Abstract
Exercise provides a number of important benefits after spinal cord injury in clinical studies and animal models. However, the amount of functional improvement in overground locomotion obtained with exercise alone has been limited thus far, for reasons that are still poorly understood. One hypothesis is that the complex network of endogenous extracellular matrix components, including chondroitin sulfate proteoglycans (CSPGs), can inhibit exercise-induced remodeling and limit plasticity of spared circuitry in the adult central nervous system. Recent animal studies have shown that chondroitinase ABC (ChABC) can enhance plasticity in the adult nervous system by cleaving glycosaminoglycan sidechains from CSPGs. In this article we review the current literature on plasticity observed with locomotor training and following degradation of CSPGs with ChABC and then present a rationale for the use of exercise combined with ChABC to promote functional recovery after spinal cord injury. We also present results of a preliminary study that tested the simplest approach for combining these treatments; use of a single intraparenchymal injection of ChABC administered to the lumbar enlargement of mice with voluntary wheel running exercise after a mid-thoracic spinal contusion injury. The results are negative, yet serve to highlight limitations in our understanding of the most effective protocols for combining these approaches. Further work is directed to identify the timing, type, and quantity of exercise and pharmacological interventions that can be used to maximize functional improvements by strengthening appropriate synaptic connections.
Keywords: locomotion, exercise, treadmill training, rehabilitation, contusion, chondroitin sulfate proteoglycans, perineuronal net, plasticity
Introduction
Limited recovery after spinal cord injury and current experimental repair approaches
The current prognosis for improvement in functional sensory and motor recovery after sustaining a spinal cord injury (SCI) remains relatively poor, despite recent marked advances in medical care and rehabilitation [1]. A wide range of promising therapies directed at preventing secondary injury and repairing the spinal cord at the site of injury have been shown to exert improvements in neuroprotection, regeneration, and/or recovery of function in animal models of SCI (rev. in [2–6]). These include single therapies, such as cell transplantation [7–10], neurotrophic factor infusion or overexpression [11–13], anti-inflammatory therapies [14–20] or administration of anti-growth inhibitory antibodies [21–23]. More dramatic improvements are observed with approaches combining two or more of these treatments [24–28], indicating that multiple mechanisms contribute to the lack of endogenous regeneration and subsequent failure of complete functional recovery. To date, however, none of these interventions or combinations has proven to be so robust and reproducible across laboratories, injury models, and animal species to provide a compelling consensus for uncontested translation to the clinical setting [29–34]. Thus, the SCI research community continues to search for effective and feasible therapeutic strategies that can be advanced to promote improved recovery of the injured adult spinal cord.
In addition to the strategies referred to above that seek to prevent secondary injury or repair the spinal cord at the site of injury, considerable recent research has been directed at understanding how the circuitry in the uninjured or “spared” segments of the spinal cord can be activated to permit reorganization or plasticity that might drive meaningful functional recovery [3;35–37]. Exercise, including locomotor training, can stimulate neural activity in appropriate muscle groups and neurological centers and can easily be included with other repair strategies to improve prognosis following SCI. However, the limited efficacy of exercise therapy alone in translation to overground walking after severe contusion injury suggests that characteristics of the mature central nervous system (CNS) contribute to an environment that is refractory to activity directed plasticity. Interventions that combine enhanced activity of appropriate circuitry with treatments that can modify the structure and efficacy of the synapses in the injured CNS show great promise for optimizing the extent and nature of functional recovery after injury. In the following pages, we describe the evidence implicating a combination of locomotor training and chondroitinase enzymatic treatment for improving functional outcomes after SCI. A review of the evidence supporting this combination and preliminary work from our lab show that further investigation is needed to define the timing, type, and amount of exercise training and pharmacological interventions that could interact or synergize to enhance useful functional recovery.
Effects of exercise on health and recovery of function after SCI
Clinical studies
Activity-based strategies seek to promote neuroplasticity and greater recovery than that induced by traditional approaches in which technology or intact body regions compensate for permanent impairments [38]. In recent years, activity based functional electrical stimulation (FES) and locomotor based exercise therapies have both been shown to induce measurable improvements in cardiovascular function [39], muscle mass and bone density [40], and improvements in reported quality of life measures [41;42]. Individual case studies also demonstrate that some SCI individuals can gain measurable sensory and/or motor function following these types of exercise based therapy even several years after injury. However, the extent of improvement is highly variable, and small effects may be compounded by the unknown role of spontaneous recovery that may not be directly mediated by the exercise paradigm [38;43–45].
For SCI individuals with cervical and thoracic injuries, treadmill training in conjunction with partial body weight support (body weight supported treadmill training, or BWSTT) has shown enhanced benefit in a number of outcomes. Similar to findings with other forms of locomotor exercise, BWSTT has been shown to induce increased muscle mass [46], and improve cardiovascular risk factors [47]. However, improvements in locomotion induced by locomotion therapy and treadmill training remain inconsistent. While treadmill performance has not yet translated to recovery of overground locomotion for people with complete SCI, studies on motor incomplete SCI show gains in gait speed, endurance and step quality [45;48–52]. In contrast, in the only balanced, prospective multicenter clinical trial to date, manually-assisted locomotor treadmill training promoted improved balance and recovery in overground assessments, but the BWSTT did not lead to better recovery than found with standard mobility training alone [44;53]. Importantly, the measurable improvements in walking speed for patients with incomplete SCI (ASIA C and D subjects) was seen in both treatment groups, indicating that locomotor activity-based exercise indeed exerts some benefits. Nevertheless, the functional improvements and changes in independence and community participation incurred through locomotor therapy alone remain limited.
The varied effects of locomotor-based rehabilitation in the clinic reflect the wide range of results obtained in locomotor training studies in animal models of SCI. Early work by Edgerton, Rossignol and others first showed that cats with complete mid-thoracic spinal cord transection could be trained to walk on a moving treadmill [54;55]. By providing initial weight support for the trunk and occasionally pinching the base of the tail for added afferent stimulation, activity measured by electromyography (EMG) recordings revealed activation of the appropriate muscle groups to produce kinematic patterns of swing and stance that were very close to those of intact animals. The injured cats were even able to adapt these movements to different treadmill speeds and after several weeks of training, they were able to step with full weight bearing, although trunk stability was poor. These studies demonstrated clearly that the isolated adult spinal cord below the level of a complete transection contains locomotor circuitry driven by coordinated central pattern generators (CPGs).
