Abstract
Objective
To study the incremental value of the ABCD2 score in predicting short term risk of ischemic stroke following thorough ED evaluation of transient ischemic attack (TIA).
Methods
This was a prospective observational study of consecutive patients presenting to the Emergency Department (ED) with a TIA. Patient underwent a full ED evaluation including CNS and carotid artery imaging, after which ABCD2 scores and risk category were assigned. We evaluated correlations between risk categories and occurrence of subsequent ischemic stroke at 7 and 90 days.
Results
The cohort consisted of 637 patients, (47% women, mean age 73, SD 13 years). There were 15 strokes within 90 days following the index TIA. At 7-days, the rate of stroke according to ABCD2 category in our cohort was: 1.1% in the low risk group, 0.3% in the intermediate risk group, and 2.7% in the high risk group. At 90-days, the rate of stroke in our ED cohort was: 2.1% in the low risk group, 2.1% in the intermediate risk group, and 3.6% in the high risk group. There was no relationship between ABCD2 score at presentation and subsequent stroke following TIA at 7 or 90 days.
Conclusion
The ABCD2 score did not add incremental value beyond an ED workup that includes CNS and carotid artery imaging in the ability to risk stratify patients with TIA in our cohort. Practice approaches that include brain and carotid artery imaging do not benefit by the incremental addition of the ABCD2 score. In this population of TIA patients, selected by emergency physicians for a rapid ED-based outpatient protocol that included early carotid imaging and treatment when appropriate, the rate of stroke was independent of ABCD2 stratification.
Introduction
Background
Transient ischemic attack (TIA) is an important warning sign of stroke, with 12–20% of all ischemic strokes heralded by a TIA1. Stroke is the number one cause of adult disability and the 3rd leading cause of death in North America2. Resource utilization and cost containment have become important considerations in clinical practice. Identifying safe yet cost effective care pathways for those with a TIA is important. In addition, there is an increasing geriatric population globally3 with the incidence and prevalence of stroke and TIA continuing to rise. Given these three parameters, clinical scores are of interest as they theoretically allow for risk stratification of patients, which is key to managing the balance between patient safety versus unduly burdensome hospitalization.
Importance
Given these three parameters, clinical scores are of interest as they theoretically allow for risk stratification of patients, which is key to managing the balance between patient safety versus unduly burdensome hospitalization. One clinical score that has recently been proposed for TIA risk stratification is the ABCD24.
Goals of the investigation
We have a robust system to evaluate TIAs in our institution, which includes brain and carotid imaging. The goal of this investigation was to determine whether the incorporation of the ABCD2 score would add incremental value to our institutional protocol in terms of risk stratification.
Methods
Study design
This is a secondary study to our Emergency Department TIA registry which is a prospective observational study of consecutive adult patients presenting to our Emergency Department with a transient ischemic attack (TIA). The secondary study involved the addition to the prospective dataset of the ABCD2 score, calculated retrospectively by trained research fellows who were blinded to patient outcome. Reporting of methods and results follow the STROBE guidelines5. STROBE provides general reporting recommendations for descriptive observational studies and studies that investigate associations between exposures and health outcomes. Patients provided written informed consent and the authors’ institutional review board approved this study.
Setting
The study was conducted in the emergency department of an academic medical center in the United States that sees 79,000 ED visits per year. The study dates were from December 2001 to December 2006. The cohort included all consecutive patients with TIA. All patients were prospectively followed up via scripted telephone interview and review of medical records for a minimum of 90 days. Follow up was achieved in 95% of the cohort, and those lost to follow up were characterized.
