Abstract
The purpose of this study is to determine 1) whether morphine postconditiong (MPostC) can attenuate the intercellular adhesion molecules-1 (ICAM-1) expression after reoxygenation injury and 2) the subtype(s) of the opioid receptors (ORs) that are involved with MPostC. Human umbilical vein endothelial cells (HUVECs) were subjected to 6 hr anoxia followed by 12 hr reoxygenation. Three morphine concentrations (0.3, 3, 30 µM) were used to evaluate the protective effect of MPostC. We also investigated blockading the OR subtypes' effects on MPostC by using three antagonists (a µ-OR antagonist naloxone, a κ-OR antagonist nor-binaltorphimine, and a δ-OR antagonist naltrindole) and the inhibitor of protein kinase C (PKC) chelerythrine. As results, the ICAM-1 expression was significantly reduced in the MPostC (3, 30 µM) groups compared to the control group at 1, 6, 9, and 12 hours reoxygenation time. As a consequence, neutrophil adhesion was also decreased after MPostC. These effects were abolished by coadministering chelerythrine, nor-binaltorphimine or naltrindole, but not with naloxone. In conclusion, it is assumed that MPostC could attenuate the expression of ICAM-1 on endothelial cells during reoxygenation via the κ and δ-OR (opioid receptor)-specific pathway, and this also involves a PKC-dependent pathway.
Keywords: Morphine, Postconditioning, Reperfusion injury, Humans, Umblical Veins, Endothelial Cells, Cell Culture
INTRODUCTION
Reperfusion injury to endothelium is mediated by neutrophils that accumulate early after reperfusion in the previously ischemic cardiac tissue (1, 2). This neutrophil adhesion is made possible by the expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1). The neutrophils adhered to the endothelial cells (ECs) potentiate the ischemic myocyte damage caused by microvascular obstruction and the generation of cytotoxic metabolites (1, 3).
Postconditioning is a novel strategy for achieving cardioprotection. It was first described by Zhao and colleagues in dogs (4), in which brief intermittent repetitive interruptions at the onset of reperfusion after a prolonged period of ischemia reduced the myocardial injury to an extent that was comparable to ischemic preconditioning (IPC), and IPC is a classic strategy for cardioprotection. The beneficial outcomes observed with postconditioning include reducing an infarct's size (4, 5), the endothelial dysfunction and the neutrophil adherence (4, 6) and the apoptosis (7). Wang et al. (8) demonstrated that morphine preconditioning attenuated neutrophil activation in an isolated rat heart model via increasing the neural endopeptidase activities and the attenuated shedding of L-selectin and ICAM-1 via a µ-opioid receptor (OR)-specific pathway. They also reported that intravenous administration of morphine decreases the serum ICAM-1 and L-selectin levels in acute myocardial infarction patients (9). This clinical setting seems to be similar to morphine postconditioning because the morphine was administered after the cardiovascular insult.
From these observations, we hypothesized that morphine postconditioning (MPostC) could attenuate the expression of adhesion molecules such as ICAM-1 during an ischemic reperfusion period. In this study, we used in vitro ischemic cell culture system by using human umbilical vein endothelial cells (HUVECs) because the numerous possible confounding factors could influence vascular function in vivo system. We also investigated the mechanisms of MPostC by blocking OR subtypes' effects on MPostC by using various antagonists.
MATERIALS AND METHODS
Cell lines and Reagents
The human umbilical vascular endothelial cell (HUVEC) line (JGC2517A, single donor) is from Lonza and the medium is EGM®-2 BulletKit® (CC-3162) from Lonza (Walkersville, MD, USA). The antibody is PE mouse anti-human CD54 from BD (cat. 555749;San Jose, California, USA), the RNeasy® RNA isolation mini kit is from Qiagen (Hilden, Germany), and all the other reagents are from Sigma Chemicals (St. Louis, MO, USA).
Cell viability
Cell viability was measured by the cell counting method. First, the cells were removed from culture and separated into a test tube. The samples were diluted four times and then they were stained by trypan blue. The stained samples were set up by a hemocytometer and cover slipped, and then they were observed under low power microscopy. Finally the cells with an intact membrane were counted.