Subsequent research demonstrated that similar locomotor patterns can be induced in other species after complete spinal transection, including rats [56] and mice [57]. Furthermore, similar patterns are reflected in the clinical recordings of patients with complete SCI during treadmill training therapy [58;59]. Thus, in addition to preventing muscle atrophy and promoting general health benefits, treadmill training holds promise for enhancing locomotor recovery after SCI by targeting functional substrates for locomotion that remain intact below the level of a complete transection.
Despite the evidence of intact locomotor CPGs in the isolated lumbar spinal cord, the animal studies indicate that treadmill training alone is insufficient to support overground locomotion following complete transection in adult rats or cats [54;60;61]. Zhang, et al. showed that rats with BWSTT, 5 days/week, starting at 5 days post-injury recovered greater hindlimb mobility at 30–45 days after transection (average BBB scores of 6, corresponding to slight to extensive movements of the three joints of the hindlimbs), than rats that did not have training (average BBB scores of 1.9–2.1, or slight hindlimb movements of one to two joints). This finding does not reflect specific locomotor movements and suggests only that treadmill training enhances excitability of the motoneuron pools. Interestingly, the authors noted anecdotally that the rats with the greatest hindlimb movements in the open field also had increased withdrawal reflex measures, suggesting that the effects of training may be simply reflect increased segmental or reflex excitability in these animals [62].
In an effort to enhance the effects of locomotor training and restore the neuromodulatory function of lost descending influence, Rossignol combined treadmill training with pharmacological interventions and showed that the kinematic patterns of hindlimb stepping could be improved in cats [63;64] and humans [65] with the addition of monoamines including serotonin, dopamine and noradrenaline agonists. Others have confirmed these results in rats transected as neonates [60] or adults [66–68]. Courtine et al. recently extended this work to show that rats with a complete transection who are treated with a combination of intrathecal monoamine stimulants, epidural electrical stimulation, and BWSTT using robotic locomotor assistance, can recover significantly better stepping patterns than those with treadmill training alone [69]. Indeed, rats with the combined treatment could even develop the ability to support their full weight in the experimental setting, a function that has not been achieved previously for rats injured as adults. Notably, the patterns of hindlimb joint excursions in the combined treatment group were very similar to those of uninjured rats, while rats that received single therapies (pharmacological stimulation or epidural stimulation only) had more irregular excursion patterns and less effective dorsiflexion, leading to more toe drags. This finding sheds a new and promising light on the capacity of the isolated spinal cord for functional recovery with training, while also emphasizing the complexity of strategies that may be required to reach this potential following injury in adult subjects. Thus, the monoamine and epidural stimulation treatments together provide a suitable condition of neuronal activation or tone that is ineffective when given alone alone, but can allow the segments below the transection level to subsequently respond to the afferent stimulation provided by the treadmill training paradigm.
Exercise therapy in animal models of incomplete SCI
Unlike the complete SCI condition, for animals and patients with incomplete SCI, treadmill training has been proposed to improve recovery by facilitating appropriate activation of the local circuitry, which has retained some ascending and descending communication with supraspinal centers [70]. After partial thoracic injuries, rats and mice show notable spontaneous recovery of hindlimb function depending on the severity of the injury, ranging from partial plantar placement to consistent weight supported stepping with a range of hindpaw and trunk deficits [71;72]. The degree of recovery seen with forced exercise after partial injury has been highly variable. In an early such study, Fouad et al. found no beneficial effect of treadmill training on locomotor recovery after a dorsal hemisection lesion in rats [73]. In contrast, Multon et al. found that daily treadmill training with manual assistance, started one day after a moderate balloon compression injury in adult rats, resulted in significant improvement in locomotor function [74]. These effects were found as early as 2 weeks post-injury and from 4–12 weeks after injury the rats that had treadmill training were able to support their body weight with occasional stepping, while those without training were not. However, two additional studies did not show improved recovery of overground locomotion by treadmill training or combined stand and treadmill training after severe thoracic contusion injury in rats [75;76]. In addition, treadmill training induced sprouting of axons at the site injury, but did not improve functional outcomes on a forelimb task in a cervical contusion injury model [77]. Thus, the ability of rats to respond to treadmill training is dependent in part on lesion severity and in part on the spinal level of the injury. Interestingly, in the negative study by Ichiyama, et al. [76], additional groups of rats with severe contusion were treated by irradiation at the lesion site, which alters the inflammatory reaction. In those groups that received the irradiation treatment, a combined treadmill and stand training paradigm facilitated functional recovery parameters of both standing and walking compared with untrained rats with irradiation alone. Thus, in addition to effects on circuitry associated directly with the CPGs or lumbar spinal cord, some of the effects of locomotor treadmill training with different lesion severities may reflect the effects of cellular reactivity and signaling cascades initiated at the site of injury.
A number of other variables may also contribute to different effects in locomotor recovery following exercise or locomotor training. Edgerton's group has shown that the extent of locomotor recovery achieved with treadmill training is also dependent upon the quantity of training, or the total number of steps executed during training sessions [78]. However, it is not known if there is an upper limit to the amount of training that is beneficial. In addition, little is known about the impact of the timing of the start of locomotor training relative to injury, the duration or “dose” of individual training sessions, or the use of a variety of training tasks. Smith, et al. [79] have recently shown that if swim training is started too early after injury, the activity can induce a prolonged opening of the blood-spinal cord barrier, leading to exacerbation of pathology and worse functional recovery than no training at all.