Selection of Participants
All patients over age 18 who had a TIA were included. A TIA was defined as per the World Health Organization (WHO) criteria6 as rapidly developed clinical signs of focal or global disturbance of cerebral function lasting fewer than 24 h, with no apparent nonvascular cause. The inclusion criteria consisted of: patients aged 18 years and older who present with symptoms suggestive of TIA; asymptomatic at the time of ED evaluation; and head CT negative for mass, bleed, or shift. The exclusion criteria were 1) patients with symptoms lasting longer than 24 h or 2) patients with acute ischemic or hemorrhagic stroke. The definition of ischemic stroke was according to the American Heart Association/American Stroke Association, as the presence of neurological symptoms persisting for more than 24 hours. All patients underwent our institution’s standard ED Observation Unit (EDOU) protocol for TIA. This protocol has been published in detail elsewhere 7. As part of this protocol, all patients undergo the same initial evaluation, including a head CT, electrocardiogram, laboratory tests, carotid Doppler study. The final determination of TIA was made by an experienced emergency medicine attending in consultation with a resident neurologist as needed.
Study size
As this is an observational study to examine the performance of a clinical scale vis a vis actual outcomes, no a priori sample size determinations were made.
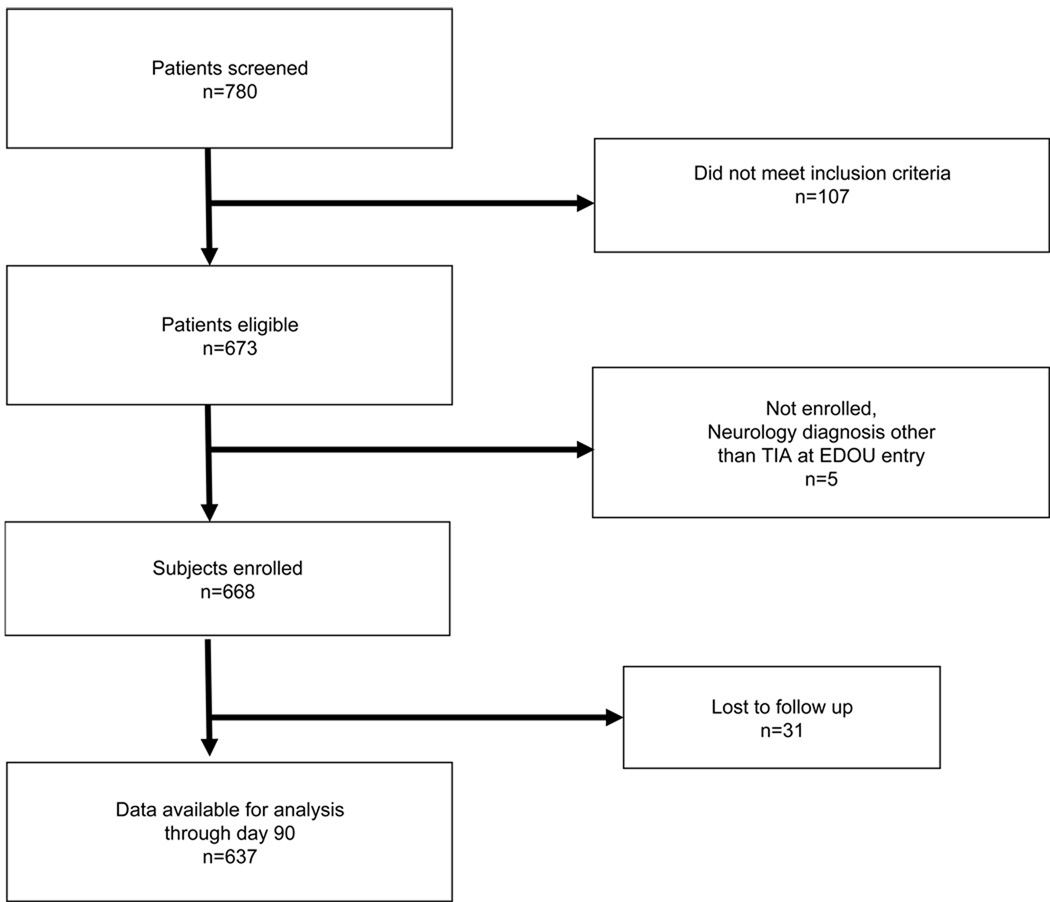
Out of a total of 673 eligible for the study, 668 were considered possible TIA by the ED physician and entered into the protocol. (See Figure 1). 31 patients did not have 90-day follow up and were excluded from the study.
