H2O2) (*p<0.05, **p<0.01).
In contrast, cytochrome oxidase activity (complex IV) was completely insensitive to all concentrations of H2O2 tested (0.125–1.0 mm), whatever TMPD (Figure 3C) or cytochrome c (Figure 3D) were used as electron donor. It must be pointed out that both TMPD and cytochrome c bind to cytochrome c oxidase in distinct sites [37]. Taken together, these results indicate that inhibition of the ETC occurs mainly at the level of the succinate-cytochrome c oxidase segment of ETC (i.e. bc1 complex).
The role of sulphydryl oxidation in the deleterious effects exerted by H2O2 on ETC was investigated. After incubation with H2O2, mitochondria were treated for 30 min with increasing concentrations of β-mercaptoethanol. Afterwards, remnant of β-mercaptoethanol and H2O2 were washed twice with fresh medium to avoid the reduction of electron acceptors by β-mercaptoethanol. Also, this ensures that the effect exerted by β-mercaptoethanol can be attributed to thiol reduction and not to the H2O2 removal from the medium. Both succinate-DCIP oxidoreductase activity (Figure 4A) and succinate-cytochrome c oxidoreductase (Figure 4B) were nearly restored (89% and 95%, respectively) when concentration of β-mercaptoethanol was twice the concentration of H2O2 used (1.0 mm for succinate-DCIP oxidoreductase and 0.5 mm for succinate-cytochrome c oxidoreductase). It must be pointed out that treatment of control samples with maximal concentrations used of β-mercaptoethanol (2.0 mm for succinate-DCIP oxidoreductase activity and 1.0 mm for succinate-cytochrome c oxidoreductase) did not have an appreciable effect on control values of enzymatic activities (inset, Figure 4A and B). These results suggest that oxidation of thiol groups on proteins from respiratory complexes could be involved in the inhibitory effect of H2O2.
Figure 4.
Effect of β-mercaptoethanol on the impairment induced by H2O2 on partial reactions of ETC. Mitochondria were incubated after H2O2 treatment with indicated concentrations of β-mercaptoethanol during 30 min at 4°C on 50 mm KH2PO4 buffer. Later, H2O2 and β-mercaptoethanol were removed twice by centrifugation and mitochondrial pellet re-suspended on fresh 50 mm KH2PO4 buffer. Concentrations of H2O2 used were: 1.0 mm H2O2 for succinate-DCIP oxidoreductase activity (A) and 0.5 mm H2O2 for succinate-cytochrome c oxidoreductase activity (B). Activities were measured as described in Materials and methods. Values from mitochondria incubated with 2.0 mmβ-mercaptoethanol (inset, panel A) or 1.0 mmβ-mercaptoethanol (inset, panel B) without H2O2 addition were taken as 100%. Results are expressed as the mean±SE of >3 independent experiments. Significantly different when compared to control (0 mm H2O2) (*p<0.05, **p<0.01).
H2O2 can also mediate oxidative damage through iron release by degrading haem proteins and iron-sulphur clusters. Released iron reacts with H2O2 to produce the highly reactive hydroxyl radical (OH•), which in turn oxidizes lipids and proteins [19,38,39]. To further investigate about the nature of the damage induced by H2O2 on ETC, the protective effect of the iron quelator EDTA and the OH• scavenger mannitol was tested. The pre-incubation during 15 min with either 10 mm mannitol or 50 mm EDTA previous to treatment with H2O2 did not protect both succinate-DCIP oxidoreductase activity (Figure 5A) and succinate-cytochrome c oxidoreductase (Figure 5B) from the inhibitory effect of H2O2. Even an augment of 10-fold in the concentration of mannitol (100 mm) did not exert protective effects (data not shown), while an augment of 2-fold (100 μm) in EDTA concentration inhibit per se control activities (data not shown). Thus, this result supports the suggestion that thiol oxidation is the main factor responsible for ETC inhibition and discards the participation of OH• radical in the effects observed.
