Abstract
Background:
The incidence of tuberculosis is increasing, and skeletal tuberculosis accounts for 10-20% of all extrapulmonary cases. Spinal tuberculosis occurs mostly in children and young adults. It causes bone destruction, spinal deformity and neural complications.
Materials and Methods:
Our study includes 37 children (below 15 years of age) with spinal tuberculosis treated in our department in the last 6 years. The demographic data, clinical profile, surgical intervention and outcome of these children are reported.
Results:
The mean age ranged from 4 to 15 years, with an average of 9.1 years, and the male/female ratio was 1.8. Thirty patients (81%) had progressive inflammatory rachialgia and only six patients (16.2%) had neurological symptoms. The lumbar spine was mostly affected (23 cases). All patients have benefited from antituberculous chemotherapy (Regimen 2SRHZ/10RH) associated with spinal immobilization during 3 months. The surgical treatment was indicated in seven patients because of the presence of large bilateral abscess of the psoas muscle in one patient and the presence of severe neurological symptoms in the six remaining patients. The evolution was favorable in all cases, including those with neurological symptoms. There was no case of death and the length of follow-up for these patients ranged between 1 and 4 years.
Conclusion:
Spinal tuberculosis is still a prevalent disease in developing countries, mainly occurring in children. Complications of the disease can be devastating because of its ability to cause bone destruction, spinal deformity and paraplegia. Therefore, an early diagnosis and establishment of treatment are necessary to expect a good outcome.
Keywords: Chemotherapy, child, neurosurgery, spine, tuberculosis
INTRODUCTION
The incidence of tuberculosis is increasing in both the developing and the developed countries.[1] It affects the spine in up to 50% of all osteoarticular tuberculosis patients.[1–8] Spinal tuberculosis occurs most commonly in children and young adults.[9–14] It causes bone destruction, spinal deformity and neural complications.[13–18] The diagnosis of this disease is based on clinical and radiological evidences, particularly in the endemic areas of the world. The treatment targets are to confirm the diagnosis, eradicate the infection, achieve a decompression of the spinal canal material and correct or prevent spinal deformity and possible sequelae.[9–18]
In this work, we present a retrospective series of 37 infants diagnosed with spinal tuberculosis and we discuss the pathophysiology as well as the clinical, paraclinical and therapeutical features of this pathology in childhood.
MATERIALS AND METHODS
This retrospective study included 37 infants below 15 years of age. These children were all hospitalized at the Neurosurgery Department of the University Hospital of Fez (Morocco) for spinal tuberculosis between January 2001 and December 2006. Only six patients were admitted in a state of compression of the neuraxis (spinal cord, cauda or nerve roots).
Hospital clinical records of each patient were reviewed; the demographic data, clinical symptoms, level involved, type of lesion, any associated lesions, biological parameters, treatment received, duration of hospital stay and outcome were all analyzed.
RESULTS
There were 123 cases of spinal tuberculosis treated in our department during the last 6 years (between January 2001 and December 2006). Thirty-seven children with spinal tuberculosis were identified. There were 24 boys and 13 girls, the male/female ratio being 1.8. The age of our patients ranged between 4 and 15 years, and the mean age was 9.1 years.
56.7% of the patients were from rural areas and the remaining patients were from cities. Bacille Calmette-Guerin (BCG) vaccination at birth was administered for all children. However, recall of vaccine was completed in two patients only.
The notion of tuberculous contagion was found in a child of the series who had an uncle under treatment for pleuro-pulmonary tuberculosis. None of our patients was previously treated for spinal or extraspinal tuberculosis.
The duration of symptoms prior to admission ranged from 3 weeks to 4 years, with a median delay of admission of 9 months.
Signs of tuberculous impregnation (anorexia, night sweats, fever and weight loss) were present in all patients. Thirty patients (81%) suffered from progressive inflammatory rachialgia. The pre-operative neurological examination was normal in 31 patients. In contrast, six patients had signs of compression of the neuraxis (i.e., syndrome of chronic spinal cord compression or cauda equina syndrome). The neurological deficits were classified according to the Frankel grading: one patient was tetraplegic and was graded B and five patients had paraparesia (four of grade C and one of grade D). The spine examination noticed a gibbosity in the front of the involved level in nine cases and a scoliosis in one patient. Neither paravertebral cold abscess nor adenopathies were recorded in our patients.
All patients had systematically benefited of the anteroposterior and lateral X-ray radiography at the level of the lesion [Figure 1]. A computed tomography (CT) scan was performed in all cases. However, magnetic resonance imaging (MRI) was only accomplished in 10 patients, including six patients with signs of compression of the neuraxis and four patients referred from other hospitals [Figure 2].
Figure 1.