Figure 1.
STROBE flow diagram
Methods of measurement
The independent variable was the calculated ABCD2 score at presentation. The dependent variables were the risk of stroke at 7 days and 90 days. These time points were chosen to be the same as those of the original ABCD2 study.
Data collection and processing
The data was collected by the research team and the ABCD2 score was calculated from the medical records. ABCD2 scores and risk category were assigned to each patient based on published criteria. The score is composed of:
Age ≥ 60years (1 point);
Blood pressure: systolic >= 140mmHg or diastolic > 90mmHg (1 point);
Clinical features: unilateral weakness (2 points) or isolated speech disturbance (1 point);
Duration of symptoms: >= 60 min. (2 points), 10–59 min. (1 point), or < 10 min. (0 points);
Diabetes (present = 1 point).
The ABCD2 score was classified as low risk (0–3 points), intermediate risk (4–5 points), and high risk (6–7 points) according to the original study4.
Primary data analysis & Statistical methods
Statistical analyses were performed in JMP 7.0, SAS institute. Contingency tables were used for binary and ordinal variables. Parametric tests were used for normally distributed variables including the ABCD2 score, and age. The actual risk of subsequent stroke in our cohort and the ABCD2 ED cohorts were plotted in simple linear fashion to assess for correlation between ABCD2 risk categories and occurrence of subsequent stroke at 7 and 90 days. Although the actual ABCD2 score was computed retrospectively, the components of the score were prospectively collected as part of the described data set.
Results
The final study cohort consisted of 637 patients, of which 47% were women. The mean age was 73.0 years, with a standard deviation of 13.3 years. The ninety day follow up rate was 95.4%. The patients lost to follow up were younger (mean age 67 years versus 73 years), same gender distribution, had lower ABCD2 scores and were less likely to be admitted to the hospital. There were a total of 15 ischemic strokes occurring within 90 days following the index TIA. The demographics of patients who had stroke versus those that did not are summarized in table 1. Table 2 summarizes the distribution of stroke events at 7 and 90 days, by ABCD2 scores. Table 3 summarizes the actual number of patients with each of the variables within the ABCD2 score.
Table 1.
Demographic characteristics by occurrence of ischemic stroke at 90 days.
| No Stroke N (%) Total n= 622 |
Stroke N (%) Total n= 15 |
Difference, (95% CI) |
|
|---|---|---|---|
| Age, years, mean SD) | 73.0 (13.3) | 74 (14) | 1 (−8, 6) |
| Females, N, % | 293 (47.1) | 5 (33) | 14% (−0.1, 0.3) |
| Prior TIA | 111 (17.8) | 3 (20) | 2% (−0.1, 0.3) |
| Prior ischemic stroke | 114 (18.3) | 2 (13) | 5% (−0.2, 0,2) |
| Hypertension | 419 (67.3) | 10 (68) | 1% (−0.2, 0.3) |
| Diabetes mellitus | 119 (19.1) | 1 (7) | 12% (−0.1, 0.2) |
| Prior atrial fibrillation | 103 (16.5) | 15 (100) | 83% (0.6, 0.9) |
Table 2.
Distribution of stroke events at 7 and 90 days, by ABCD2 scores
| ABCD2 score | 7 days | stroke event rate |
90 days | stroke event rate |
||
|---|---|---|---|---|---|---|
| Stroke N=6 |
No stroke N=631 |
0.94 | Stroke N=15 |
No stroke N=622 |
2.35 | |
| Low (0–3) | 2 | 187 | 1.06 | 4 | 185 | 2.12 |
| Intermediate (4–5) | 1 | 335 | 0.30 | 7 | 329 | 2.08 |
| High (6–7) | 3 | 109 | 2.68 | 4 | 108 | 3.57 |
Table 3.