Anoxia/Reoxygenation
Anoxia was performed in an anaerobic chamber (model Innova CO-14; New Brunswick Scientific, Hertfordshire, UK). To induce abrupt anoxia, the cell culture medium was deoxygenated before the experiments by placing the cell culture in the chamber for 12 hr. With this technique, the oxygen tension in the anoxic medium was 0.5 mmHg (i.e., > 99% decrease of the Po2). During periods of anoxia, the cells were kept in an incubator (37℃) within the anaerobic chamber. Reoxygenation was obtained by removing the anoxic medium and changing it to a normal, oxygenated culture medium (95 mmHg) and by returning the ECs to the normal cell incubator.
Experimental protocols
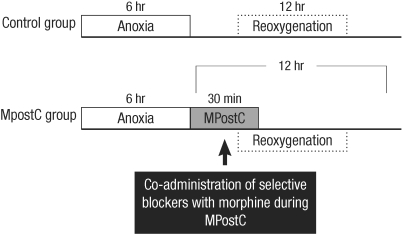
MPostC was performed during 30 min and, after 6 hr anoxia, by using 0.3, 3, and 30 µM doses of morphine. The prolonged anoxia was set at 6 hr, and the ICAM-1 expression was evaluated at 0, 1, 3, 6, 9, and 12 hr reoxygenation (Fig. 1). The duration of anoxia was chosen based on a study by Beauchamp et al. (10), who showed a marginal increase of ICAM-1 after 6 hr anoxia, so we used 6 hr because it was the shortest anoxic time and the concentrations of morphine were chosen (0.3, 3.0, 30 µM) based on a study by Chen and Zhang. (11), who revealed the effect of MPostC in an isolated heart model, and Dershwitz et al.'s (12) clinical practice. The possible roles of ORs were evaluated: the cells were morphine (3 µM) postconditioned by coadministering the inhibitor of protein kinase C (PKC) chelerythrine (1, 5, 25 µM) or the antagonist of the OR subtypes (nonselective, but highly affinitive µ-OR antagonist naloxone (2, 10, 50 µM), a κ-OR antagonist nor-binaltorphimine (1, 5, 25 µM) and a δ-OR antagonist naltrindole (1, 5, 25 µM)), and all dosages of the blockers were chosen based on previous studies (11, 13). The ICAM-1 expressions of blocker added groups were then compared to that of the control group at 6 hr reoxygenation time when the ICAM-1 expression of the control group reached the peak value.
Fig. 1.
Experimental protocol. MPostC represents morphine postconditioning.
Flow cytometry
The surface expressions of adhesion molecules were measured by flow cytometry (Facsflow from BD) by using an ICAM-1 specific fluorescein isothiocyanate-labeled antibody (BD, Franklin lake, NJ, USA). Briefly, the cells were washed with phosphate buffered saline (PBS) and then they were trypsinized. After detaching the cells and transferring them into tubes, the digestion was stopped by the addition of medium 199 that contained 10% fetal calf serum. After centrifugation at 218 g, (4℃, 5 min), the tubes were washed with PBS. The cells were incubated with PE Mouse Anti-Human CD54 for 30 min at 4℃, and the cells were washed again with PBS and resuspended in a volume of 500 µL PBS for the flow cytometric analysis. For the adjusting the instrument settings, the control cells were prepared by performing the procedure as described above, but by omitting addition of the antibody. At least three different sets of experiments with cells from different isolations were performed.
