Abstract
Background:
To evaluate the effectiveness of cilostazol in patients with intracranial arterial stenosis, we used magnetic resonance angiography (MRA). The drug's effect on the cerebral blood flow (CBF) was examined by single photon emission computed tomography (SPECT).
Methods:
In this retrospective study, we evaluated the clinical outcomes of 20 patients with stenosis in the M1 segment of the middle cerebral artery (MCA) who had suffered ischemic stroke within 12 weeks or manifested asymptomatic stenosis exceeding 50%. All patients received cilostazol (100 mg twice daily). MRA and SPECT (at rest and after acetazolamide challenge) studies were performed before and 6 and 12 months after the start of cilostazol treatment.
Results:
In 5 patients the stenotic lesion showed improvement on MRA. Mean stenosis before cilostazol therapy was 71.7 ± 4.9%, which improved to 39.0 ± 3.2% at 6 months and to 27.2 ± 2.8% at 12 months. SPECT study showed that CBF was improved in 3 patients; in one there was improvement at rest and the other 2 manifested improvement upon acetazolamide challenge.
Conclusions:
Cilostazol had a remodeling effect on stenotic lesions due to arteriosclerotic changes and improved CBF in some patients.
Keywords: antiplatelet therapy, cerebral blood flow, intracranial artery stenosis, single photon emission computed tomography
INTRODUCTION
Antiplatelet drugs are effective for the secondary prevention of cerebral infarction. The long-term daily administration of aspirin is useful in efforts to prevent serious vascular events in survivors of ischemic stroke; it reduces the recurrence rate and improves survival.[3–5] Cilostazol (Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan) has been shown to be useful for the prevention of ischemic stroke. It is a phosphodiesterase 3 inhibitor with both antiplatelet and vasodilating effects, and its mechanisms of action differ from those of aspirin.[9,21] Huang et al. observed no significant difference in the recurrence rate in patients with ischemic stroke who were randomly assigned to either cilostazol or aspirin therapy, and suggested cilostazol as a more effective and safer alternative to aspirin.[8] Cilostazol has been shown to be effective in the secondary prevention of stroke[6] and it prevented the occurrence of re-stenosis after coronary angioplasty[15] and stenting and decreased the carotid intima media thickness in diabetic patients.[1] Katakami et al. reported that compared with aspirin, cilostazol potently inhibited the progression of carotid intimamedia thickening.[10]
In their cilostazol stroke prevention study, a placebo-controlled double-blind trial, Shinohara et al. found that cilostazol effectively prevented the recurrence of vascular events in patients with lacunar infarction.[16] Kwon et al. who performed a trial of cilostazol in their symptomatic intracranial arterial stenosis study, reported that it prevented progression of the stenotic region.[12] They used magnetic resonance angiography (MRA) to evaluate intracranial arterial stenosis. Although MRA is useful for assessing longitudinal changes of intracranial arterial stenosis, it does not yield any information on the cerebral blood flow (CBF).
We performed a retrospective study on patients with symptomatic intracranial arterial stenosis who were treated with cilostazol. We used MRA to evaluate improvement of their stenoses and single photon emission computed tomography (SPECT) to study their CBF.
MATERIALS AND METHODS
Our retrospective study included 20 patients with stenosis in the M1 segment of the middle cerebral artery (MCA) who were treated between April 2007 and March 2010. All patients had suffered ischemic stroke within 12 weeks or presented with asymptomatic stenosis exceeding 50%. None of the patients had other stenotic lesions in the other internal carotid or vertebro basilar arteries. Inclusion criteria were as follows: (1) almost independent pursuit of the activities of daily living (ADL) (modified Rankin scale 0—2), (2) no or small ischemic lesions on magnetic resonance images (MRI), (3) absence of enhanced ischemic lesions on MRI, and (4) stable general condition without heart failure. Patients with malignant tumor, liver dysfunction, renal failure, heart failure, or respiratory dysfunction were excluded, as were patients who had suffered cardiac infarct within 6 months, were treated for diabetes and had a fasting blood glucose level exceeding 300 mg/dl, manifested artery to artery embolism or cardioembolic infarction, congestive heart failure, uncontrolled hypertension (diastolic blood pressure >110 mmHg), bleeding tendency, or hypersensitivity to cilostazol.