Figure 5.
Effect of mannitol and EDTA on the impairment induced by H2O2 on partial reactions of ETC. Triton-solubilized mitochondria were incubated during 15 min on 50 mm KH2PO4 buffer at 4°C with 10 mm mannitol or 50 μm EDTA previous to treatment during 30 min with 1.0 mm H2O2 for succinate-DCIP oxidoreductase activity (A) and 0.5 mm H2O2 for succinate-cytochrome c oxidoreductase activity (B). Activities were measured as described in Materials and methods. Values from incubated controls (mitochondria incubated during 45 min without EDTA, mannitol or H2O2 addition, data not shown) were taken as 100% (dotted line). Results are expressed as the mean±SE of >3 independent experiments. Significantly different when compared to 10 mm mannitol (*p<0.05, **p<0.01) and 50 μm EDTA (+p<0.05).
Next, to locate a putative site in the bc1 complex where H2O2 exerted its effects, difference absorption spectra of cytochromes were recorded. Experiments were conducted in the presence of different quinone redox sites inhibitors of bc1 complex. The amount of reducible cytochromes c (550 nm peak) using dithionite was similar in controls (6.8)× 10−4 mm) (Figure 6, solid line) and in mitochondria subjected to oxidative stress (7.0)×10−4 mm) (Figure 6, dotted line). In addition, the content of cytochrome b (562 nm peak) was 3.1×10−4 mm in the control and 3.4)×10−4 mm in treated mitochondria. These results suggest that the oxidative stress induced by H2O2 exposure did not cause the release of cytochromes from the bc1 complex.
Figure 6.

Effect of H2O2 on cytochromes levels of yeast mitochondria using dithionite as electron donor. Mitochondria were pre-incubated during 30 min on 50 mm KH2PO4 buffer at 4°C in the absence (solid line) or the presence of 0.5 mm H2O2 (dotted line) Later, H2O2 was removed twice by centrifugation and mitochondrial pellet re-suspended on fresh 50 mm KH2PO4 buffer. A small amount of dithionite was used as electron donor. Spectra recording was performed as described in Materials and methods. Experiments were repeated at least three times. Data are from a representative experiment.
Antimycin A is a bc1 complex inhibitor that prevents re-oxidation of cytochromes b at Qi site, which is reflected as a decrease from 2:1 (Figure 7A, continuous lines) to ~1.5:1 (Figure 7A, dotted lines) in the absorbance ratio of the reduced cytochromes c vs b. This was observed both in control mitochondria and in H2O2-treated mitochondria in the presence of succinate as electron donor. Thus, H2O2 did not change the effect of antimycin A on bc1 complex.
Figure 7.
Effect of H2O2 on difference absorption spectra of cytochromes from yeast mitochondria in the presence of bc1 complex inhibitors. Mitochondria were pre-incubated during 30 min on 50 mm KH2PO4 buffer at 4°C in the absence or the presence of 0.5 mm H2O2. Later, H2O2 was removed twice by centrifugation and mitochondrial pellet re-suspended on fresh 50 mm KH2PO4 buffer. Inhibitors were added 5 min before spectra recording and 10 mm succinate was used as electron donor. Spectra were performed as described in Materials and methods. (A) Spectra in absence (solid line) or the presence (dotted lines) of antimycin A, (B) spectra in the presence of myxothiazol in control (solid line) and treated (dotted line) mitochondria, (C) spectra in the presence of stigmatellin in control (solid line) and treated (dotted line) mitochondria. Experiments were repeated at least three times. Data are from a representative experiment.
Myxothiazol is a bc1 complex inhibitor that prevents ubiquinol oxidation at the proximal position of QO site. The effect of myxothiazol is reflected as a decrease from 2:1 to ~0.8:1 in the absorbance ratio of reduced cytochromes c vs b (Figure 7B, continuous line). In contrast to antimycin A, H2O2 modified the effects of myxothiazol, inducing a full resistance to inhibition and allowing observing a ratio of 2:1 of reduced cytochromes c to reduced cytochromes b (Figure 7B, dotted line).