Assessment of the ICAM-1 gene expression by quantitative real-time PCR
RNA was prepared using an RNeasy® RNA isolation Mini kit (Qiagen, Hilden, Germany). Reverse-transcription polymerase chain reaction (RT-PCR) was performed using a one-step RT-PCR kit from Roche. The RT-PCR experiments were performed with the primers for the respective target enzyme ICAM-1: 5'-AGT-GGT-GGG-GGA-GAC-ATA-GC-3' (sense); 3'-GTG-TGGGCC-TTT- GTG-TTT-TG-5' (antisense). The conditions were as follows: ICAM-1:1 cycle at 95℃ for 10 min; 50 cycles (for quantitative real-time PCR or qRT-PCR) at 95℃ for 15 sec, 55℃ for 5 sec and 72℃ for 6 sec; 1 cycle at 65℃ for 15 sec and a final extension cycle at 40℃ for 30 sec. The specificity of the qRT-PCR amplification was verified by a melting curve analysis (from 50℃ to 90℃) using SYBR Premix Ex Taq (Roche Diagnostic, Mannheim, Germany). The relative gene expression levels were calculated as ratios with using β-actin for normalization.
Neutrophil adhesion
The adhesion of neutrophils was assessed by using human neutrophils. The neutrophils were taken from healthy volunteers. The neutrophils were isolated with the use of the MiniMACS™ Separator (Miltenyl Biotec, Bergisch Gladbach, Germany) and they were suspended in DMEM with 10% serum. The cell viability, assessed with Trypan blue, was always > 96%. The suspension of neutrophils was then incubated with reoxygenated ECs at 6 hr reoxygenation under static conditions for 15 min at 37℃. After the dishes were washed with PBS, they were incubated with PE Mouse Anti-Human CD15 for 20 min at 4℃. Then they were fixed with 2% paraldehyde, and examined by optical microscopy (Carl Zeiss Ins., Berlin, Germany). The adherent neutrophils were counted on a minimum of 5 microscopic fields. The results are expressed ratio of adhesion of neutrophils to the ECs.
Statistical analysis
We used SPSS for Windows version 10.0 (SPSS, Chicago, IL, USA) for the statistical analysis. All the data, which is expressed as means ± SDs, is representative of six different experiments. The results for the flow cytometry were evaluated by a one-way ANOVA. Comparisons between individual data were made using the paired t-test. All P values < 0.05 were considered significant.
RESULTS
Cell viability
The cell survival rate after prolonged anoxia followed by reoxygenation was 92%. This was calculated as a mean value.
The ICAM-1 expression on the HUVEC cells after reperfusion ischemic injury
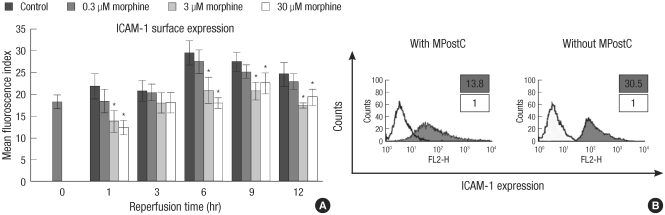
ICAM-1 protein expression was measured each different dosage of MPostC (0.3, 3, 30 µM) groups in consecutive order. As demonstrated in Fig. 2, the ICAM-1 protein expression was attenuated at 1, 6, 9, and 12 hr in the 3 and 30 µM MPostC groups, as compared to that of the control group. There was no significant difference between the control group and the 0.3 µM group.
Fig. 2.
Attenuation of the ICAM-1 protein expression in the HUVEC cells by MPostC. (A) The intercellular adhesion molecules-1 (ICAM-1) expression in the HUVECs is compared between the morphine postconditioning (MPostC) groups and the control group after 6 hr anoxia. The numbers of viable cells was 1 × 105 and the cell viability was 92%. The groups were divided to the control group and the 0.3, 3, and 30 µM MPostC groups. The mean fluorescence index (MFI) from each group was recorded at 0, 1, 3, 6, 9, and 12 hr. The valus are the mean ± SD of 6 experiments. *P < 0.05. (B) Phenotypical graph of the HUVECs. Flow cytometry analysis was done to characterize the ICAM-1 expressions on the HUVECs. PE Mouse Anti-Human CD54 monoclonal antibody was used to detect the ICAM-1 expression. The isotype antibody was used as the negative control (bold). The values were measured at 6 hr reperfusion time.