Consequently, 20 patients underwent cilostazol treatment (100 mg twice daily). They were 16 men and 4 women (mean age 64.5 ± 6.5 years); 12 (60%) had suffered atherothrombotic infarction and 8 (40%) were asymptomatic. Patients manifesting palpitation, tachycardia, or headache after cilostazol trial were changed to 100 mg cilostazol per day for a week, and then 100 mg twice a day. If their symptoms failed to improve, cilostazol treatment was stopped.
Patients included in this study were evaluated for ischemic stroke recurrence, hemorrhagic stroke including hemorrhagic infarction, additional stroke of unknown etiology, and death. All analyses were performed in our outpatient department. Follow-up studies were performed 6 and 12 months after the start of cilostazol treatment.
MRI scans were inspected for changes in ischemic lesion, for the development of new cerebrovascular incidents, and for unknown nonspecific changes. MRA studies to assess the degree of stenosis were performed with a 3D time-of-flight gradient echo technique for intracranial arteries. CBF was evaluated on SPECT scans using 99m Tc- hexomethyl propylene amine oxime (HMPAO, 740 MBq). After completion of the 1st scan, within less than 10 min, stress was induced with an intravenous (iv) injection of 1g acetazolamide; a 2nd scan was obtained 5 min after the 2nd iv delivery of 99m Tc-HMPAO.
Patients with pre-treatment MRA and SPECT evidence of lesions were re-examined 6 and 12 months after the start of cilostazol treatment using the same neuroimaging techniques. Changes in the M1 stenosis were analyzed by MRA. Continuous data are shown as the mean ± standard deviation (SD). The unpaired t test was used to compare continuous variables in 2 patient groups, those in whom cilostazol was effective (stenosis improved to less than 50%) and patients in whom it was not (stenosis did not improve to less than 50%). The χ2 test or Fisher's exact test was used to compare ratios. Statistical significance was defined as P < 0.05.
RESULTS
Three patients experienced drug-induced tachycardia or headache (tachycardia, n = 1; mild headache, n = 2). In these three patients we changed the administration method (100 mg per day for a week followed by 100 mg twice daily). Consequently, two patients experienced symptom abatement and they were continued on cilostazol for 12 months. The other patient was changed to aspirin. Therefore, 19 patients received cilostazol treatment and were evaluated by MRI, MRA, and SPECT 6 and 12 months after the start of cilostazol therapy.
The overall rate at 6 months for stroke, myocardial infarction, and death was 5.1% (1/19); the affected patient suffered an ipsilateral atherothrombotic infarction. At 12-month follow-up there were no additional cases of stroke. Consequently, the overall 1-year outcome for stroke, myocardial infarction, and death remained at 5.1%. No patient suffered hemorrhagic complications.
Cilostazol was effective in 5 patients. Their mean stenosis pre-treatment was 71.7 ± 4.9% on MRA; it improved to 39.0 ± 3.2% at 6 months and to 27.2 ± 2.8% at 12 months. Cilostazol was not effective in 14 patients; their mean stenosis pre-treatment was 75.8 ± 5.3%; it was 71.6 ± 4.7% at 6 months and 70.3 ± 4.8% at 12 months [Figure 1]. The difference between the two groups at 6 and 12 months was significant (P < 0.001 and P < 0.001, respectively).
Figure 1.
