Abstract
Influenza vaccines are the primary method for controlling influenza and its complications. This study was conducted as a phase 3, randomized, double-blind, controlled, multi-center trial at seven university hospitals to evaluate the immunogenicity and safety of an inactivated, split, trivalent influenza vaccine (GC501, Green Cross Corporation, Yongin, Korea), which was newly manufactured in Korea in 2008. Between September 21 and 26, a total of 329 healthy subjects were recruited for the immunogenicity analysis, while 976 subjects were enrolled for the safety analysis. The GC501 vaccine met both FDA and EMEA criteria with ≥ 80% of subjects achieving post-vaccination titers ≥ 40 for all three subtypes, even in the elderly. The vaccine was well tolerated with only mild systemic and local adverse events. In summary, GC501 showed excellent immunogenicity and a good safety profile in both young adults and the elderly. The licensure of GC501 might be an important basis in preparation for the future influenza pandemic.
Keywords: Influenza Vaccines; Influenza, Human; Vaccines, Inactivated
INTRODUCTION
Influenza and its complications have a large social impact including increased demands on the healthcare system and patient disability and mortality. Annually, the World Health Organization (WHO) estimates that there are 3-5 million cases of severe influenza illness, resulting in 250,000-500,000 deaths in industrialized countries (1). In the Republic of Korea (ROK), the Korean Influenza Surveillance Scheme (KISS) has been operational in public health centers and private clinics since the year 2000, and about 20% of the general population is presumed to get the flu every year (2, 3). In addition to its social impact, influenza also has a significant economic impact, including lost or reduced productivity in the work place, work and school absenteeism, and increased healthcare costs.
Influenza vaccines are the primary method for controlling influenza and its complications. In the ROK, the influenza vaccine coverage rate in the general population is 34.3%, while the coverage rate in the priority group is 61.3%; higher than the rates of other countries (4). Despite the high vaccination rate in the ROK, all influenza vaccines prior to 2008 were imported from global vaccine manufacturers. In 2008, the Green Cross Corporation of the ROK started to produce an influenza vaccine. Herein, a multi-center, randomized trial of inactivated trivalent influenza vaccine was conducted for the purposes of licensure.
MATERIALS AND METHODS
Study design
This study was conducted as a randomized, double-blind, controlled, multi-center, phase 3 trial at seven university hospitals in the ROK to evaluate the immunogenicity and safety of an inactivated, split, trivalent influenza vaccine (GC501, Green Cross Corporation) newly manufactured in Korea in 2008 (Clinical trial number-KCT0000034). Immunogenicity of the GC501 was compared to that of the control vaccine (Fluarix®, GlaxoSmithKline Biologicals, Rixensart, Belgium). Subjects (healthy adults aged ≥ 18 yr) were excluded for the following reasons: allergy to eggs, history of Guillain-Barre syndrome, immunodeficiency or receipt of immunosuppressive therapy, active neoplastic diseases, and previous influenza vaccination within six months prior to the study. Subjects who met the entry criteria for the study were stratified into two age groups (18-59 and ≥ 60 yr) and randomized to receive either the study vaccine or the control vaccine.
After a baseline blood sample was collected, the vaccine was administered as a single intramuscular injection into the deltoid muscle, and subjects were observed for 30 min. At day 21 after vaccination, a second blood sample was obtained.
Vaccines
The study vaccine (GC501) was a trivalent, inactivated split vaccine loaded into pre-filled syringes containing 15 µg of hemagglutinin and was produced in embryonated chicken eggs by the Green Cross Corporation (Yongin, ROK) according to the guidelines of the WHO. The control vaccine was a 0.5 mL commercial trivalent, inactivated split vaccine (Fluarix®) in pre-filled syringes containing 15 µg of hemagglutinin. The vaccines were each composed of the following influenza strains: A/Brisbane/59/2007 IVR-148 (H1N1), A/Uruguay/716/2007 NYMC X-175C (H3N2), and B/Brisbane/3/2007.