In order to further explore the effect of H2O2 on the QO site of cytochrome bc1, another QO site inhibitor was tested. Stigmatellin is an inhibitor that completely abolishes electron transfer at the distal niche of QO site. This effect can be observed in difference absorption spectra as the disappearance of the absorption peak of cytochromes c at 550 nm (Figure 7C, continuous line). In contrast to myxothiazol, H2O2 did not modify the effect of stigmatellin (Figure 7C, dotted line). Taken together, these results suggest that oxidative stress induced by H2O2 exposure alters selectively the proximal niche of QO site at complex bc1. The effect on the QO site pro bably is the mechanism underlying the observed decrease in the rate of electron transfer at the level of the succinate cytochrome c oxidoreductase region of ETC.
In mammalian mitochondria subjected to oxidative stress, lipid peroxidation has been reported as the main factor affecting ETC function [11-14]. We evaluated lipid peroxidation to explore whether it could be involved in the harmful effects of H2O2 on yeast ETC. Lipid peroxidation in yeast mitochondria did not rise by treatment with H2O2 (Figure 8) but, instead, diminished TBARS formation in 0.27 nmoles (0.74 vs 0.47 nmoles TBARS/mg of protein in control vs treated mitochondria, respectively). To verify whether H2O2 was an appropriate inducer of mitochondrial lipid peroxidation, mitochondria from rat liver and brain were challenged with H2O2 in the same manner as yeast mitochondria. First, it was observed that basal levels of lipid peroxidation were higher in rat mitochondria than yeast mitochondria. Second, H2O2 stimulated TBARS level increased 72% in liver mitochondria (1.26 vs 2.17 nmoles TBARS/mg of protein in control vs treated mitochondria, respectively) and 61% in brain mitochondria (2.26 vs 3.64 nmoles TBARS/mg of protein in control vs treated mitochondria, respectively). These results suggest that H2O2 is able to induce lipid peroxidation in isolated mitochondria and that such a process may not be involved in the deleterious effects of H2O2 on yeast mitochondrial ETC.
Figure 8.

Effect of H2O2 on lipid peroxidation levels in yeast and rat mitochondria. Mitochondria were pre-incubated during 30 min on 50 mm KH2PO4 buffer at 4°C in the absence (black bars) or the presence (grey bars) of 0.5 mm H2O2. Measurements were made as described in Materials and methods. Data are presented as mean±SE of three independent experiments. Significantly different when compared to yeast mitochondria treated with H2O2 (*p<0.05, **p<0.01).
Discussion
The results obtained in this study describe the effects of oxidative stress generated by H2O2 on yeast ETC functionality. H2O2 has been considered the most important ROS at cellular level [18], whereas mitochondria are the major site of H2O2 generation due to the presence of Mn and CuZn-SOD isoforms [18], which convert superoxide to H2O2 byproduct of respiration. In addition, activation of NADPH oxidases on immune cells (e.g. macrophages) increase cellular levels of H2O2 [40], supporting the rationale of using this oxidant as an oxidative stress inducer.
H2O2 decreases oxygen consumption rate in all the respiratory states (Figure 1), even in the lowest concentration used (0.125 μm). This result can be interpreted as a consequence of selective damage on some segment of ETC, as was evidenced by the full lost in activity of succinate cytochrome c reductase (Figure 2B). In agreement with this idea, it has been demonstrated that, due to their susceptibility of certain proteins of yeast, mitochondria undergo oxidative modifications by H2O2 exposure [41]. These modifications include methionine residues oxidation, irreversible −SH groups oxidation, carbonylation, protein cross-linking, etc. [42]. The inhibition observed in complex II–complex III segment could be due to one of those modifications: the recovery of the activity obtained with β-mercaptoethanol (Figure 4) and the null protective effect of mannitol and EDTA on enzymatic activities suggests that oxidation of −SH groups are the main event responsible for the inhibition. In addition, given the hydrophilic nature of β-mercaptoethanol, it is likely that the damage to succinate cytochrome c reductase was exerted in a hydrophilic region.