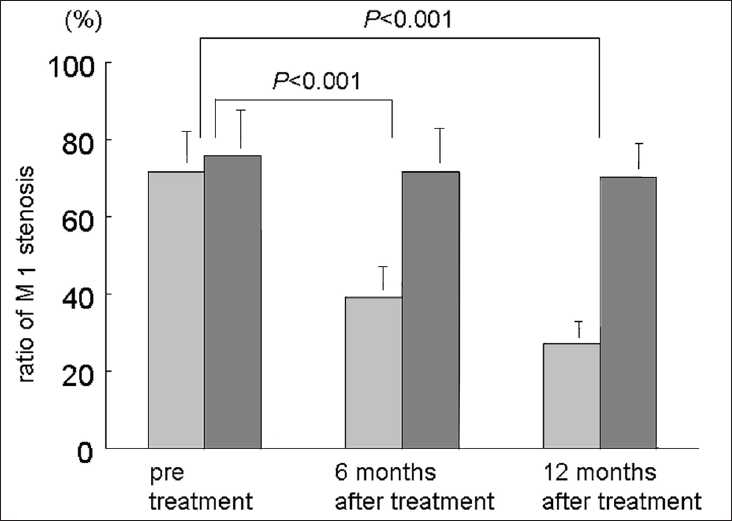
Burr graph demonstrating changes in M1 stenosis before, and 6 and 12 months after cilostazol treatment. Light and dark bars show the suspected mean ratio of M1 stenosis in 5 responders and 14 non-responders.
Three of five patients in whom cilostazol was effective showed improvements in CBF on SPECT study; one manifested improvement at rest and the other 2 improved upon acetazolamide challenge. The other two patients in the effective group and all 14 patients in the non-effective group showed no improvement in CBF on SPECT study at rest and upon acetazolamide challenge.
REPRESENTATIVE CASES
Case 1 - Figure 2
Figure 2.
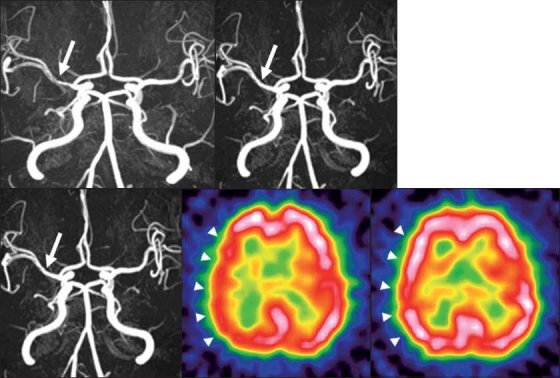
2A: Pretreatment magnetic resonance angiography (MRA) showing a stenotic lesion (60%) at the right M1 (white arrow).
2B: Magnetic resonance imaging (MRI) scan acquired 6 months after treatment shows improvement of the stenotic lesion (20%) at the right M1(white arrow).
2C: MRA obtained 12 months after the start of cilostazol treatment shows improvement of the stenotic lesion (16.7%) at the right M1 (white arrow).
2D: Pretreatment single photon emission computed tomography (SPECT) shows a decrease in CBF in the right cerebral hemisphere (white arrowhead).
2E: SPECT obtained 12 months after the start of cilostazol treatment shows improvement of the CBF in the right cerebral hemisphere (white arrowhead).
This 50-year-old man had a history of transient motor weakness on the left side. MRA confirmed right MCA stenosis (60%); it improved to 20% and 16.7% at 6 and 12 months after the start of cilostazol treatment (200 mg per day). Before cilostazol therapy, his CBF at rest was decreased in the right cerebral hemisphere; it improved at 12-month follow-up and he was free of symptoms.
Case 2 - Figure 3
Figure 3.