The previous work also raises the possibility that differences in the role of locomotor exercise on recovery after incomplete injury may be attributed to whether the activity is forced, as with treadmill training, swim training or automated running wheels, or voluntary, where the individual or animal participates in exercise as self-selected activity. Indeed, recent studies by Engesser-Cesar et al. demonstrated that voluntary wheel running can provide improvements in functional recovery in rat and mouse models of contusive SCI [80–82]. This approach takes advantage of the innate motivation of rodents to sustain prolonged periods of locomotion when given unlimited access to a wheel. However, the positive effects of voluntary wheel running after SCI have not been universal [83]. Post-injury exposure to running wheels was sufficient to induce a significant trend in improved recovery that was linearly related to the number of days per week of access to wheels. However, the final locomotor recovery scores between the 0, 3, and 7 day/week groups were small and did not reach statistical significance using a single endpoint ANOVA. Taken together, the limited positive effects and the lack of consistency between labs suggests that even voluntary running, which can go for anywhere from a few minutes to several hours each day, is only modestly effective for improving overground locomotion after partial SCI in adult animals.
Cellular substrates of plasticity and reorganization mediated by locomotor training
Neural plasticity can be mediated by biochemical changes affecting synaptic efficacy or neural excitability and by structural changes such as axon sprouting or synaptic reorganization. Exercise enhances expression of mRNA and protein levels of growth factors in both the brain and spinal cord, including brain derived growth factor (BDNF), neurotrophin-3, fibroblast growth factor-2 (FGF-2) and insulin-like growth factor (IGF-1) ([75;84]; rev. in [85]). Each of these growth factors represents a potent player mediating plasticity of the central nervous system, with critical roles in regulating synaptic efficacy, growth cone extension, progenitor cell proliferation, and differentiation. Considerable evidence points especially to a critical role of BDNF in exercise-mediated improvements in locomotion and stepping after SCI. Gomez-Pinilla et al. first showed that just 3 days of voluntary wheel running increased levels of BDNF, its receptor trkB, and downstream effectors including phosphorylation of synapsin I and expression of cyclic AMP response element-binding protein (CREB) in the intact rat spinal cord [86]. This group then showed that hemisection injury causes a reduction in BDNF caudal to the site of the lesion, an effect that was restored by voluntary wheel running [84]. To confirm a role of BDNF in motor recovery in this model, they then infused a truncated BDNF receptor IgG fusion protein (trkB-IgG) as an inhibitor of endogenous BDNF and showed that BDNF is important for locomotor recovery after hemisection [87]. However, one recent study suggests that it is the selective distribution of BDNF in subpopulations of motoneurons, rather than total BDNF protein, which changes in response to treadmill training after complete transection [88]. Thus, subtle changes in the amount or distribution of BDNF expression and synaptic composition may modulate efficacy in appropriate circuits that contribute to stepping or locomotion.
Exogenous BDNF can also enhance stepping activity after SCI. Our group has found that intrathecal BDNF infusions [89;90] and bone marrow stromal cell grafts that secrete BDNF [91] each induce spontaneous stepping patterns in adult rats after SCI and improve the rate of recovery of stepping, but not the final extent of recovery. Other groups have also shown that untrained spinal cats with grafts of fibroblasts secreting BDNF and NT-3 improve stepping on a treadmill as well as cats that were trained [92]. These effects may be mediated by a number of downstream changes in the spinal cord. For example, BDNF is associated with structural changes including increased sprouting of serotonergic axons in rat brain [93]. This is consistent with observations that BDNF secreting grafts enhance sprouting of serotonergic fibers at the site of injury, and that voluntary wheel running can induce increased serotonin fiber density in the lumbar spinal cord of mice after moderate contusion injury [82]. Exogenously applied BDNF has also been associated with increased growth of motoneuron processes after contusion injury [89] or ventral root avulsion [94]. Another effect of BDNF release may be related to the phosphorylation and enhanced activation of excitatory NMDA receptors [95]. Thus, the effects of exercise are likely due in part to effects of BDNF on synaptic circuitry and excitation of locomotor pathways.
Exercise also alters the activity of inhibitory transmitter function after injury. Levels of the inhibitory transmitter, glycine, and GAD-67, a synthetic enzyme for γ-amino butyric acid (GABA), are both increased at chronic time points after spinal cord injury [96;97]. These changes have been seen in both dorsal and ventral horn, and recently Khristy et al. [98] demonstrated changes in the expression of the gamma2 subunit of the GABA(A) receptor in specific populations of motoneurons after spinal cord transection. This adaptation in inhibitory tone may reflect a modulatory response to the loss of descending inhibition. However, both glycine and GAD-67 levels and the changes in expression of the GABA-B receptors are restored to normal levels by treadmill step training. In addition, inhibition of glycinergic activity with the antagonist strychnine can reverse the negative effects of stand training on stepping patterns in injured cats [99].
Together, these structural and synaptic changes mediated by treadmill training after spinal cord injury provide substantial support for the hypothesis that repetitive step training can restore excitability levels closer to normal and enhance reorganization of spared circuitry. However, while there is substantial evidence for increased BDNF and trophic factor changes, the evidence for structural reorganization is more subtle and limited. This suggests that step training alone has limited effects on reorganization of the adult neural circuitry in the absence of descending control. We are left with the question of why these effects are limited. It is reasonable to infer that characteristics of the mature CNS are refractory to experience dependent reorganization. From an adaptive viewpoint, a refractory nervous system would favor long term stabilization of beneficial behaviors during developmental learning. However, the effort to alter and ultimately improve the course of recovery after injury might be enhanced by addressing the components of the mature nervous system that limit plasticity during the window of time when rehabilitation could be maximized.
Chondroitinase ABC enhances sprouting and recovery after SCI in adult mammals
The adult mammalian central nervous system (CNS) is more restricted in its ability to support sprouting and recovery after injury and to change in response to perturbations in synaptic activity than is the developing nervous system. For example, in primary visual cortex, early monocular deprivation can dramatically alter the formation of ocular dominance columns and disrupt binocular vision [100], while similar deprivation in the adult has little effect on synaptic reorganization. The time point when the neuropil is sensitive to reorganization is termed the “critical period”. Likewise, both the extent of axonal sprouting and the degree of functional recovery is greater after spinal cord injury in neonatal or young animals than in adults [101;102].