Immunogenicity assessment
Serum samples were assessed for hemagglutination-inhibition (HI) antibodies to each hemagglutinin of the H1N1, H3N2 and B strains contained in the vaccine using a standard assay with use of chicken erythrocytes at the Korea University Guro Hospital (5). HI antibody titers that were below the detection limit (i.e., < 1:10) were assigned a value of 1:5, and all other titers over 1:5,120 were assigned a value of 1:5,120.
The three co-primary immunogenicity end points after vaccination were the proportion of subjects with antibody titers of 1:40 or more on HI assays (seroprotection rate), the proportion of subjects with either seroconversion or a four-fold or more increase in antibody titer (seroconversion rate), and geometric mean titer ratio (GMTR) (i.e., the ratio of the geometric mean titer after vaccination to the geometric mean titer before vaccination) (6). Serologic response, as measured by HI antibody titers, was assessed using the criteria set by both the United States Food and Drug Administration (FDA) and the Committee for Proprietary Medicinal Products (CPMP) of the European Agency for the Evaluation of Medicinal Products (EMEA). According to the FDA criteria, the lower bound of the two-sided 95% confidence interval (CI) of seroprotection should meet or exceed 70% in adults aged < 65 yr (60% in adults ≥ 65 yr), and the lower bound of the two-sided 95% CI of seroconversion should meet or exceed 40% in adults aged < 65 yr (30% in adults ≥ 65 yr) (7). In order to confirm protective immunogenicity based on the EMEA criteria, one of the following criteria must be met: seroprotection rates > 70% for subjects aged 18-60 yr and > 60% for subjects over 60 yr, seroconversion rates > 40% for subjects aged 18-60 yr and > 30% for subjects over 60 yr, or GMT ratios > 2.5 for subjects aged 18-60 yr and > 2.0 for subjects over 60 yr (6).
Safety assessment
At first visit, enrolled subjects were given a digital thermometer and a diary card containing of a list of solicited adverse events and their grades. On the immunization day (day 0), subjects were observed at the study site for a period of 30 min after vaccination to detect any immediate adverse reactions. For the next 7 days, subjects were educated to record the severity of solicited local adverse events and solicited systemic adverse events, axillary temperature and concomitant medications in the diary card. Subjects used a standard scale to grade adverse events (8).
Solicited local adverse events were pain, tenderness, redness and swelling, and solicited systemic adverse events were fever, headache, malaise, shivering, fatigue, sweating, myalgia and arthralgia. We collected unsolicited reports during the study. Serious adverse events were reported within 24 hr. Any adverse events were possibly considered to be related to the vaccine for up to six months after vaccination.
Statistical analysis
The sample size was chosen to meet the immunogenicity endpoints based on the data of the Fluarix® trial (9). All statistics were generated using SPSS 11.5. The Student t-test (Mann Whitney U-test for less than 30 samples) was used to compare HI titers between the two groups. HI titers obtained before and after vaccination were compared using the Wilcoxon test (two-tailed), and categorical variables were analyzed using the chi-square test. Results were considered statistically significant if the P value was less than 0.05.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and the standards of Good Clinical Practice by the International Conference on Harmonization. The protocol and consent forms were approved by the institutional review board of each participating study site. Informed written consent was obtained from all participants following a detailed explanation of schedules and study contents.