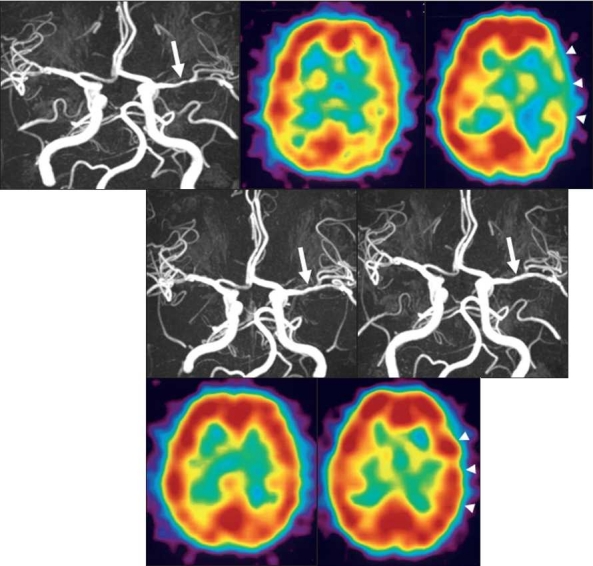
3A: Pretreatment MRA shows a stenotic lesion (85%) at the left M1 (white arrow)
3B: Pretreatment SPECT shows no laterality in CBF at rest.
3C: Pretreatment SPECT shows hypovasoreactivity in the left temporo-occipital region after acetazolamide challenge (white arrow head).
3D: MRA obtained 6 months after the start of cilostazol treatment shows improvement of the stenotic lesion (30%) at the left M1 (white arrow).
3E: MRA acquired 12 months after the start of cilostazol treatment shows improvement of the stenotic lesion (20%) at the left M1 (white arrow).
3F: SPECT obtained 12 months after the start of cilostazol treatment shows increased CBF in the left cerebral hemisphere.
3G: SPECT obtained 12 months after the start of cilostazol treatment shows CBF improvement in the left cerebral hemisphere after acetazolamide challenge (white arrowhead).
This 60-year-old woman had a history of mild sensory disturbance on the right side. MRA revealed left MCA stenosis (85%). Before treatment, her CBF at rest manifested no marked laterality. However, acetazolamide challenge disclosed extensive CBF reduction in the left temporo-occipital region. Her stenosis improved to 30% and 20% at 6 and 12 months after the start of cilostazol administration (200 mg per day). Acetazolamide challenge at 12-month follow-up showed a CBF increase in the right cerebral hemisphere and she was free of ischemic events.
DISCUSSION
Stenosis of intracranial arteries is causative in ischemic stroke and antiplatelet therapy is important for its prevention.[3–5] Although aspirin has been the commonly used antiplatelet drug, other medications have been introduced. Whether their benefits exceed those of aspirin remains an open debate. Hankey et al.[7] found that clopidogrel was slightly more effective than aspirin and Shinohara et al.[16] reported cilostazol to be useful for the prevention of recurrent vascular events in patients with lacunar infarction. In a cilostazol stroke prevention study to assess the safety and efficacy of cilostazol vs. aspirin, their effectiveness was observed to be similar with respect to the prevention of recurrent stroke in patients with noncardioembolic stroke; cilostazol was more efficacious and safer than aspirin for the prevention of secondary stroke.[17] Kwon et al. reported that cilostazol prevented progression of the intracranial stenotic region.[12] Their study addressed the effect of cilostazol on the progression of intracranial arterial stenosis (IAS) in the M1 segment of MCA. The degree of stenosis was assessed on MRA images. In patients treated with cilostazol, 3 of 45 (6.7%) symptomatic IAS progressed and 11 (24.4%) regressed; the rates were 15 (28.8%) and 8 (15.4%) in patients given a placebo and the difference between the two patient groups was significant (P < 0.008). CBF in patients with regression of IAS was not evaluated.
Kim et al.[11] attempted stent-assisted angioplasty in 14 patients with symptomatic high-grade stenosis (>60%) on the proximal portion of MCA. These patients had suffered recurrent transient ischemic attacks resistant to medical therapy or one or more strokes and 11 manifested perfusion defects on SPECT. Follow-up SPECT showed improved perfusion in the affected MCA territory after stent-assisted angioplasty.[11] Birk et al. presented the first results on the effect of cilostazol on the cerebral hemodynamics in normal individuals.[2] In their double-blind, randomized, crossover study, cilostazol (100 mg twice daily) or placebo was administered orally to 12 healthy participants. They reported that cilostazol had no effect on regional CBF within a 4-h observation period. On the other hand, according to Oishi et al., CBF in the cortex was significantly higher after than before cilostazol administration and cilostazol had a better effect on the cerebral circulation than ticlopidine hydrochloride in patients in the chronic stage of cerebral infarction 2 weeks and 3 months after onset.[14] However, their observations may be attributable to a period effect.