One important factor contributing to the limitation in plasticity and sprouting as CNS development proceeds is the maturation of the extracellular matrix (ECM), which is composed of multi-adhesive macromolecules, including hyaluronan, proteoglycans, and additional link proteins, that participate in a wide range of cell-cell interactions [103;104]. After injury to the CNS, a number of ECM molecules which inhibit axonal outgrowth in vitro, including chondroitin sulfate proteoglycans (CSPGs) and tenacins, are upregulated within the neuropil surrounding the lesion site in a well described and orchestrated manner [105;106]. This local environment is known to contribute to the failure of axonal regeneration at the lesion site. Indeed, chronic repeated intrathecal infusions of chondroitinase ABC, a bacterial enzyme that cleaves the glycosaminoglycan sidechains from CSPGs, results in increased growth of axons through or around the lesion site and improved functional recovery [107]. The effects of repeated intrathecal ChABC application at the lesion site include increased sprouting of both sensory and motor axons through loss of action of inhibitory growth molecules, release of growth factors sequestered within the glial extracellular matrix, neuroprotection including both reduced dieback of injured axons and reduced atrophy of distant cell bodies, and enhanced conduction through white matter tracts (reviewed in [108], and Bradbury, et al., this volume).
Intraparenchymal injections of ChABC eliminate perineuronal nets and facilitate plasticity
In addition to their role in the local response to injury, CSPGs also participate in the activity-dependent formation and maintenance of ECM structures that surround specific subpopulations of neurons and stabilize synapses. These structures, which are enriched in regions of highly active neurons, are termed perineuronal nets, or PNNs [92–96]. PNNs are composed of highly crosslinked conjugates of CSPGs, hyaluronan, tenacin-R and link proteins, and they can be identified in tissue sections by their ability to bind to the lectin, Wisteria floribunda agglutinin (WFA), or staining with iron colloid [109;110]. The development of PNNs in the cortex corresponds closely to the end of the critical period for functional plasticity, suggesting that PNNs contribute to the restricted plasticity found in the adult brain and spinal cord.
While chronic infusions may be required to reduce inhibitory factors at the injury site, recent work has demonstrated that microinjections of just a few microliters of ChABC (1–4 μl of approximately 50 U/ml), when administered directly into the neuropil of intact adult CNS gray matter, are sufficient to target and disrupt PNNs and re-activate plasticity in response to physiological stimuli in the adult visual [111] and somatosensory systems [112]. Following monocular deprivation, the removal of PNNs by ChABC is sufficient to reactivate cortical binocular functions in the adult, while microinjections placed into the body of the nucleus cuneatus permitted functional plasticity and expansion of forepaw representation after a cervical dorsal column lesion. In the spinal cord, Galtrey et al. demonstrated that 2 single intraparenchymal microliter injections of ChABC to the cervical spinal cord enlargement were sufficient to induce long term changes in CSPGs and hyaluronan in the spinal cord and improve appropriate functional recovery after crossed reinnervation of forelimb peripheral nerves in adult rats [113], indicating that CSPG-GAGs in the intact spinal cord can inhibit recovery of relevant and appropriate behaviors. Houlé and colleagues [114;115] have recently shown that microinjections of ChABC into the parenchyma below a peripheral nerve graft can be combined with application of neurotrophic factors to support axonal growth out of the graft, across an established glial scar, and into the spinal cord gray matter to form functional synapses. These studies are exciting because they demonstrate that intraparenchymal chondroitinase represents a potentially safe and effective component of a treatment for longstanding SCI. To date, however, discrete microinjections of ChABC alone have not been found to be sufficient to improve functional recovery when administered as a single therapy after SCI [116].
Combining ChABC with rehabilitation in animal models of SCI
Based on the observation that disruption of PNNs in adult cortex, spinal cord and dorsal column nuclei are sufficient to permit functional reorganization of sensory systems, we and others have proposed that a combination of ChABC and functionally appropriate exercise would support repair and recovery where neither therapy alone was sufficient. In a recent such combination study, Garcia-Alias et al. [117] took advantage of the ability of ChABC to digest CSPG-GAGs and perineuronal nets to optimize the effects of repetitive motion training on a forelimb task in rats following SCI. In this study, rats learned to shell sunflower seeds as a manual dexterity task and then received a cervical hemisection lesion that disrupted descending cortical control of the distal motoneurons. After injury, the rats received microinjections above and below the injury and intrathecal infusion of ChABC or control enzyme, and then they were either placed in a chamber to perform the seed shelling task or housed in an enriched cage environment to pursue general locomotor training. The rats with ChABC combined with training sessions in a seed manipulation task showed greater functional improvement in a related forelimb manual dexterity task. In contrast, rats that received ChABC with more general locomotor exercise did better in a ladder walk test, but did not perform as well on a skilled reaching task. These results demonstrate that recovery on a specific functional task following SCI can be improved significantly by combining ChABC to enhance plasticity with specific motor training to strengthen appropriate neural circuits. This provides initial proof of the novel principle that ChABC plus a specific activity can shape functional recovery. Notably, these results also suggest that exercise without specific task training can compromise the beneficial effects of a task-specific training paradigm. However, the SCI lesion was restricted to the dorsal columns. In addition, the very specific nature of this task does not address whether a similar approach (repetitive activity) could be harnessed to facilitate recovery on a more global task such as overground locomotor recovery, particularly following a larger moderate or severe contusion injury.