RESULTS
Study subjects
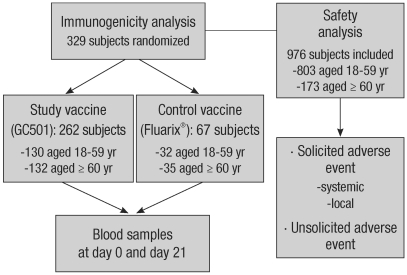
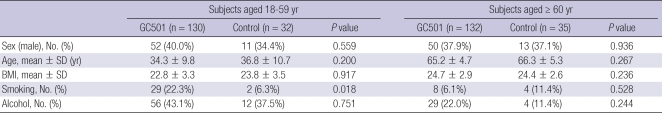
Between September 21 and 26, 2008, a total of 329 healthy subjects were recruited for the analysis of immunogenicity, while 976 subjects were enrolled for the safety analysis (Fig. 1). Three hundred and nineteen subjects were randomized to receive either the study vaccine (GC501, 130 subjects aged 18-59 yr and 132 subjects aged ≥ 60 yr) or the control vaccine (Fluarix®, 32 subjects aged 18-59 yr and 35 subjects aged ≥ 60 yr). The demographic and baseline characteristics of the two vaccine groups were well matched, as shown in Table 1. All 976 subjects in the safety analysis received the study vaccine.
Fig. 1.
Flowchart of subjects through the study.
Table 1.
Demographic characteristics of the study subjects
BMI, body mass index.
Immunogenicity
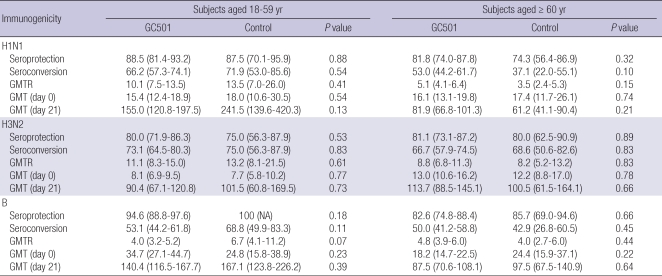
Pre-vaccination GMTs were similar between the GC501 and control vaccine groups in both subjects aged 18-59 yr and in subjects aged ≥ 60 yr (Table 2). Table 2 presents the immunogenicity data on the seroprotection, seroconversion and GMTR of 329 subjects by intent-to-treat (ITT) analysis. For each strain of the three subtypes, the seroprotection rates, seroconversion rates and GMTRs were comparable between the GC501 and control vaccine groups, irrespective of age (P > 0.05). The GC501 vaccine met both the FDA and EMEA criteria. In terms of the GC501 vaccine, seroprotection rates were 88.5% for A/H1N1, 80.0% for A/H3N2 and 94.6% for B in subjects aged 18-59 yr and were 81.8% for A/H1N1, 81.1% for A/H3N2 and 82.6% for B in subjects aged ≥ 60 yr. Likewise, seroconversion rates were 66.2% for A/H1N1, 73.1% for A/H3N2 and 53.1% for B in subjects aged 18-59 yr and were 53.0% for A/H1N1, 66.7% for A/H3N2 and 50.0% for B in subjects aged ≥ 60 yr. GMTRs ranged between 4.0 and 16.1 and were lower with regard to subtype B compared to those of A/H1N1 and A/H3N2.
Table 2.
Immune responses after immunization with trivalent inactivated influenza vaccines, as measured using a hemagglutination-inhibition (HI) assay: GC501 versus control vaccine (Fluarix®)
Geometric mean ratios are the ratios of the antibody levels at day 21 and at day 0. Seroconversion was defined as a pre-vaccination antibody titer of 1:10 or less and a post-vaccination titer of 1:40 or more.
Safety
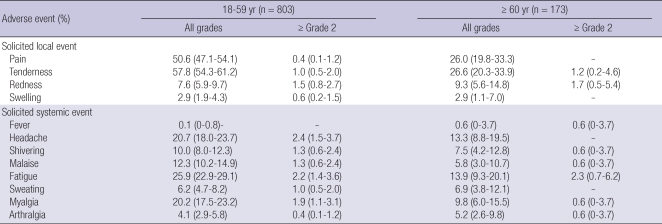
Solicited local and systemic adverse events reported within seven days of vaccination are shown in Table 3. Following the vaccination, 595 (61.0%) of the 976 subjects reported solicited local adverse events, while 388 subjects (39.8%) reported solicited systemic adverse events. Overall, solicited adverse events were less common in the elderly group aged ≥ 60 yr (82 among 173 subjects, 47.4%) compared to those of the subjects aged 18-59 yr (587 among 803 subjects, 73.1%). The most common local adverse events were pain (451, 46.2%) and tenderness (510, 52.3%), while the most frequent systemic event was fatigue (232, 23.8%), followed by headache (189, 19.4%) and myalgia (179, 18.3%). Most subjects reported systemic adverse events as grade 1 (noticeable but did not interfere with daily activity) that resolved within three days. High-grade systemic adverse events (≥ grade 2) were observed in 6.9% of subjects: fatigue (22, 2.3%), headache (19, 1.9%), myalgia (16, 1.6%), malaise (11, 1.1%) and shivering (11, 1.1%).