The resistance towards quinone redox sites inhibitors of bc1 complex in native [43] and mutant organisms [44,45] has been used to identify these sites, as well as to establish structure–activity relationships. A similar approach was used to identify which redox site in bc1 complex underwent alterations during oxidative stress. Stigmatellin is a QO site competitive inhibitor that fully avoids ubiquinol oxidation at the distal position [46]. H2O2 did not modify the effects of this inhibitor (Figure 7C), suggesting that stigmatellin binding occurred. However, the myxothiazol-resistant cytochrome c reduction, observed in the difference spectra (Figure 7B), reflected some kind of alteration caused by H2O2 on the proximal niche of QO site. The resistance towards myxothiazol could be explained by either (a) the myxothiazol binding site was modified and thus, inhibitor binding was not possible or (b) an increase in superoxide formation was promoted, leading to the non-catalytic reduction of cytochrome c. Muller et al. [46] demonstrated that interaction of myxothiazol with the proximal niche of QO site allows for partial ubiquinol oxidation, but prevents the transfer of a second electron from ubisemiquinone, allowing for its accumulation and the reduction of cytochrome c through superoxide formation. Likewise, it is possible that H2O2 modified the environment in the proximal niche, allowing ubisemiquinone escape and non-catalytic cytochrome c reduction. Therefore, Q-cycle would suffer a diminution in its efficiency, limiting partially electron flow towards cytochrome c oxidase in the succinate cytochrome c oxidoreductase segment of the respiratory chain, as reflected by the partial decline observed in respiratory rate even at the highest concentration of H2O2 used.
The idea of a direct interaction between H2O2 and proteins of the bc1 complex is supported by the null effects obtained with H2O2 on yeast mitochondria lipid peroxidation (Figure 8). In bovine heart submitochondrial particles [11], rat synaptosomes [12] and rat heart mitochondria [13,14], mitochondrial lipid peroxidation has been identified as a key factor in ETC inhibition. In our hands, mitochondrial yeast lipid peroxidation did not increase due to oxidative stress while the same treatment promoted lipid peroxidation in rat liver and brain mitochondria. It is important to emphasize that conditions used in this work to induce oxidative stress were different from those used in the above reports. Even though H2O2 does not induce lipid peroxidation by itself, peroxides can induce this process through iron release by degrading hem proteins (e.g. cytochromes c and b) [19]. Iron reacts with H2O2 through Fenton’s chemistry to produce the highly reactive hydroxyl radical (OH•) [38]. The latter can initiate lipid peroxidation readily through hydrogen abstraction from double bonds of phospholipids unsaturated hydrophobic chains [39]. Consequently, it is possible that H2O2 could have promoted lipid peroxidation by this mechanism in rat mitochondria but not in yeast mitochondria.
In agreement with this argument, different absorption spectra revealed that treatment with H2O2 did not diminish the content of either cytochromes c or b, since its reduction in the presence of dithionite was similar to control experiment results (Figure 6), which means that iron was not released, at least from proteins containing this sort of cytochromes (e.g. bc1 complex). Otherwise, cytochrome reduction would not have been possible.
The unsaturation degree of fatty acids from mitochondrial membranes has been positively correlated with their vulnerability to peroxidation [24]. Fatty acid content from membranes of yeast mitochondria grown on lactate consists mainly of the monounsaturated fatty acids, palmitoleic (C16:1) and oleic (C18:1) acids [27], whereas rat mitochondria membranes contain the highly unsaturated fatty acids, docosahexaenoic acid (22:6) and arachidonic acid (20:4) [23,24]. Thus, the lower double bond content in lipids from yeast mitochondria could increase their resistance against lipid peroxidation, which would explain, in part, the differences observed in this parameter between yeast and rat mitochondria.