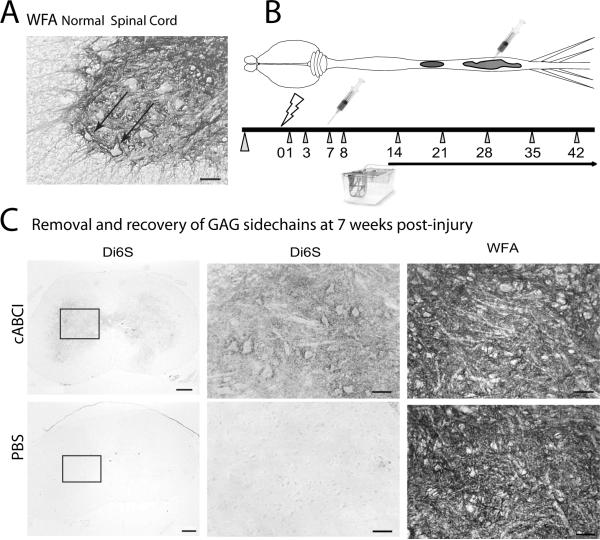
For optimal clinical translation, the application of ChABC and exercise should be minimally invasive and easy to access, in order to maximize safety and feasibility. In addition, the effects should be robust and applicable to a range of injury severities. In our lab, we were interested in taking a risk and determining if the absolutely simplest possible clinical application of ChABC and exercise would be sufficient to improve functional recovery after a moderate contusion injury. We therefore tested the effects of a single microinjection of ChABC to the gray matter in the spinal cord lumbar enlargement, placed one week after mid-thoracic injury, alone or combined with exercise by voluntary wheel running. The targeted site for ChABC digestion was the neuropil and PNNs of the L1–L5 spinal cord, where the CPG circuitry and the motoneurons and interneurons required for hindlimb locomotor movements are located. Histological staining of the untreated spinal cord using the lectin, WFA, reveals the dense distribution of N-acetylgalactosamine sites associated with PNNs in the mouse lumbar spinal cord (Figure 1A). The hypothesis was that if CPG and the lumbar spinal cord ECM was refractory to exercise-mediated plasticity following SCI, then the animals with ChABC plus running wheels would show use-dependent improvements in locomotion.
Figure 1.
Distribution of PNNs in mouse lumbar spinal cord and their digestion with ChABC.
A. Ventral horn of a normal mouse L4 lumbar spinal cord section stained with WFA lectin reveals dense PNNs surrounding neurons in the intermediate gray and ventral horn (Arrows; Scale = 50 μm).
B. Illustration of experimental design for the described study. Mice received a contusion injury at the mid-thoracic spinal cord (T9 vertebral level, lightning bolt on timeline). One week after injury, the lumbar cord was exposed and a single microinjection of ChABC injected into the lumbar enlargement (syringe). Behavioral testing was performed prior to injury and at 1,3,7 and 8 days post injury (dpi) and weekly thereafter (triangles). At 8 days after injury, mice were housed in cages with or without running wheels. The number of revolutions was recorded daily. Mice were perfused at 42 dpi. C. Histological analysis of representative transverse sections through the lumbar enlargement of mice. Di6S staining was performed using a monoclonal antibody to the 6S stubs of CSPGs (clone 3B3) on tissues obtained at 4 or 7 weeks after injury. Mice that received ChABC showed lasting exposure of Di6S stubs (top left, middle panel), while mice with vehicle injections showed no stub staining (bottom left, middle). By 7 weeks post-injury, the N-acetylgalactosamine staining of WFA was recovered and not distinguished from the untreated specimens, revealing a recovery of GAG epitopes. Scale, left panels = 200 μm; middle and right panels = 50 μm.
In this experiment, adult female C57BL/6 mice received a moderate contusion injury using the Ohio State ESCID device, as described in [118;119] (n=6–10/group over 2 study trials). At 1 week after injury, we exposed the L4–L5 spinal levels and injected the mice with 1.0 μl of ChABC in PBS or PBS vehicle (ChABC-I provided by Acorda Therapeutics, Inc.) (Figure 1B). A previous pilot study (not shown) confirmed that this ChABC dose was sufficient to digest the 6-sulfated GAGs found on CSPGs, yielding the expression of GAG “stubs” that extended all the way from the caudal lesion border to the lower spinal cord regions, as confirmed in tissues obtained at one week after the injections.
On the day following the intraparenchymal injections, the mice were returned to their cages, and half of the ChABC mice and half of the vehicle-injected mice were housed with running wheels that had counters to monitor the number of revolutions of the wheel each day. We then evaluated locomotor recovery using the BMS locomotor rating scale developed for mouse contusion injury (BMS; [71]). We also tested responses to mechanical (von Frey hair), thermal (Plantar Heat), cold (Ice) or proprioceptive stimuli applied to the hindlimbs, and responses to mechanical stimuli applied to the trunk [120].
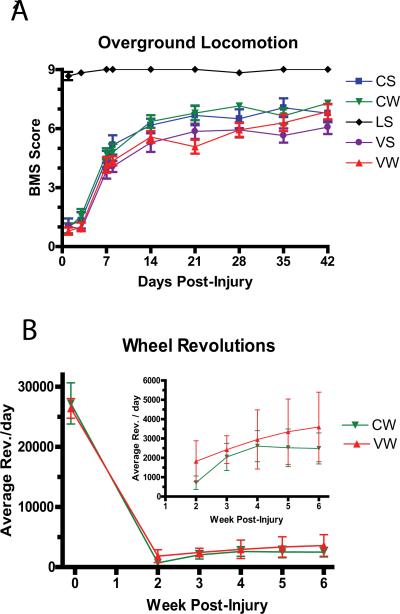
Under these conditions, neither exercise alone, ChABC alone, nor the combination of both treatments resulted in a significant improvement in locomotor function compared with mice that had no wheels and intraparenchymal vehicle injections (Figure 2A). There were also no effects of the experimental conditions on any of the sensory outcome measures (not shown). Both of the groups with access to running wheels used them with similar numbers of wheel revolutions per day (Figure 2B). The lack of effect on these outcomes demonstrates that the limitations of exercise effects after spinal cord injury are not restricted solely by the presence of CSPG-GAG chains in the lumbar neuropil. Although digestion of the perineuronal nets in other areas of the intact CNS is sufficient to permit functional reorganization when combined with physiological stimulation or nerve regeneration, a single lumbar injection of ChABC is insufficient to permit synaptic reorganization by wheel running alone. It will be important to determine if anatomical or biochemical indices support the possibility that ChABC facilitated some form of plasticity or sprouting in the lumbar cord that was not sufficient for improved overground locomotion or sensory recovery.
Figure 2.
Time course of locomotor recovery and wheel revolutions in pilot study. A. There was no significant difference in the extent of recovery between the treatment groups over time. Data represents combined results of two smaller studies with a total n= 6–10 mice per group. CS= ChABC + Sedentary (no wheel); CW= ChABC + Wheel; LS = Laminectomy + Sedentary; VS = Vehicle + Sedentary; VW= Vehicle + Wheel. B. Number of wheel revolutions per day was not different between the CW and VW groups. Note very few voluntary wheel revolutions after injury as compared with the pre-injury acclimation period.