Neutrophil adhesion to ECs after reperfusion ischemic injury
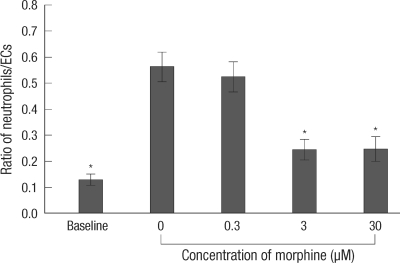
The neutrophil adhesion to ECs was increased in the control group at 6 hr reoxygenation when a peak response of ICAM-1 expression had been observed, as compared to that of the control group at 0 hr reoxygenation (baseline). Ischemia induced neutrophil adhesion to ECs of all groups was compared at 6 hr reoxygenation. The neutrophil adhesion to ECs was reduced in the 3 and 30 µM MPostC group as compared to that of the control group (Fig. 3).
Fig. 3.
Ratio of adhesion neutrophils to ECs. The ratio of adhesion neutrophils to ECs was measured at 6 hr reoxygenation. Baseline meant the value of the control group at 0 hr reoxygenation. The valus are the mean ± SD of 6 experiments. *is P < 0.05.
ICAM-1 mRNA synthesis after reperfusion ischemic injury
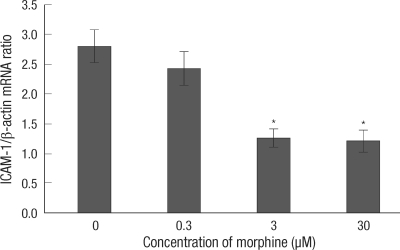
Ischemia induced messenger RNA (mRNA) expression of ICAM-1 of all groups was compared at 6 hr reoxygenation. mRNA expression of ICAM-1 was decreased in the 3, 30 µM MPostC groups as compared to that of the control group (Fig. 4).
Fig. 4.
Attenuation of the ICAM-1 mRNA level in the HUVEC cells by MPostC. qRT-PCR was performed to measure the ICAM-1 mRNA levels with using SYBR Premix Ex Taq. The relative gene expression levels were calculated as ratios by using β-actin for normalization. The value of the 0 hr control was baseline and it was calculated as a ratio of 1, and the others were recalculated as ratios relevant to a ratio of 1. All the values were compared to the value of the control group at 6 hr reoxygenation. The values are the mean ± SD of 6 experiments. *P < 0.05.
ICAM-1 expression of the MPostC (3 µM) group with added selective blockers
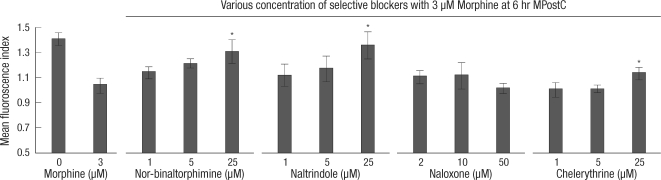
The ICAM-1 protein expressions of the MPostC (3 µM) group with added selective blockers were measured at 6 hr reperfusion time. As demonstrated in Fig. 5, the ICAM-1 protein expression was increased in the chelerythrine (25 µM) + MPostC (3 µM) group, the naltrindole (25 µM) + MPostC (3 µM) group and the nor-binaltorphimine (25 µM) + MPostC (3 µM) group, as compared to that of the 6 hr MPostC (3 µM) group.
Fig. 5.
Selective OR antagonists reverse the attenuation of the ICAM expression induced by MPostC. The intercellular adhesion molecule-1 (ICAM-1) expression was measured in the 3 µM morphine postconditioned cells in the presence of various concentrations of selective blockers. The mean florescence index of ICAM-1 was measured at 6 hr reoxygenation time. The dosages of each of the blockers were increased five folds at each time. The value of the control group at 0 hr reoxygenation was baseline and it was calculated as a ratio of 1, and the others were recalculated as ratios relevant to a ratio of 1. All the values were compared to the value of the 3 µM MpostC. The valus are the mean ± SD of 6 experiments. *P < 0.05.