Table 3.
Solicited local and systemic adverse events within seven days after GC501 vaccination
*Data are presented as proportion (95% confidence interval) of participants who reported having a solicited local or systemic adverse event. Pain was grade 0 (absent), grade 1 (does not interfere with activity), grade 2 (repeated use of non-narcotic pain reliever > 24 hr or interferes with activity), grade 3 (any use of narcotic pain reliever or prevents daily activity), or grade 4 (emergency room visit or hospitalization). Tenderness was grade 0 (absent), grade 1 (mild discomfort to touch), grade 2 (discomfort with movement), grade 3 (significant discomfort at rest), or grade 4 (emergency room visit or hospitalization). Other adverse events were grade 1 (does not interfere with activity), grade 2 (interferes with activity), grade 3 (prevents daily activity), or grade 4 (emergency room visit or hospitalization).
During the 21 days after vaccination, unsolicited adverse events were reported by 86 (8.8%) of the 976 subjects. Of these, respiratory system disorder (31, 3.2%) was the most common, and 32 (3.3%) of the events were considered to be related to vaccination. On the other hand, two severe adverse events of atelectasis and convulsion were reported. Atelectasis was not considered to be related to vaccination, while the episode of convulsion was possibly related. All subjects with unsolicited adverse events recovered without sequelae, and no death was reported.
DISCUSSION
This study showed that the immunogenicity of the new influenza vaccine (GC501) is largely comparable with that of the commercial trivalent, inactivated split vaccine (Fluarix®, GlaxoSmith-Kline Biologicals, Rixensart, Belgium), which was well tolerated.
Up until the early 21st century, about 85% of the world's supply of influenza vaccine had been produced by nine vaccine companies located in nine different countries: France, Germany, Italy, the Netherlands, Switzerland, the United Kingdom, the United States, Canada, and Australia (10). In the situation of large influenza epidemic, any country would be confronted with vaccine shortage. Concerning vaccine shortage, some Asian countries had tried to have their own manufacturing system. Japan already has four national influenza vaccine manufacturers, and China recently achieved licensure of an influenza vaccine. The GC501 vaccine in this study is the first Korean influenza vaccine which is expected to contribute to lowering the risk of vaccine shortages in the many vaccine-limited countries.
For the licensure of a new influenza vaccine, the FDA and EMEA provide definite criteria that must be achieved (6, 7). In the present study, GC501 met the FDA requirements with consistent rates of seroprotection (lower bound of the two-sided 95% CI ≥ 70%) and seroconversion (lower bound of the two-sided 95% CI ≥ 40%) for all three subtypes, irrespective of age. As for the EMEA criteria, GC501 exceeded the requirements with ≥ 80% of subjects achieving post-vaccination titers ≥ 40 for all three subtypes, even in the elderly.
In general, local reactions after inactivated influenza vaccination are known to be relatively frequent (10%-65%) (11). Consistent with previous reports, GC501 showed mild local reactions in 61.0% of subjects. Though the frequency of systemic adverse reactions to GC501 (39.8%) was higher than those in previous reports (15% in average) (10), most adverse reactions were grade 1 and were resolved spontaneously within a few days. One episode of convulsion was reported by a vaccine recipient (0.1%) and was considered to be possibly vaccine-related. However, the subject recovered without sequelae, and the episode was not recurrent.
