Abstract
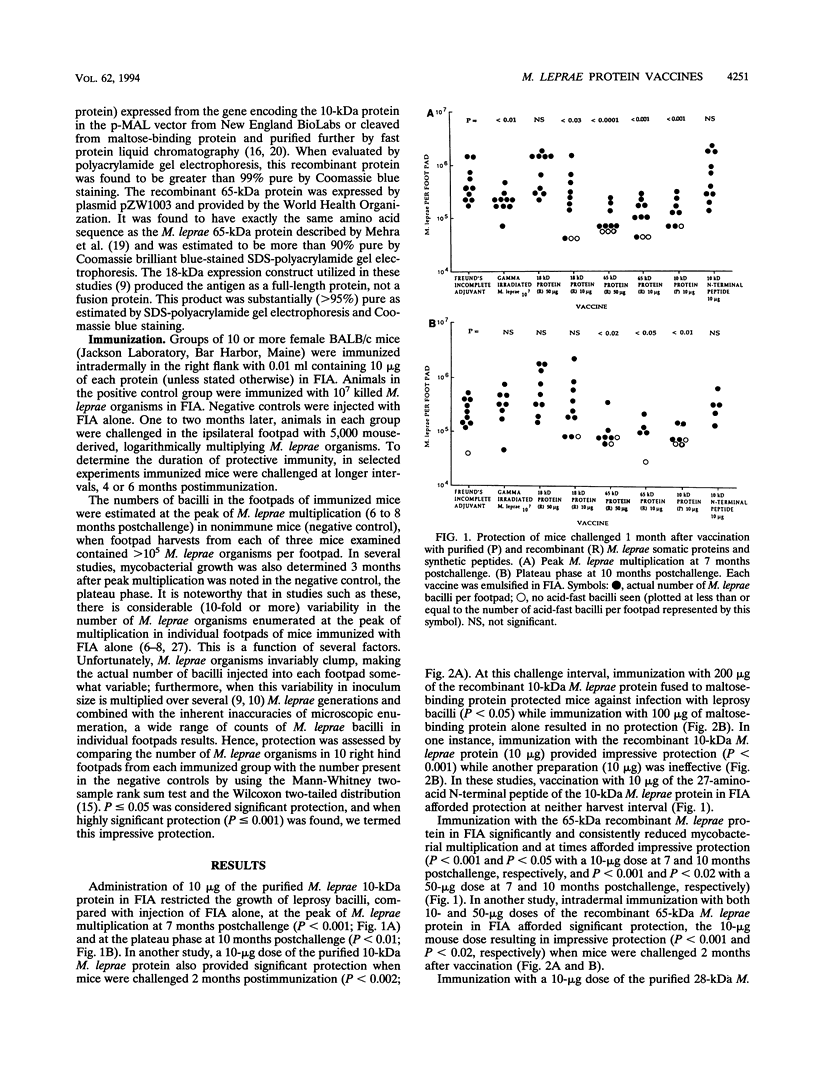
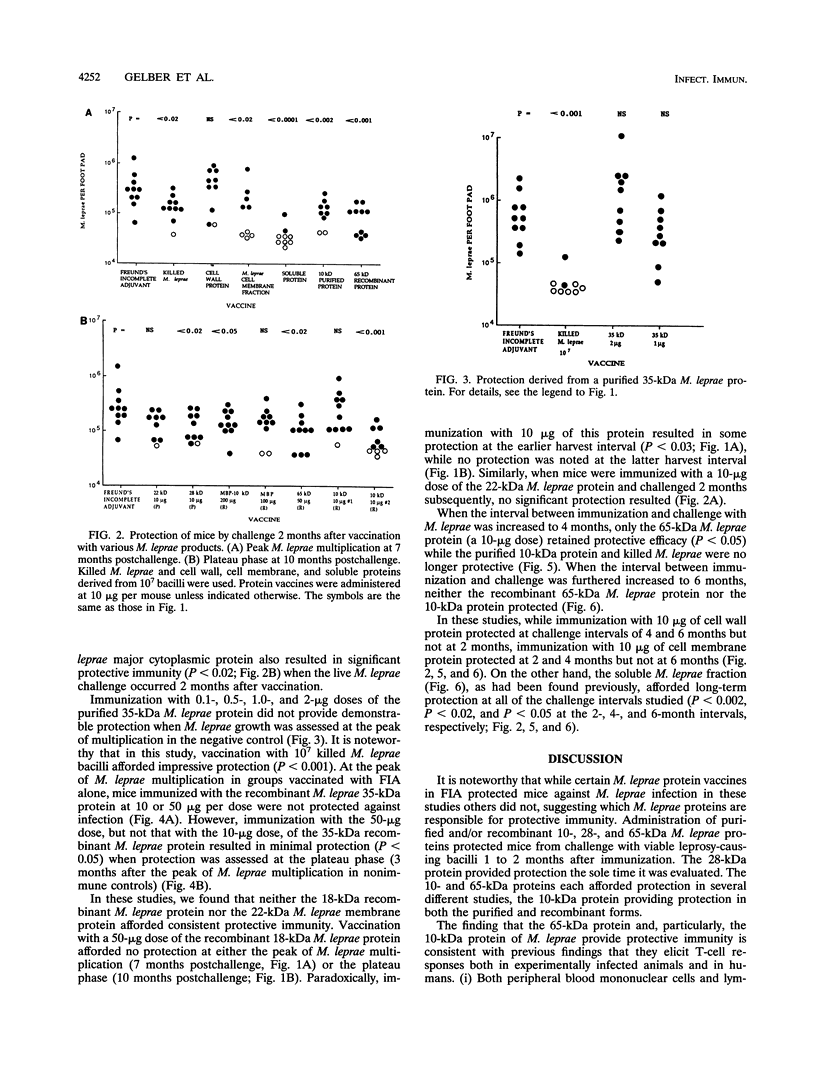
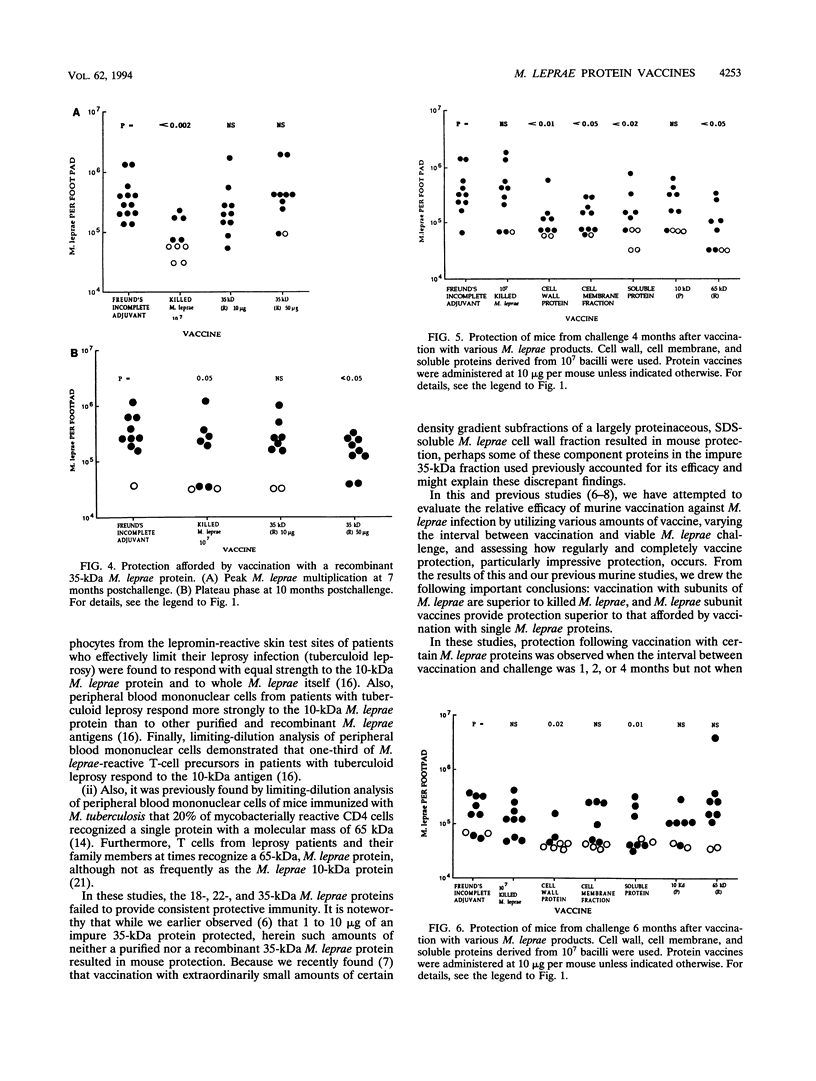
In this study, we evaluated vaccination with a number of purified, as well as recombinant, Mycobacterium leprae proteins for protective efficacy in mice. BALB/c mice were immunized intradermally with various native somatic (purified) or recombinant M. leprae proteins and their synthetic polypeptides emulsified in Freund's incomplete adjuvant. The protective efficacy of these preparations was assessed by enumeration of bacilli in the footpads of mice challenged with viable M. leprae 1 to 2 months following immunization. Protection was afforded by the purified and recombinant 10-kDa M. leprae cytoplasmic heat shock protein, the recombinant cell wall-associated 65-kDa M. leprae heat shock protein, and to a lesser extent, the purified 28-kDa M. leprae cytoplasmic protein (superoxide dismutase). Vaccination with either the purified or recombinant 35-kDa M. leprae cell membrane protein, the synthetic 27-amino-acid N-terminal peptide of the 10-kDa protein, the recombinant 18-kDa M. leprae protein, or the purified 22-kDa cell membrane protein was ineffective. When the interval between immunization and challenge was increased to 6 months, the purified 10-kDa M. leprae protein and the recombinant 65-kDa M. leprae protein lost vaccine efficacy, while a sodium dodecyl sulfate-soluble protein fraction of the M. leprae cell wall (soluble proteins), as had been found previously, continued to protect, suggesting that multiple M. leprae protein epitopes are critical for solid vaccine protection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993 Mar;61(3):844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Convit J., Sampson C., Zúiga M., Smith P. G., Plata J., Silva J., Molina J., Pinardi M. E., Bloom B. R., Salgado A. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet. 1992 Feb 22;339(8791):446–450. doi: 10.1016/0140-6736(92)91056-e. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Stoker N. G., Lee S. P., Jackson M., Grant K. A., Jouy N. F., Lucas S. B., Hasan R., Hussain R., McAdam K. P. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989 Jul;57(7):1979–1983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Gelber R. H., Brennan P. J., Hunter S. W., Munn M. W., Monson J. M., Murray L. P., Siu P., Tsang M., Engleman E. G., Mohagheghpour N. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect Immun. 1990 Mar;58(3):711–718. doi: 10.1128/iai.58.3.711-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber R. H., Murray L., Siu P., Tsang M. Vaccination of mice with a soluble protein fraction of Mycobacterium leprae provides consistent and long-term protection against M. leprae infection. Infect Immun. 1992 May;60(5):1840–1844. doi: 10.1128/iai.60.5.1840-1844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Kalpana G. V., Cirillo J. D., Pascopella L., Snapper S. B., Udani R. A., Jones W., Barletta R. G., Bloom B. R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Lwin K., Sundaresan T., Gyi M. M., Bechelli L. M., Tamondong C., Garbajosa P. G., Sansarricq H., Noordeen S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull World Health Organ. 1985;63(6):1069–1078. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Bajardi A. C., Grisso C. L., Sieling P. A., Alland D., Convit J., Fan X. D., Hunter S. W., Brennan P. J. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992 Jan 1;175(1):275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Samperio P., Lamb J., Bothamley G., Stanley P., Ellis C., Ivanyi J. Molecular study of the T cell repertoire in family contacts and patients with leprosy. J Immunol. 1989 May 15;142(10):3599–3604. [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- ROSENTHAL S. R., LOEWINSOHNE, GRAHAM M. L., LIVERIGHT D., THORNE G., JOHNSON V. BCG vaccination against tuberculosis in Chicago. A twenty-year study statistically analyzed. Pediatrics. 1961 Oct;28:622–641. [PubMed] [Google Scholar]
- STEIN S. C., ARONSON J. D. The occurrence of pulmonary lesions in BCG-vaccinated and unvaccinated persons. Am Rev Tuberc. 1953 Nov;68(5):695–712. doi: 10.1164/art.1953.68.5.695. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C., van Landingham R. M., Walker L. L., Ye S. Z. Comparison of the immunogenicity of vaccines prepared from viable Mycobacterium bovis BCG, heat-killed Mycobacterium leprae, and a mixture of the two for normal and M. leprae-tolerant mice. Infect Immun. 1983 Jun;40(3):1096–1103. doi: 10.1128/iai.40.3.1096-1103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schooten W. C., Ottenhoff T. H., Klatser P. R., Thole J., De Vries R. R., Kolk A. H. T cell epitopes on the 36K and 65K Mycobacterium leprae antigens defined by human T cell clones. Eur J Immunol. 1988 Jun;18(6):849–854. doi: 10.1002/eji.1830180604. [DOI] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