As with most negative findings, there are a number of factors that can contribute to limiting the beneficial effects of the approach. For example, natural plasticity in mice following a moderate injury might be high enough through endogenous mechanisms, so that effects of ChABC and running were masked. Alternatively, the intensity of training may have been insufficient; the mice may not have run enough steps per night to provide the repetitive function required for reorganization [78]. Voluntary wheel running offers advantages including motivation and low stress on the subjects, but does not allow standardization or maximal stepping of injured animals. Indeed, some mice could be characterized as sedentary on those nights when had moved nesting materials into their wheels and run <50 revolutions. Finally, repeated administration of ChABC may be required to maintain a responsive environment. Removal of GAGs within the lumbar enlargement is likely overcome by rapid neosynthesis. Thus, while a single injection represents the simplest and ideal translatable therapeutic approach, repeated intraparenchymal injections are clinically feasible and can be examined as the next step to defining a relatively non-invasive therapy.
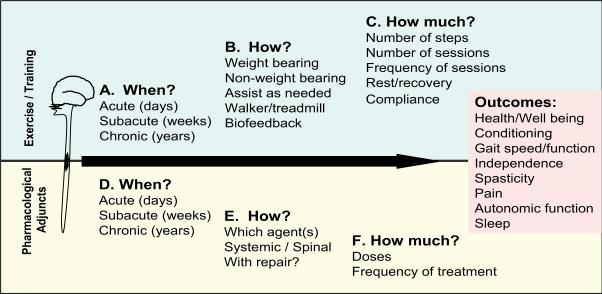
The results of this preliminary study are important because they emphasize those key parameters that must be addressed as this very plausible approach moves forward (Figure 3). Interventions that include exercise and pharmacology have potential to improve quality of life across a vast spectrum of outcome measures (“Outcomes”, Figure 3), ranging from general health and well being to specific modulation of complications such as spasticity and pain.
Figure 3.
Critical questions to resolve in a combined exercise and enzyme therapy approach designed to improve quality of life, including a wide range of Functional Outcomes (right panel). Top: The optimal exercise/rehabilitation paradigm must consider when to begin therapy, how to direct the therapy, and how much therapy to administer. Bottom: Likewise, optimizing ChABC therapy requires additional insight into when, how and how much to administer. With increased understanding of the nature and mechanisms of these approaches, a combination can be directed toward targeted improvements in functional outcome measures ranging from general health and well being to specific amelioration of symptoms of spasticity, pain, and autonomic dysfunctions.
Three major factors are still unknown as these rehabilitation adjunct therapies are expanded. First, the timing of initiation of locomotor therapy (When?) will be critical. Training too early may be detrimental, while starting too late may be ineffective. For example, Endo et al. [121] have shown that assisted stepping exercise immediately after SCI can induce allodynia in rats. In addition, as described above [79] training too early after injury can exacerbate tissue loss and decrease functional recovery. Secondly, the manner of training (How?) will also be important. For example, passive exercise, such as that obtained with stationary cycling, may engage different sensory and postural afferent inputs than partial body weight supported treadmill training or overground locomotor training. In addition, the motivational state and hypothalamo-pituitary axis activities are also likely to be critical determinants of effectiveness of exercise therapies, especially if high frequency or high intensity training is required. From a modeling perspective, this may be addressed by directly comparing effects of forced locomotor training (treadmill, swimming, moving wheel) and unforced locomotion (free running wheels). Thirdly, the amount or intensity of training required for optimal recovery (How much?) is also unknown. The consequences of either insufficient (undertraining) or overly ambitious training may be critical to the extent of recovery. Concomitant effects are also important to monitor. For example, if long sessions of treadmill training make patients too flaccid, it may impair some functions and prevent the full possibility of recovery.
In addition to critical questions regarding the rehabilitation paradigm, there is also need for further understanding of the timing, methods, and dosing needed for successful translation of ChABC as a biological therapeutic in combination with exercise and rehabilitation to clinical use [34]. From the animal studies reviewed here, we could conclude that the intrathecal bolus infusion approach most commonly employed may be necessary for many of the beneficial effects of ChABC on functional recovery, including neuroprotection, enhanced sprouting, and functional regeneration in tissues surrounding the lesion. However, therapies that specifically target plasticity and removal of inhibitory PNNs in denervated regions of the CNS may still be important, but may require repeated intraparenchymal injections critically timed with regard to time post-injury and coinciding with relevant rehabilitation strategies in order to maximize training effects.
Conclusions and Future Directions
Physical rehabilitation and exercise therapies represent feasible and important components of future strategies to enhance functional recovery after SCI. However, despite the clear presence of intact circuitry for locomotion in the distal segments of the spinal cord, exercise therapy alone has provided only limited improvements for individuals with SCI, and incomplete translation of benefits from the treadmill to overground walking. The addition of enzyme therapy that could be applied directly to the parenchyma of the lumbar spinal cord is a logical and clinically possible avenue to reduce the inhibitory influence of the mature ECM and permit rehabilitation-dependent plasticity. To realize this potential, specific and directed studies are needed to define the time, method, and intensity of exercise therapy and the timing, route of administration, and dosing of ChABC.
Acknowledgements
The authors are grateful for technical assistance from Todd Lash, Qin Feng Yin and Lesley Fischer, and editorial comments and discussions from Dr. Ellen Andrews and Ms. Rebekah Richards. ChABC-I was generously provided through a Materials Transfer Agreement with Acorda Therapeutics, Inc., Hawthorne, NY. This work was supported by grants from Spinal Research (STR100) and NINDS (NS043246 and NS045758).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare no conflicts of interest.