DISCUSSION
We observed that the expression of ICAM-1 on ECs and neutrophil adhesion to ECs were attenuated by MpostC during reoxygenation period. The effect of MpostC was abolished by selective blockers (the inhibitor of PKC chelerythrine, κ-OR antagonist nor-binaltorphimine or δ-OR antagonist naltrindole).
Clinically, it is difficult to apply ischemic postconditioning. Pharmacological postconditioning, which only requires that a drug that is given as adjunctive treatment be present could be readily applied to all clinical scenarios of myocardial ischemia-reperfusion and this is a particularly promising therapy (14). Intravenous administration of morphine remains an essential step in the initial treatment of acute myocardial infarction (15) and it is usually injected after the ischemic insult. Since Zhao et al. (4) first described the cardioprotective effect of postconditoning, the mechanism and effect of postconditiong have been studied in many articles (5, 6, 11, 14). Wang et al. (8) recently showed that posttreatment with morphine may decrease the size of infarction by mimicking ischemic postconditioning in the isolated rat heart, and both the mito-KATP channels and the κ-ORs, but not the δ-ORs, mediated the cardioprotection produced by MPostC. However, the effect of MPostC on ECs has not yet been investigated, and so our study is the first that has demonstrated the effect of MPostC on ECs.
ICAM-1 is known to be a major ligand on ECs for the adhesion of activated leukocytes and the subsequent passage of leukocytes into the myocardium (16-18). The serum ICAM-1 levels are elevated in the patients with acute myocardial infarction, and this suggests that both rolling and postrolling events play an important role in ischemic myocardial injury (19). The expression of ICAM-1 can be induced by an inflammatory stimulus, and it is particularly responsive to tumor necrosis factor-α (TNF-α) and interleukin-1 (20). Nuclear factor-κB is a latent gene regulatory protein (21) that is sequestered, in an inactive form, in the cytoplasm of most cells. Nuclear factor-κB has been implicated in most inflammatory responses. After being activated, this protein upregulates the genetic expression of ICAM-1, among various other proinflammatory mediators. The ICAM-1 expression also could be increased by reactive oxygen species (22) and this was supported by Beauchamp et al.'s experiment (10). As demonstrated in Fig. 2, the effects of MPostC seemed to reach their maximum at 3 µM, and the increment of the morphine dosage (30 µM) did not further attenuate the ICAM-1 expression. This suggests that MPostC might have a ceiling effect when morphine over 3 µM is used for postconditioning. Hsiao et al. (23) reported that apoptosis was increased in HUVEC cells when they were incubated with more than 10 µM morphine throughout 48 to 72 hr period. In this study, we treated the HUVEC cells with 0.3, 3, 30 µM morphine in a period of 30 min, and we did not observe a further decrease of cell viability after morphine postconditioning.
Ichikawa et al. (24) reported that the neutrophil adherence to ECs was increased after 1 hr anoxia, and they showed a biphasic increase in neutrophil adhesion to HUVECs, with the peak responses occurring at 30 min (phase 1) and 4 hr after reoxygenation (phase 2). This two-phase neutrophil-EC adhesion response involved the transcription-independent (phase 1) and transcription-dependent (phase 2) surface expression of different EC adhesion molecules. In our study, the ICAM-1 expression showed a monophasic pattern, with a peak response occurring at 6 hr reoxygenation.
Although several endothelial adhesion molecules might be involved in the neutrophil adhesion to ECs (for example P and E selectins), Yoshida et al. (25) reported increment of reperfusion-induced neutrophil-endothelial interactons was mediated through the interacton between neutrophil and ICAM-1. We also confirmed that the neutrophil adhesion to ECs was increased in the control group at 6 hr reoxygenation as compared to the control group at 0 hr reoxygenation (Fig. 3). These results strongly supported that the upregulated ICAM-1 expression increased neutrophil adhesion to ECs. Therefore it is important to attenuate the ICAM-1 expression for reducing neutrophil induced ischemic reperfusion injury to the endothelium. We also confirmed that neutrophil adhesion to ECs was attenuated by MPostC (Fig. 3), and the mRNA expression of ICAM-1 was decreased in the 3 and 30 µM MPostC groups, as compared to that of the control group (Fig. 4). The mRNA expression of ICAM-1 was similar to phase 2 of a previous study (24). This suggests that MPostC may attenuate the ICAM-1 expression at 6 hr reoxygenation by a transcription-dependent mechanism.