Actual number and percentages of patients with each of the variables within the ABCD2 score.
| Low (0–3) n=189 |
Intermediate(4–5) n=336 |
High (6–7) n=112 |
|
|---|---|---|---|
| A (Age); 1 point for age >60 years |
135 (71%) |
299 (89%) |
107 (96%) |
| B (Blood pressure > 140/90 mmHg); 1 point for hypertension at the acute evaluation |
100 (53%) |
249 (74%) |
96 (86%) |
| C (Clinical features); 2 points for unilateral weakness, 1 for speech disturbance without weakness |
22 (2 points) (12%) 50 (1 point) (26%) |
163 (2 points) (49%) 121 (1 point) (36%) |
106 (2 points) (95%) 6(1 point) (5%) |
| D (symptom Duration); 1 point for 10–59 minutes, 2 points for >60 minutes |
25 (2 points) (13%) 73 (1 point) (39%) |
152 (2 points) (45%) 138 (1 point) (41%) |
100 (2 points) (89%) 12 (1 point) (11%) |
| D (Diabetes); 1 point | 20 (11%) |
67 (20%) |
32 (29%) |
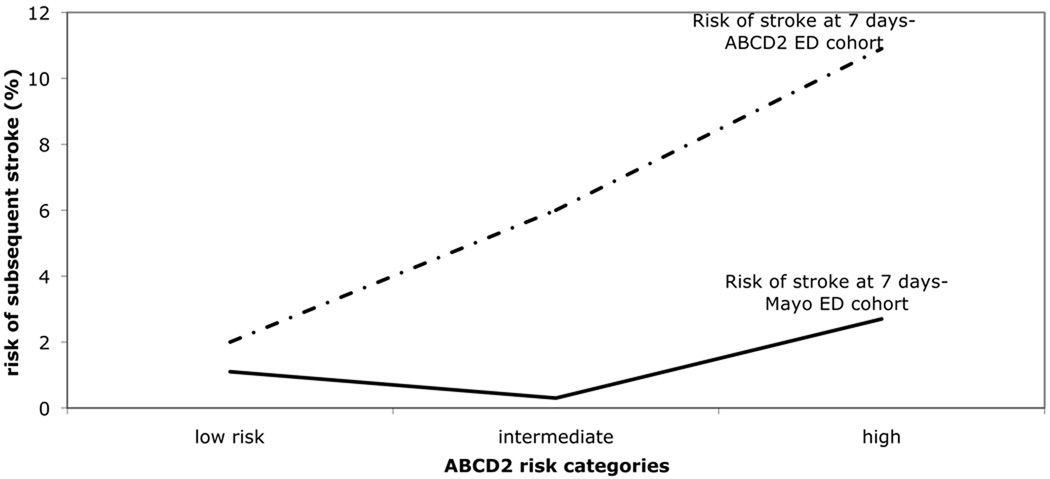
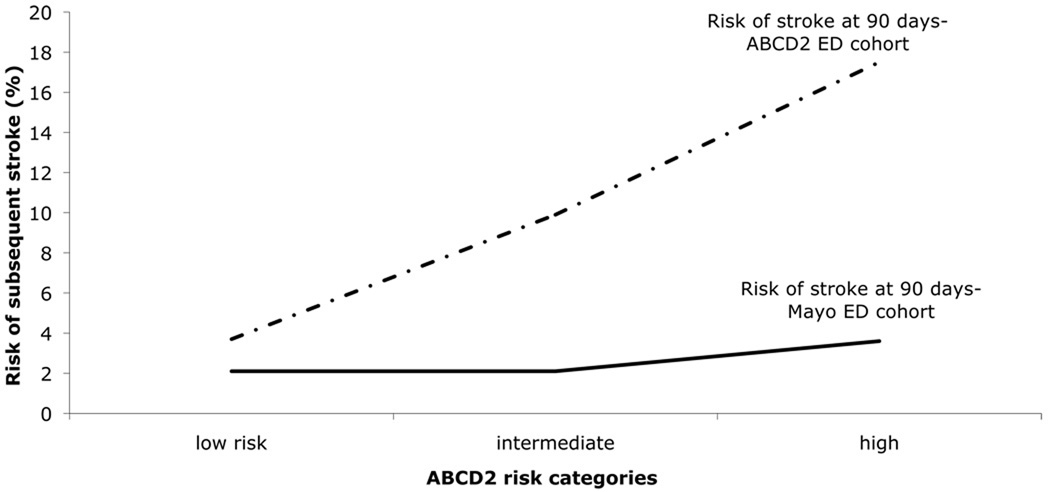
The incidence of short term risk of ischemic stroke according to ABCD2 score is summarized in figure 3. At 7-days, the rates of stroke according to ABCD2 category in the ABCD2 ED original cohort3 and our ED cohorts respectively were: 2.0% and 1.1% (95% CI 0.29–3.78) in the low risk group, 6.0% and 0.3% (95% CI 0.05–1.67) in the intermediate risk group, and 10.9 and 2.7% (95% CI 0.92–7.60) in the high risk group. At 90-days, the rates of stroke according to ABCD2 category in the ABCD2 ED and our ED cohorts respectively were: 3.7% and 2.1% in the low risk group, 9.9% and 2.1% in the intermediate risk group, and 17.5% and 3.6% in the high risk group. In other words, in the ED patients with a full TIA workup, there was no statistically significant relationship between ABCD2 score at presentation and subsequent stroke following TIA at 7 or 90 days in the current cohort.
Figure 3.
Risk of stroke at 7 and 90 days in ABCD2 ED and our ED cohorts
Limitations
Limitations include ascertainment bias secondary to the very nature of a TIA and how it is diagnosed. This ascertainment bias is somewhat reduced by the fact that patients in our cohort, by being in an academic medical center, underwent evaluation by multiple providers, including a medical student, resident, staff physician and consult neurologist before the final diagnosis was made. Another limitation