A contributor to the resistance of yeast mitochondria against oxidative stress could be the Hsp60 molecular chaperone. Cabiscol et al. [20] demonstrated that Hsp60 protects proteins containing Fe/S clusters from damage by H2O2, avoiding in this manner the release of iron from these proteins and allowing greater cell viability than that observed in cells with low Hsp60 levels.
In summary, the results show a high resistance of yeast mitochondria against oxidative stress, as was suggested first by Machida et al. [47]. The study of complex bc1 inhibition by oxidative stress in terms of the Q cycle dysfunction must also be considered and not only in function of lipid peroxidation and ubiquinone and cardiolipin loss as discussed above, the first could lead to a greater production of ROS. On the other hand, it would be interesting to evaluate whether the differences observed in lipid peroxidation propensity between mammalian and yeast mitochondria are involved in the dissimilarities observed in some important physiological processes where lipid peroxidation has been implicated (e.g. programmed cell death).
Acknowledgements
The authors appreciate the technical assistance of Norma Sánchez-Suárez (IFC-UNAM). This study was supported partially by grants from CONACYT (AS-M: 43705, 64277; SM-A: 64308) and CIC-UMSNH (2.16) and by the P01 AG 021830 (IB) from the NIH/NIA and P01 AI062885-01 (IB) from the NIAID.
References
- [1].Beckman KB, Ames BN. The free radical theory of aging matures. Phys Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [2].Luo X, Pitkänen S, Kassovska-Bratinova S, Robinson BH, Lehotay DC. Excessive formation of hydroxyl radicals and aldehydic lipid peroxidation products in cultured skin fibro-blasts from patients with complex I deficiency. J Clin Invest. 1997;99:2877–2882. doi: 10.1172/JCI119481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pitkänen S, Robinson BH. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nishikawa T, Edelstein D, Du XL, Yamagishi SI, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes H-P, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- [5].Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- [6].Guo J, Lemire BD. The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide. J Biol Chem. 2003;278:47629–47635. doi: 10.1074/jbc.M306312200. [DOI] [PubMed] [Google Scholar]
- [7].Lee YI, Hwang JM, Im JH, Lee YI, Kim NS, Kim DG, Yu DY, Moon HB, Park SK. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem. 2004;279:15460–15471. doi: 10.1074/jbc.M309280200. [DOI] [PubMed] [Google Scholar]
- [8].Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- [9].Wei Y-H, Lu CY, Lee H-C, Pang C-Y, Ma Y-S. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann NY Acad Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- [10].Wei Y-H, Lu CY, Wei CY, Ma YS, Lee H-C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol. 2001;44:1–11. [PubMed] [Google Scholar]
- [11].Forsmark-Andreé P, Lee CP, Dallner G, Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med. 1997;22:391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- [12].Cardoso SM, Pereira C, Oliveira CR. Mitochondrial function is differentially affected upon oxidative stress. Free Radic Biol Med. 1999;26:3–13. doi: 10.1016/s0891-5849(98)00205-6. [DOI] [PubMed] [Google Scholar]
- [13].Petrosillo G, Ruggiero FM, Di Vennosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- [14].Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- [15].Giulivi C, Sarcansky M, Rosenfeld E, Boveris A. The photodynamic effect of rose bengal on proteins of the mitochondrial inner membrane. Photochem. Photobiol. 1990;52:745–751. doi: 10.1111/j.1751-1097.1990.tb08676.x. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJA. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 2002;265:16330–16336. [PubMed] [Google Scholar]