The OR family is comprised of three primary subtypes: µ, κ, and δ. The ORs are guanine nucleotide binding protein (G protein)-coupled receptors that inhibit adenylyl cyclase (Gi coupled). Both OR activation, which has been implicated in IPC, and the exogenous activation of ORs have been well documented to confer both acute and delayed cardioprotection against ischemia-reperfusion injury (26). The mechanisms by which OR activation confers cardioprotection are typically thought to involve the phosphatidylinositol 3-kinase, MAPK (mitogen-activated protein kinases), PKC (protein kinase C) and GSK3-pathways (26, 27), as well as both the sarcolemmal and mitochondrial ATP-sensitive K (mitoKATP) channels (26). Fu et al. (28) revealed the cardioprotective effects of IPC in isolated rat hearts via the κ-ORs and δ-ORs. Zhang et al. (29) suggested that the remifentanil-conferred myocardial protection against ischemic injury occurred through a mechanism that was similar to IPC and the involved PKC pathway; Peart and Gross (30) described that chronic morphine preconditioning was mediated by a PKC-independent pathway, and this involved PKA, β2-AR and Gs proteins, whereas acute morphine preconditioning was mediated via the Gi proteins and the PKC pathway. Wang et al. (8) first reported the effect of morphine preconditioning on ECs. They revealed that in an isolated rat heart model, morphine preconditioning with naloxone (a µ-OR antagonist) abolished the cardioprotective effect and increased the ICAM-1 expression. But chelerythrine (a PKC inhibitor) or glibenclamide (an ATP-sensitive potassium channel antagonist) did not alter the action of morphine preconditioning on the neutrophil-endothelium activation. Thus, they concluded that morphine preconditioning attenuated the ICAM-1 expression via a µ-OR-specific pathway. Further, they reported that morphine preconditioning did not involve the PKC pathway or the ATP-sensitive potassium channels. That was different from the opioid preconditioning pathway for the cardiomyocytes, which was thought to be related to the κ-OR and δ-OR pathways (15, 26, 30), with involvement of the PKC pathway.
As demonstrated in Fig. 5, the ICAM-1 protein expression was increased in the chelerythrine, naltrindole or nor-binaltorphimine + MPostC (3 µM) groups. This result means that κ-OR and δ-OR could be involved in the effects of MPostC on the ICAM-1 expression instead of µ-OR, which is unlike the pathway of morphine preconditioning that was previously described (8). Wang et al. (8) reported that morphine preconditioning increases the activities of neutrophil endopeptidase to attenuate ICAM-1 via a µ-opioid receptor-specific pathway. However Chen et al. (11) concluded that morphine postconditioning protected isolated rat hearts via both the mito-KATP channel and κ-OR but not δ-OR, yet Gross et al. (27) demonstrated that BW373U86 (a selective δ-OR ligand) conferred protection when administered at reperfusion in isolated rat hearts. Although there have been conflicts and debates about the mechanism of morphine postconditioning, on the basis of our observations, we believe that the effects of MPostC on the ICAM-1 expression of ECs could be mediated by both κ and δ-ORs, and it also involves a PKC-dependent pathway. However, it is still remained to determine whether blockade of MPostC was mimicked by direct effects of antagonists. Therefore, we plan to enhance understanding of antagonists' direct effects to ICAM-1 expression by using the siRNA blocking or knock-out mice in a future study.
In conclusion, MPostC could attenuate the expression of ICAM-1 on ECs during reoxygenation period via the κ and δ-OR (opioid receptor)-specific pathway, involving a PKC-dependent pathway.