is the external validity of our study; our cohort was predominantly Caucasian reflecting the demographic make-up of our area. Finally, there is now a new definition of TIA8, which is tissue based rather than time based, and reads “a brief episode of neurological dysfunction caused by focal brain or retinal ischemia, with clinical symptoms typically lasting less than one hour, and without evidence of acute infarction.”
Discussion
The results of the current study demonstrate that an ED TIA workup, which includes brain and carotid imaging, is an appropriate initial diagnostic strategy; when performed in conjunction with the initiation of proper prevention strategies (i.e., antithrombotics) does an excellent job of risk stratifying patients. ABCD2 score did not add any additional value in predicting stroke risk in our cohort. Similar non-correlation is noted both at 7 and 90 days. This lack of correlation is also noted in recent study9 of the North Dublin TIA registry, in which the authors suggest caution in using a historical risk stratification tool such as the ABCD2, supporting the argument that the clinical ABCD2 score may not be appropriate to use in ED’s, which have the ability to provide a full TIA workup.
The strengths of the current study are that it was a consecutive cohort and the researchers were not involved in the care provided to the patients, which should decrease measurement and selection biases. The researchers calculating the scores were blind to the outcome of the patient. Another strength is Mayo Clinic’s catchment area (Olmsted and the nine surrounding counties) and consequently our very small lost to follow up rate (5%) allowing us to calculate incidence of ischemic stroke in this cohort. We have characterized the cohort lost to follow up, and found them to be younger, have lower ABCD2 scores, and less likely to be admitted to the hospital, which suggests they would not contradict our current findings. Random and systematic errors were reduced by enhancing the precision and accuracy of the variables collected via the use of a standardized measurement method, and pre-designed data abstractions forms. Finally, the ABCD2 score was calculated based on the medical records of a prospectively enrolled consecutive TIA cohort, similar to the original Johnston study.