Reference List
- 1.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 2.Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J. Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos. Trans. R. Soc. Lond B Biol. Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi SL, Keirstead HS. Stem cells and spinal cord regeneration. Curr. Opin. Biotechnol. 2009;20:552–562. doi: 10.1016/j.copbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Houle JD, Amin A, Cote MP, Lemay M, Miller K, Sandrow H, Santi L, Shumsky J, Tom V. Combining peripheral nerve grafting and matrix modulation to repair the injured rat spinal cord. J. Vis. Exp. 2009;20(33) doi: 10.3791/1324. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa K, Chang YW, Li H, Berlin Y, Ikeda O, Kane-Goldsmith N, Grumet M. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp. Neurol. 2005;193:394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJ. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 2006;5:7. doi: 10.1186/jbiol35. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes BT, Reier PJ. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol. 1992;116:1–12. doi: 10.1016/0014-4886(92)90171-l. [DOI] [PubMed] [Google Scholar]
- 10.Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin A, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 11.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houweling DA, Lankhorst AJ, Gispen WH, Bar PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp. Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovich PG, Guan Z, Wei P, Huitinga I, van RN, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 16.Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 2009;109:1658–1667. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- 17.Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young W, Decrescito V, Flamm ES, Blight AR, Gruner JA. Pharmacological therapy of acute spinal cord injury: studies of high dose methylprednisolone and naloxone. Clin.Neurosurg. 1988;34:675–97. [PubMed] [Google Scholar]
- 19.Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp. Neurol. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates--re-examination and extension of behavioral data. Eur. J. Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P, Huang L, Zou J, Yu Z, Wang Y, Wang X, Xu L, Liu X, Xu XM, Lu PH. Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats. Neurobiol. Dis. 2008;32:535–542. doi: 10.1016/j.nbd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. see comments. [DOI] [PubMed] [Google Scholar]
- 24.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, Sandhu F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 26.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. see comments. [DOI] [PubMed] [Google Scholar]
- 27.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 28.Nothias JM, Mitsui T, Shumsky JS, Fischer I, Antonacci MD, Murray M. Combined effects of neurotrophin secreting transplants, exercise, and serotonergic drug challenge improve function in spinal rats. Neurorehabil. Neural Repair. 2005;19:296–312. doi: 10.1177/1545968305281209. [DOI] [PubMed] [Google Scholar]
- 29.Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp. Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steward O, Sharp K, Selvan G, Hadden A, Hofstadter M, Au E, Roskams J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp. Neurol. 2006;198:483–499. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Yoshihara H, Shumsky JS, Neuhuber B, Otsuka T, Fischer I, Murray M. Combining motor training with transplantation of rat bone marrow stromal cells does not improve repair or recovery in rats with thoracic contusion injuries. Brain Res. 2006;1119:65–75. doi: 10.1016/j.brainres.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 32.Kwon BK, Okon EB, Hillyer J, Mann C, Baptiste DC, Weaver L, Fehlings M, Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma. 2010 Apr. doi: 10.1089/neu.2009.1149. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet W, Tsai E, Baptiste DC, Smithson LJ, Kawaja MD, Fehlings M, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma. 2010 Apr. doi: 10.1089/neu.2009.1177. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon BK, Okon EB, Plunet W, Baptiste DC, Fouad K, Hillyer J, Weaver L, Fehlings M, Tetzlaff W. A systematic review of directly applied biologic therapies for acute spinal cord injury. J. Neurotrauma. 2010 Apr. doi: 10.1089/neu.2009.1150. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci. 2008;31:410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev. Disabil. Res Rev. 2009;15:112–116. doi: 10.1002/ddrr.61. [DOI] [PubMed] [Google Scholar]
- 39.Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch. Phys. Med. Rehabil. 1992;73:1085–1093. [PubMed] [Google Scholar]
- 40.Valent LJ, Dallmeijer AJ, Houdijk H, Slootman HJ, Janssen TW, Post MW, van der Woude LH. Effects of hand cycle training on physical capacity in individuals with tetraplegia: a clinical trial. Phys. Ther. 2009;89:1051–1060. doi: 10.2522/ptj.20080340. [DOI] [PubMed] [Google Scholar]
- 41.Hicks AL, Adams MM, Martin GK, Giangregorio L, Latimer A, Phillips SM, McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- 42.Anneken V, Hanssen-Doose A, Hirschfeld S, Scheuer T, Thietje R. Influence of physical exercise on quality of life in individuals with spinal cord injury. Spinal Cord. 2010;48:393–399. doi: 10.1038/sc.2009.137. [DOI] [PubMed] [Google Scholar]
- 43.McDonald JW, Becker D, Sadowsky CL, Jane JA, Sr., Conturo TE, Schultz LM. Late recovery following spinal cord injury. Case report and review of the literature. J. Neurosurg. 2002;97:252–265. doi: 10.3171/spi.2002.97.2.0252. [DOI] [PubMed] [Google Scholar]
- 44.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behrman AL, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys. Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- 46.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- 47.Ditor DS, Kamath MV, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J. Appl. Physiol. 2005;98:1519–1525. doi: 10.1152/japplphysiol.01004.2004. [DOI] [PubMed] [Google Scholar]
- 48.Wernig A, Müller S, Nanassy A, Cagol E. Laufband Therapy Based on `Rules of Spinal Locomotion' is Effective in Spinal Cord Injured Persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 49.Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30:229–238. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- 50.Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J. Neurol. Phys. Ther. 2005;29:127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- 51.Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, Basso M, Behrman A, Harkema S, Saulino M, Scott M. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil. Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nooijen CF, Ter HN, Field-Fote EC. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J. Neuroeng. Rehabil. 2009;6:36. doi: 10.1186/1743-0003-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbeau H, Basso M, Behrman A, Harkema S. Treadmill training after spinal cord injury: good but not better. Neurology. 2006;67:1900–1901. doi: 10.1212/01.wnl.0000249080.15391.6d. [DOI] [PubMed] [Google Scholar]
- 54.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 55.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 56.De Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog. Brain Res. 2002;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- 57.Leblond H, L'Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- 59.Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- 60.De Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrauma. 2006;23:1147–1163. doi: 10.1089/neu.2006.23.1147. [DOI] [PubMed] [Google Scholar]