AUTHOR SUMMARY
Morphine Postconditioning Attenuates ICAM-1 Expression on Endothelial Cells
Too Jae Min, Joong-il Kim, Jae-Hwan Kim, Kyung Hee Noh, Tae Woo Kim, Woon-Young Kim, Yoon-Sook Lee, and Young Cheol Park
In this study we demonstrated that MPostC attenuates the expression of ICAM-1 on endothelial cells and the neutrophil adhesion to Endothelial cells. Our results suggest that the inhibitory effects of MPostC on the ICAM-1 expression of ECs could be mediated by both κ and δ-Ors. Also, a PKC-dependent pathway appears to be involved.
References
- 1.Ma XL, Tsao PS, Lefer AM. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991;88:1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kukielka GL, Hawkins HK, Michael L, Manning AM, Youker K, Lane C, Entman ML, Smith CW, Anderson DC. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993;92:1504–1516. doi: 10.1172/JCI116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 5.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583–1593. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang TL, Chang H, Hung CR, Tseng YZ. Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc Res. 1998;40:557–563. doi: 10.1016/s0008-6363(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997;80:1532–1535. doi: 10.1016/s0002-9149(97)00788-1. [DOI] [PubMed] [Google Scholar]
- 10.Beauchamp P, Richard V, Tamion F, Lallemand F, Lebreton JP, Vaudry H, Daveau M, Thuillez C. Protective effects of preconditioning in cultured rat endothelial cells: effects on neutrophil adhesion and expression of ICAM-1 after anoxia and reoxygenation. Circulation. 1999;100:541–546. doi: 10.1161/01.cir.100.5.541. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res. 2008;145:287–294. doi: 10.1016/j.jss.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Dershwitz M, Walsh JL, Morishige RJ, Connors PM, Rubsamen RM, Shafer SL, Rosow CE. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000;93:619–628. doi: 10.1097/00000542-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Cao CM, Xia Q, Tu J, Chen M, Wu S, Wong TM. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J Pharmacol Exp Ther. 2004;309:560–567. doi: 10.1124/jpet.103.061135. [DOI] [PubMed] [Google Scholar]
- 14.Yellon DM, Opie LH. Postconditioning for protection of the infarcting heart. Lancet. 2006;367:456–458. doi: 10.1016/S0140-6736(06)68157-9. [DOI] [PubMed] [Google Scholar]
- 15.Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Distinct components of morphine effects on cardiac myocytes are mediated by the kappa and delta opioid receptors. J Mol Cell Cardiol. 1997;29:711–720. doi: 10.1006/jmcc.1996.0313. [DOI] [PubMed] [Google Scholar]
- 16.Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 18.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 19.Shyu KG, Chang H, Lin CC, Kuan P. Circulating intercellular adhesion molecule-1 and E-selectin in patients with acute coronary syndrome. Chest. 1996;109:1627–1630. doi: 10.1378/chest.109.6.1627. [DOI] [PubMed] [Google Scholar]
- 20.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 22.Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 23.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ Res. 1997;81:922–931. doi: 10.1161/01.res.81.6.922. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida N, Granger DN, Anderson DC, Rothlein R, Lane C, Kvietys PR. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992;262:H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- 26.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35:709–718. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 27.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 28.Fu LL, Xia Q, Shen YL, Wong TM. Involvement of endogenous opioids in cardioprotective effects of ischemic preconditioning in the isolated rat heart. Sheng Li Xue Bao. 1998;50:603–610. [PubMed] [Google Scholar]
- 29.Zhang Y, Chen ZW, Girwin M, Wong TM. Remifentanil mimics cardioprotective effect of ischemic preconditioning via protein kinase C activation in open chest of rats. Acta Pharmacol Sin. 2005;26:546–550. doi: 10.1111/j.1745-7254.2005.00100.x. [DOI] [PubMed] [Google Scholar]
- 30.Peart JN, Gross GJ. Cardioprotective effects of acute and chronic opioid treatment are mediated via different signaling pathways. Am J Physiol Heart Circ Physiol. 2006;291:H1746–H1753. doi: 10.1152/ajpheart.00233.2006. [DOI] [PubMed] [Google Scholar]