The overall risk of subsequent stroke is significantly lower in our ED cohort than in the ABCD2 ED cohort. This is despite similar reported population based stroke incidence in the Oxfordshire and Rochester communities10,11. One explanation may be that our emergency physicians used a low threshold for making the diagnosis of TIA for this EDOU pathway, and that as a result our event rate was low across the board. This is potentially important because it implies that the ABDC2 will not stratify risk well when applied to all ED comers with a possible TIA. However, we postulate that reason for this lower risk of stroke may be due to our practice model7 for the evaluation of patients with suspected TIA in the ED. Briefly, all patients who present to our ED with a history consistent with TIA undergo laboratory testing, computed tomography, electrocardiography and carotid Doppler imaging. They are evaluated by a senior neurology resident, given aspirin or an alternative antithrombotic agent, and provided with education regarding warning signs for stroke. Patients with high grade carotid stenoses or new onset atrial fibrillation are hospitalized for urgent intervention. If laboratory and imaging testing does not suggest that the patient requires emergent hospitalization, then patients are discharged to home with neurology follow up in an outpatient TIA Clinic within 72 hours. In essence, the nature of our ED observation unit TIA protocol allows for real time identification and treatment of high risk patients, which ultimately result in a lower subsequent stroke risk. Our low stroke rate mirrors the experience of others’ where TIA is treated as an emergency, as noted in a systematic review and metaanalysis on the topic13. The SOS-TIA study14 and the EXPRESS study15 from the United Kingdom reports similar study findings in outpatient TIA clinics with 24 hour access, where the rapid access to daily TIA clinic and administration of an antithrombotic agents was provided to all patients with suspected brain ischemia. SOS-TIA reported 90-day stroke rate of 1.24% (95% CI 0.72–2.12), and EXPRESS study reported stroke risk of 2.1%, whereas the rate predicted from ABCD2 scores was 5.96%.
A few institutions have developed protocols where the entire initial evaluation for TIA is performed in the ED, rather than setting up special outpatient TIA clinics. 16, 17. The reported 90 day stroke risks from these studies are 1.3% and 3.3% respectively. Although further studies may be helpful to assess our findings in different patient populations and care systems, we believe that this study’s results are compatible with earlier research on the derivation and validation of the ABCD2 score. The ABCD2 has merit in countries and care system settings where there are limited resources, and definitive imaging studies are not readily available in the clinic or ED. In these settings, the ABCD2 may help to risk stratify patients to determine the urgency of definitive work up.
Fortunately for most patients in the United States, most ED’s have the ability to obtain both brain and carotid artery imaging. In this setting, the definitive work up is being provided in the acute setting, and the results of this testing provide decision making guidance beyond that of a prediction tool, as one would expect. Thus, it appears that a full ED work up of TIA patients is the preferred approach, and that in this setting, the ABCD2 score does not provide incremental value.