We found that the 12-month administration of cilostazol improved stenosis in the M1 segment in five of 19 patients (26.3%). In three of these, SPECT showed improved CBF. Although the prevention of intracranial arterial stenosis by cilostazol has been reported, that study did not address CBF.[12] Therefore, ours is the first documentation that cilostazol prevents the progression of stenosis and improves CBF and we suggest that its beneficial effect on CBF is reflected in the improvement of the stenotic lesions in the M1 segment. Therefore, the improved CBF may be due to not only the vasodilatation effect of cilostazol but also its remodeling effect on the M1 stenotic lesion.
What mechanisms participate in improving M1 arterial stenosis? Other antiplatelet drugs exhibited no remodeling effect on intracranial arteriosclerotic lesions. The mechanisms of action of cilostazol were multimodal. One effect is the inhibition of type III phosphodiesterase activity in platelets and the increase of intracellular levels of cyclic adenosine monophosphate (cAMP).[13,18,20] The increase in the intraplatelet concentration of cAMP inhibits the production of thromboxane A2 and platelet aggregation by inhibiting phospholipase and cyclooxygenase. In addition, it acts as an arterial vasodilator, probably via its direct action on vascular smooth muscle. Intracellular cAMP blocks the release of calcium ions from intracellular storage granules in smooth muscle cells, thereby inhibiting the function of contractile proteins.[21] Cilostazol also suppresses the proliferation of smooth muscle cells, an effect that may also be mediated via an increase in the level of intracellular cAMP in smooth muscle cells.[19]
In the present study, cilostazol decreased triglycerides, low density lipoprotein, and total cholesterol and increased high density lipoprotein cholesterol.[22] Although the magnitude of its lipid-altering effect is small, it adds to the potential clinical utility of the drug.
Factors involved in the remodeling of the stenotic M1 segment after cilostazol administration remain to be elucidated. Large trials are needed to identify the role of cilostazol in the improvement of symptomatic intracranial artery stenosis.
CONCLUSIONS
Our preliminary retrospective investigation of the effectiveness of cilostazol in a small series of patients with stenosis in the M1 segment of MCA. The drug had a remodeling effect on stenotic lesions due to arteriosclerotic changes and improved CBF in some of these patients.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/8/76145
Contributor Information
Yutaka Kai, Email: ykai@fc.kuh.kumamoto-u.ac.jp.
Masaki Watanabe, Email: wasakiwata@yahoo.co.jp.
Motohiro Morioka, Email: mmorioka@kumamoto-u.ac.jp.
Teruyuki Hirano, Email: terry07@kumamoto-u.ac.jp.
Shigetoshi Yano, Email: yanos@kumamoto-u.ac.jp.
Yuki Ohmori, Email: ohmori@fc.kuh.kumamoto-u.ac.jp.
Takayuki Kawano, Email: tkawano-nsu@umin.ac.jp.
Jun-Ichiro Hamada, Email: jhamada@ns.m.kanazawa-u.ac.jp.
Jun-Ichi Kuratsu, Email: jkuratsu@kumamoto-u.ac.jp.