- [17].Sen T, Sen N, Tripathi G, Chatterjee U, Chakrabarti S. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem Int. 2006;49:20–27. doi: 10.1016/j.neuint.2005.12.018. [DOI] [PubMed] [Google Scholar]
- [18].O’Brien KM, Dirmeier R, Engle M, Poyton RO. Mitochondrial protein oxidation in yeast mutants lacking manganese-(MnSOD) or copper- and zinc-containing Superoxide Dis-mutase (CuZnSOD): evidence that MnSOD and CuZnSOD have both unique and overlapping functions in protecting mitochondrial proteins from oxidative damage. J Biol Chem. 2004;279:51817–51827. doi: 10.1074/jbc.M405958200. [DOI] [PubMed] [Google Scholar]
- [19].Paller MS, Jacob HS. Cytochrome P-450 mediates tissue-damaging OH• formation during reoxygenation of the kidney. Proc Natl Acad Sci USA. 1994;91:7002–7006. doi: 10.1073/pnas.91.15.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cabiscol E, Belli G, Tamarit J, Echave P, Herrero E, Ros J. Mitochondrial Hsp60, resistance to oxidative stress, and the labile iron pool are closely connected in Saccharomyces cerevisiae. J Biol Chem. 2002;277:44531–44538. doi: 10.1074/jbc.M206525200. [DOI] [PubMed] [Google Scholar]
- [21].Pamplona R, Portero-Otin M, Ruiz C, Gredilla R, Herrero A, Barja G. Double bond content of phospholipids and lipid peroxidation negatively correlate with maximum longevity in the heart of mammals. Mech Ageing Dev. 2000;112:169–183. doi: 10.1016/s0047-6374(99)00045-7. [DOI] [PubMed] [Google Scholar]
- [22].Pamplona R, Portero-Otin M, Riba D, Requena JR, Thorpe SR, Lopez-Torres M, Barja G. Low fatty acid unsaturation: a mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans. J Gerontol A Biol Sci Med Sci. 2000;55:286–291. doi: 10.1093/gerona/55.6.b286. [DOI] [PubMed] [Google Scholar]
- [23].Herrero A, Portero-Otin M, Bellmunt MJ, Pamplona R, Barja G. Effect of the degree of fatty acid unsaturation of rat heart mitochondria on their rates of H2O2 production and lipid and protein oxidative damage. Exp Gerontol. 2004;39:725–733. doi: 10.1016/s0047-6374(01)00214-7. [DOI] [PubMed] [Google Scholar]
- [24].Pamplona R, Portero-Otin M, Requena JR, Thorpe SR, Herrero A, Barja G. A low degree of fatty acid unsaturation leads to lower lipid peroxidation and lipoxidation-derived protein modification in heart mitochondria of the longevous pigeon than in the short-lived rat. Mech Ageing Dev. 1999;106:283–296. doi: 10.1016/s0047-6374(98)00121-3. [DOI] [PubMed] [Google Scholar]
- [25].Luttik MAH, Overkamp KM, Kötter P, de Vries S, van Dijken JP, Pronk JT. The Saccharomyces cerevisiaeNDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J Biol Chem. 1998;273:24529–24534. doi: 10.1074/jbc.273.38.24529. [DOI] [PubMed] [Google Scholar]
- [26].de Vries S, Grivell LA. Purification and characterization of a rotenone-insensitive NADH:Q6 oxidoreductase from mitochondria of Saccharomyces cerevisiae. Eur J Biochem. 1988;176:377–384. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]
- [27].Tuller G, Nemec T, Hrastnik C, Daum G. Lipid composition of subcellular membranes of an FY 1679-derived haploid yeast wild-type strain grown on different carbon sources. Yeast. 1999;15:1555–1564. doi: 10.1002/(SICI)1097-0061(199910)15:14<1555::AID-YEA479>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [28].Avéret N, Fitton V, Bunoust O, Rigoulet M, Guérin B. Yeast mitochondrial metabolism: from in vitro to in situ quantitative study. Mol Cell Biochem. 1998;184:67–79. [PubMed] [Google Scholar]