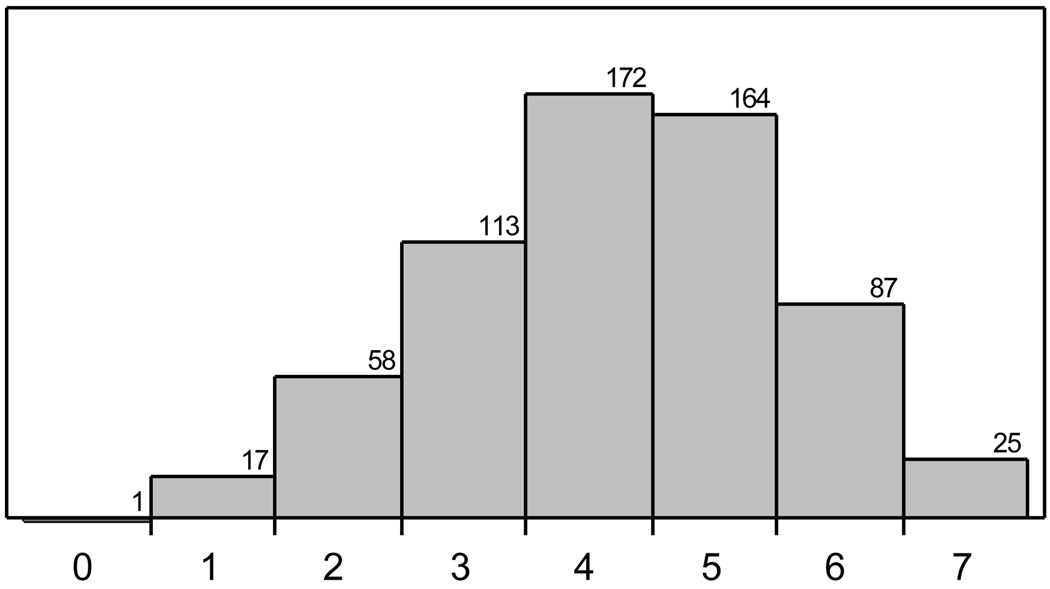
Figure 2.
Distribution of ABCD2 score
Acknowledgements
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Dr. Stead is supported through a Mayo Foundation Emergency Medicine Research Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hackam DG, Kapral MK, Wang JT, et al. Most stroke patients do not get a warning: A population-based cohort study. Neurology. 2009;73:1074–1076. doi: 10.1212/WNL.0b013e3181b9c8e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Arias E. National Vital Statistics Reports. No 6. Vol 53. Hyattsville, MD: National Center for Health Statistics; 2004. United States Life Tables, 2002. [PubMed] [Google Scholar]
- 4.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 7.Stead LG, Bellolio MF, Suravaram S, et al. Evaluation of Transient Ischemic Attack in an Emergency Department Observation Unit. Neurocrit Care. 2009;10(2):204–208. doi: 10.1007/s12028-008-9146-z. [DOI] [PubMed] [Google Scholar]
- 8.Easton DL, Albers GW, Alberts GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. Epub 2009 May 7. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of the ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack. The North Dublin TIA study. Stroke. 2010 March 18;41 doi: 10.1161/STROKEAHA.109.571844. 2010; epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Sciolla R, Meis F. Rapid identification of high risk transient ischemic attacks. Prospective validation of the ABCD score. Stroke. 2008;39:297–302. doi: 10.1161/STROKEAHA.107.496612. [DOI] [PubMed] [Google Scholar]
- 11.Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21(6):848–853. doi: 10.1161/01.str.21.6.848. [DOI] [PubMed] [Google Scholar]
- 12.Stead LG, Bellolio MF, Suravaram S, et al. Evaluation of Transient Ischemic Attack in an Emergency Department Observation Unit. Neurocrit Care. 2009;10(2):204–208. doi: 10.1007/s12028-008-9146-z. [DOI] [PubMed] [Google Scholar]
- 13.Giles MF, Rothwell PM. Risk of early stroke after transient ischemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 14.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Giles MF, Chandrateva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 16.Ross MA, Compton S, Medado P, et al. An emergency department diagnostic protocol for patients with transient ischemic attack: a randomized controlled trial. Ann Emerg Med. 2007;50:109–119. doi: 10.1016/j.annemergmed.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Brown MD, Reeves MJ, Glynn T, et al. Implementation of an emergency department based transient Ischemic attack clinical pathway: a pilot study in knowledge translation. Acad Emerg Med. 2007;14(11):1114–1119. doi: 10.1197/j.aem.2007.04.019. [DOI] [PubMed] [Google Scholar]