- 61.Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Ji SR, Wu CY, Fan XH, Zhou HJ, Liu GL. Observation of locomotor functional recovery in adult complete spinal rats with BWSTT using semiquantitative and qualitative methods. Spinal Cord. 2007;45:496–501. doi: 10.1038/sj.sc.3102013. [DOI] [PubMed] [Google Scholar]
- 63.Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res. Bull. 1993;30:387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- 64.Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- 65.Rossignol S, Barbeau H. Pharmacology of locomotion: an account of studies in spinal cats and spinal cord injured subjects. J. Am. Paraplegia. Soc. 1993;16:190–196. doi: 10.1080/01952307.1993.11735900. [DOI] [PubMed] [Google Scholar]
- 66.Feraboli-Lohnherr D, Barthe J-Y, Orsal D. Serotonin-Induced Activation of the Network for Locomotion in Adult Spinal Rats. J. Neurosci. Res. 1999;55:87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 67.Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 68.Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 69.Courtine G, Gerasimenko Y, van den BR, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain Res Bull. 2009;78:4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 72.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 73.Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 74.Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J. Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- 75.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 76.Ichiyama R, Potuzak M, Balak M, Kalderon N, Edgerton VR. Enhanced motor function by training in spinal cord contused rats following radiation therapy. PLoS. One. 2009;4:e6862. doi: 10.1371/journal.pone.0006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma. 2007;24:1000–1012. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- 79.Smith R, Brown EH, Shum-Siu A, Whelan A, Burke D, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma. 2009 Jan; doi: 10.1089/neu.2008-0829. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J. Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- 81.Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 82.Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- 83.Erschbamer MK, Pham TM, Zwart MC, Baumans V, Olson L. Neither environmental enrichment nor voluntary wheel running enhances recovery from incomplete spinal cord injury in rats. Exp. Neurol. 2006;201:154–164. doi: 10.1016/j.expneurol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 86.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 87.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macias M, Nowicka D, Czupryn A, Sulejczak D, Skup M, Skangiel-Kramska J, Czarkowska-Bauch J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC. Neurosci. 2009;10:144. doi: 10.1186/1471-2202-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- 90.Ankeny DA, McTigue DM, Guan Z, Yan Q, Kinstler OB, Stokes BT, Jakeman LB. Pegylated brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Exp. Neurol. 2001;170:85–100. doi: 10.1006/exnr.2001.7699. [DOI] [PubMed] [Google Scholar]
- 91.Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp. Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 92.Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J. Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- 93.Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J. Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blits B, Carlstedt TP, Ruitenberg MJ, de WF, Hermens WT, Dijkhuizen PA, Claasens JW, Eggers R, van der SR, Tenenbaum L, Boer GJ, Verhaagen J. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp. Neurol. 2004;189:303–316. doi: 10.1016/j.expneurol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Slack SE, Thompson SW. Brain-derived neurotrophic factor induces NMDA receptor 1 phosphorylation in rat spinal cord. Neuroreport. 2002;13:1967–1970. doi: 10.1097/00001756-200210280-00027. [DOI] [PubMed] [Google Scholar]
- 96.Edgerton VR, McCall GE, Hodgson JA, Gotto J, Goulet C, Fleischmann K, Roy RR. Sensorimotor adaptations to microgravity in humans. J. Exp. Biol. 2001;204:3217–3224. doi: 10.1242/jeb.204.18.3217. [DOI] [PubMed] [Google Scholar]
- 97.Tillakaratne NJ, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J. Neurosci Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 98.Khristy W, Ali NJ, Bravo AB, de LR, Roy RR, Zhong H, London NJ, Edgerton VR, Tillakaratne NJ. Changes in GABA(A) receptor subunit gamma 2 in extensor and flexor motoneurons and astrocytes after spinal cord transection and motor training. Brain Res. 2009;1273:9–17. doi: 10.1016/j.brainres.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb Locomotor and Postural Training Modulates Glycinergic Inhibition in the Spinal Cord of the Adult Spinal Cat. J. Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- 100.Wiesel TN, Hubel D. Hl Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 101.Bregman BS, Goldberger ME. Infant lesion effect. III. Anatomical correlates of sparing and recovery of function after spinal cord damage in newborn and adult cats. Dev. Brain Res. 1983;9:137–154. doi: 10.1016/0165-3806(83)90047-0. [DOI] [PubMed] [Google Scholar]
- 102.Kunkel-Bagden E, Dai HN, Bregman BS. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp. Neurol. 1992;116:40–51. doi: 10.1016/0014-4886(92)90174-o. [DOI] [PubMed] [Google Scholar]
- 103.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 104.Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008;27:1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- 105.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 106.Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 107.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 108.Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp. Neurol. 2007;206:159–171. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Murakami T, Kosaka M, Sato H, Ohtsuka A, Taguchi T. The intensely positively charged perineuronal net in the adult rat brain, with special reference to its reactions to oxine, chondroitinase ABC, hyaluronidase and collagenase. Arch. Histol. Cytol. 2001;64:313–318. doi: 10.1679/aohc.64.313. [DOI] [PubMed] [Google Scholar]
- 110.Murakami T, Ohtsuka A, Matsuoka H, Taguchi T, Murakami T, Abe K, Ninomiya Y. Intensely positively charged perineuronal nets in the adult rat brain as detected by staining with anionic iron colloid. Arch. Histol. Cytol. 2001;64:45–50. doi: 10.1679/aohc.64.45. [DOI] [PubMed] [Google Scholar]
- 111.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 112.Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130:926–939. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- 114.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge“ and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J. Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tom VJ, Kadakia R, Santi L, Houle JD. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J. Neurotrauma. 2009;26:2323–2333. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 118.Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- 119.Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB. Behavioral and histological outcome following graded contusion injury in C57Bl/6 mice. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- 120.Hoschouer EL, Basso DM, Jakeman LB. Aberrant sensory responses are dependent on lesion severity after spinal cord contusion injury in mice. Pain. 2010;148:328–342. doi: 10.1016/j.pain.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Endo T, Ajiki T, Inoue H, Kikuchi M, Yashiro T, Nakama S, Hoshino Y, Murakami T, Kobayashi E. Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem. Biophys. Res Commun. 2009;381:339–344. doi: 10.1016/j.bbrc.2009.02.043. [DOI] [PubMed] [Google Scholar]