In summary, GC501 showed excellent immunogenicity and a good safety profile in both young adults and the elderly. The licensure of GC501 might be an important basis in preparation for the future influenza pandemic.
Footnotes
Funding statement: Green Cross Corporation funded this research.
AUTHOR SUMMARY
Immunogenicity and Safety of Trivalent Inactivated Influenza Vaccine: A Randomized, Double-Blind, Multi-Center, Phase 3 Clinical Trial in a Vaccine-Limited Country
Joon Young Song, Hee Jin Cheong, Heung Jeong Woo, Seong-Heon Wie, Jin-Soo Lee, Moon-Hyun Chung, Yang Ree Kim, Sook In Jung, Kyung-Hwa Park, Tae Hyong Kim, Soo-Taek Uh, and Woo Joo Kim
This study is a phase 3, randomized, double-blind, controlled, multi-center trial to evaluate the immunogenicity and safety of an inactivated, split, trivalent influenza vaccine (GC501, Green Cross Corporation), which was newly manufactured in Korea in 2008. During study periods, a total of 329 healthy subjects were recruited for the immunogenicity analysis, while 976 subjects were enrolled for the safety analysis. The GC501 vaccine met both FDA and EMEA criteria with ≥ 80% of subjects achieving post-vaccination titers ≥ 40 for all three subtypes, even in the elderly. The vaccine was well tolerated with only mild adverse events. In summary, GC501 showed excellent immunogenicity and a good safety profile in both young adults and the elderly. The licensure of GC501 might be an important basis in preparation for the future influenza pandemic.
References
- 1.Wilschut JC, McElhaney J, Palache B. Influenza. 2nd ed. Philadelphia, PA: Elsevier Ltd; 2006. pp. 117–132. [Google Scholar]
- 2.Park SC, Choeng HJ, Sohn JW, Choi SJ, Eom JS, Woo HJ, Chun BC, Kim WJ. Efficacy of influenza vaccination among chronic ill patients: retrospective case control study. Infect Chemother. 2004;36:207–212. [Google Scholar]
- 3.Lee JS, Shin KC, Na BK, Lee JY, Kang C, Kim JH, Park O, Jeong EK, Lee JK, Kwon JW, Park SC, Kim WJ. Influenza surveillance in Korea: establishment and first results of an epidemiological and virological surveillance scheme. Epidemiol Infect. 2007;135:1117–1123. doi: 10.1017/S0950268807007820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, Choi WS, Jo YM, Seo YB, Kim WJ. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect. 2007;55:273–281. doi: 10.1016/j.jinf.2007.04.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929–3935. doi: 10.1016/j.vaccine.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 6.European Committee for Proprietary Medicinal Products. London, UK: The Europen Agency for the Evaluation of Medicinal Products; c1997. [accessed on 17 Aug 2010]. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96) Available at http://www.ema.europa.eu/pdfs/human/bwp/021496en.pdf. [Google Scholar]
- 7.Guidance for industry: clinical data needed to support the licensure of trivalent inactivated influenza vaccines. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. c2006. [accessed on 17 Aug 2010]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091985.pdf.
- 8.Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. U.S. Department of Health and Human Services FDA, Center for Biologics Evaluation and Research. 2007. [accessed on 17 Aug 2010]. Available at http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRe.
- 9.El Sahly HM, Keitel WA. Clinical data on Fluarix: an inactivated split seasonal influenza vaccine. Expert Rev Vaccines. 2008;7:713–719. doi: 10.1586/14760584.7.6.713. [DOI] [PubMed] [Google Scholar]
- 10.Fedson DS. Pandemic influenza and the global vaccine supply. Clin Infect Dis. 2003;36:1552–1561. doi: 10.1086/375056. [DOI] [PubMed] [Google Scholar]
- 11.Cheong HJ. Safety of influenza vaccine. J Korean Med Assoc. 2007;50:267–273. [Google Scholar]