REFERENCES
- 1.Ahn CW, Lee HC, Park SW, Song YD, Huh KB, Oh SJ, et al. Decrease in carotid intima media thickness after 1 year of cilostazol treatment in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52:45–53. doi: 10.1016/s0168-8227(00)00235-7. [DOI] [PubMed] [Google Scholar]
- 2.Birk S, Kruuse C, Petersen KA, Jonassen O, Tfelt-Hansen P, Olesen J. The phosphodiesterase 3 inhibitor cilostazol dilates large cerebral arteries in humans without affecting regional cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:1352–8. doi: 10.1097/01.WCB.0000143536.22131.D7. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative overview of randomised trials of antiplatelet therapy-I Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. Antiplatelet Trialists' Collaboration. [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborative overview of randomised trials of antiplatelet therapy-II Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994;308:159–68. Antiplatelet Trialists' Collaboration. [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative overview of randomised trials of antiplatelet therapy-III Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ. 1994;308:235–46. Antiplatelet Trialists′ Collaboration. [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh F. A placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis. 2000;19:147–57. doi: 10.1053/jscd.2000.7216. [DOI] [PubMed] [Google Scholar]
- 7.Hankey GJ, Sudlow CL, Dunbabin DW. Thienopyridines or aspirin to prevent stroke and other serious vascular events in patients at high risk of vascular disease.A systematic review of the evidence from randomized trials? Stroke. 2000;31:1779–84. doi: 10.1161/01.str.31.7.1779. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: A randomised, double-blind, pilot study. Lancet Neurol. 2008;7:494–9. doi: 10.1016/S1474-4422(08)70094-2. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda Y, Kikuchi M, Murakami H, Satoh K, Murata M, Watanabe K, et al. Comparison of the inhibitory effects of cilostazol, acetylsalicylic acid and ticlopidine on platelet functions ex vivo.Randomized, double-blind cross-over study. Arzneimittelforschung. 1987;37:563–6. [PubMed] [Google Scholar]
- 10.Katakami N, Kim YS, Kawamori R, Yamasaki Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus: Principal results of the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) study: A randomized trial. Circulation. 2010;121:2584–91. doi: 10.1161/CIRCULATIONAHA.109.892414. [DOI] [PubMed] [Google Scholar]
- 11.Kim JK, Ahn JY, Lee BH, Chung YS, Chung SS, Kim OJ, et al. Elective stenting for symptomatic middle cerebral artery stenosis presenting as transient ischaemic deficits or stroke attacks: short term arteriographical and clinical outcome. J Neurol Neurosurg Psychiatry. 2004;75:847–51. doi: 10.1136/jnnp.2003.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, et al. Cilostazol prevents the progression of symptomatic intracranial arterial stenosis: The multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–6. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–86. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 14.Oishi M, Mochizuki Y, Shikata E, Satho Y. Effect of cilostazol on cerebral blood flows in chronic stage of cerebral circulation. Keio J Med. 2000;49:145–7. [PubMed] [Google Scholar]
- 15.Park SW, Lee CW, Kim HS, Lee NH, Nah DY, Hong MK, et al. Effects of cilostazol on angiographic restenosis after coronary stent placement. Am J Cardiol. 2000;86:499–503. doi: 10.1016/s0002-9149(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara Y, Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, et al. Antiplatelet cilostazol is beneficial in diabetic and/or hypertensive ischemic stroke patients.Subgroup analysis of the cilostazol stroke prevention study. Cerebrovasc Dis. 2008;26:63–70. doi: 10.1159/000135654. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara Y, Katayama K, Uchiyama S, Yamagichi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (CSPS 2): An aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–68. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 18.Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–56. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Oida K, Fujiwara R, Maeda H, Hayashi Y, Takai H, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20:900–6. doi: 10.1097/00005344-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Gotoh F, Fukuuchi Y, Amano T, Uematsu D, Kawamura J, et al. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke. 1989;20:668–73. doi: 10.1161/01.str.20.5.668. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Ishikawa T, Hagiwara M, Onoda K, Itoh H, Hidaka H. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacol. 1988;36:313–20. doi: 10.1159/000138400. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Mitani K, Kotosai K, Nozako M, Miyakoda G, Yabuuchi Y. Antiatherogenic effects of cilostazol and probucol alone, and in combination in low-density lipoprotein receptor-deficient mice fed with a high fat diet. Horm Metab Res. 2008;40:473–8. doi: 10.1055/s-2008-1065348. [DOI] [PubMed] [Google Scholar]