- [29].Guérin B, Labbe P, Somlo M. Preparation of yeast mitochondria (Saccharomyces cerevisiae) with good P/O and respiratory control ratios. Methods Enzymol. 1979;55:149–159. doi: 10.1016/0076-6879(79)55021-6. [DOI] [PubMed] [Google Scholar]
- [30].Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- [31].Thakar JH, Hassan MN. Effects of 1-methyl-4phenyl-1, 2, 3, 6- tetrahydropyridine (MTPT), cyperquat (MPP+) and paraquat on isolated mitochondria from rat stratium cortex and liver. Life Sci. 1988;43:143–149. doi: 10.1016/0024-3205(88)90291-3. [DOI] [PubMed] [Google Scholar]
- [32].González-Hernández JC, Aguilera-Aguirre L, Pérez-Vázquez V, Ramírez J, Clemente-Guerrero M, Cortés-Rojo C, Saavedra-Molina A. Effect of D-amino acids on some mitochondrial functions in rat liver. Amino Acids. 2003;24:163–169. doi: 10.1007/s00726-002-0317-5. [DOI] [PubMed] [Google Scholar]
- [33].Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–765. [PubMed] [Google Scholar]
- [34].Uribe S, Ramírez J, Peña A. Effects of β-pinene on yeast membrane functions. J Bact. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hallberg EM, Shu Y, Hallberg RL. Loss of mitochondrial Hsp60 function: non equivalent effect on matrix-targeted and intermembrane targeted proteins. Mol Cell Biol. 1993;13:3050–3057. doi: 10.1128/mcb.13.5.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- [37].Yoshida T, Fee JA. Studies on cytochrome c oxidase activity of the cytochrome c1aa3 complex from Thermus thermophilus. J Biol Chem. 1984;259:1031–1036. [PubMed] [Google Scholar]
- [38].Halliwell B, Gutteridge JMC. The chemistry of free radicals and related reactive species. In: Halliwell B, Gutteridge JMC, editors. Free radicals in biology and medicine. 3rd ed. Oxford University Press; Oxford: 1999. pp. 36–104. [Google Scholar]
- [39].Barber DJW, Thomas JK. Reactions of radicals with lecithin bilayers. Radiat Res. 1978;74:51–65. [Google Scholar]
- [40].Nathan C, Nogueira N, Juangbhanich C, Ellis J, Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979;149:1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Costa VMV, Amorim MA, Quintanilha A, Moraidas-Ferreira P. Hydrogen peroxide-induced carbonylation of key metabolic enzymes in Saccharomyces cerevisiae: the involvement of the oxidative stress response regulators Yap1 and Skn7. Free Radic Biol Med. 2002;33:1507–1515. doi: 10.1016/s0891-5849(02)01086-9. [DOI] [PubMed] [Google Scholar]
- [42].Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- [43].Esposti ME, Ghelli A, Butler G, Roberti M, Mustich A, Cantatore P. The cytochrome b of the sea urchin Paracentrotus lividus is naturally resistant to myxothiazol and mucidin. FEBS Lett. 1990;263:245–247. doi: 10.1016/0014-5793(90)81384-z. [DOI] [PubMed] [Google Scholar]
- [44].Thierbach G, Michaelis G. Mitochondrial and nuclear myxothiazol resistance in Saccharomyces cerevisiae. Mol Gen Genet. 1982;186:501–506. doi: 10.1007/BF00337956. [DOI] [PubMed] [Google Scholar]
- [45].Kessl JJ, Lange BB, Merbitz-Zahradnik T, Zwicker K, Hill P, Meunier B, Palsdottir H, Hunte C, Meshnick S, Trumpower BL. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J Biol Chem. 2003;278:31312–31318. doi: 10.1074/jbc.M304042200. [DOI] [PubMed] [Google Scholar]
- [46].Muller F, Crofts AR, Kramer DM. Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry. 2002;41:7866–7874. doi: 10.1021/bi025581e. [DOI] [PubMed] [Google Scholar]
- [47].Machida K, Tanaka T, Fujita K-I, Taniguchi M. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J Bact. 1998;180:4460–4465. doi: 10.1128/jb.180.17.4460-4465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]