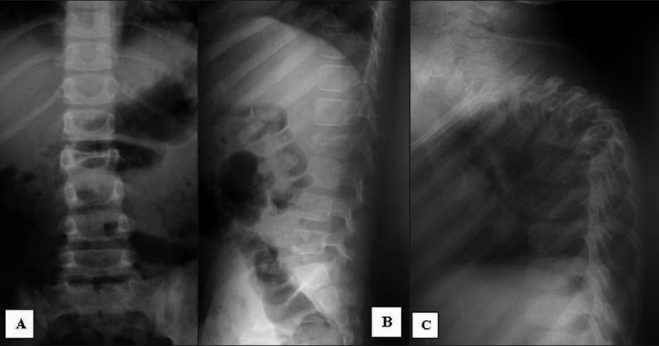
Anteroposterior (A) and lateral (B) X-ray of the lumbar spine showing spondylitis of the second lumbar vertebral body (L2) and lateral X-ray of the thoracic spine (C) of another patient showing a severe kyphosis as a consequence of T5-T6 spondylitis
Figure 2.
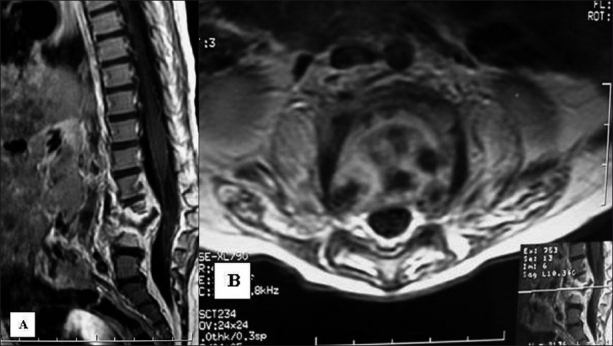
Magnetic resonance imaging of the lumbar spine in sagittal (A) and axial (B) slices after injection of Godolinium demonstrating L3-L4 spondylitis associated with intraspinal abscess causing a moderate kyphosis and a slight compression of the cauda equina
The lumbar spine was mostly affected in 23 cases. The thoracolumbar junction (T11-L1) was involved in seven cases, the thoracic spine in five cases and the cervical spine in two cases. There were three patients with multiple spine level involvement. Patterns of vertebral column involvement were divided into two types: spondylodiscitis with simultaneous vertebral and discal involvement was seen in 33 cases (89%) and spondylitis with vertebral lesion was observed in four cases (11%). An X-ray of the chest was routinely accomplished and was normal in all cases.
The biological assessment includes inflammatory tests consisting of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), full blood count, hepatic assessment, tuberculin intradermal test and exploration of mycobacterium in the spittle or in the gastric liquid. The biological inflammatory syndrome was present in 33 cases and the intradermal test of tuberculin was positive (>6 mm of induration) in 28 cases. Inflammatory anemia was present in seven patients. However, test of mycobacterium was always negative.
All patients benefited from antituberculous chemotherapy. The regimen adopted was 2SRHZ/10RH in all cases: Streptomycin (1 mg/kg/day), Rifampicin (10 mg/kg/day), Isoniazid (5 mg/kg/day) and Pyrazinamide (30 mg/kg/day) were administrated during 2 months and then Rifampicin and Isoniazid were continued during the following 10 months. A hepatic assessment of the control was systematically carried out every 3 months until the end of the treatment protocol.
A spinal immobilization by corset, orthesis or minerva was accomplished according to the affected level, and this was set for 3 months in all patients.
Only seven patients of our series were treated surgically: one patient was operated for a large bilateral abscess of the psoas muscle and the six remaining patients were operated as they had neurological symptoms secondary to compression of the spinal cord. All these patients were operated via an anterior approach (one cervicotomy, three thoracotomies and two thoracophrenotomies). Only the patient operated by cervicotomy had benefited from the anterior osteosynthesis by a corset-associating bone graft. The five other patients operated by the anterior approach profited from arthrodesis by iliaque graft only after spinal cord decompression. In the operated cases, the diagnosis of tuberculosis was confirmed by a histological study. Rehabilitation was an essential therapeutic complement among all patients who had a neurological deficit.
After discharge from the hospital, the patients benefited from clinical, biological and radiological control routinely. All patients were seen and examined three times a month for the first 3 months and then at 3-month intervals thereafter until the treatments were finished. The X-ray of the control was systematically carried out during the first and the second 3 months of treatment, which allowed supervising the radiological consolidation of the heart tuberculous site. The CT scan of the control was taken in two of the operated patients by thoracotomy.
There was no recurrent infection and no multidrug-resistant bacillus types in this series. The evolution was favorable in all cases, including in those with neurological symptoms. All patients were able to walk without any support or assistance after a median duration of 5 months. Signs of radiographic consolidation were observed by the end of the third month of treatment in all cases. In our series, the mean duration of hospital stay was 11 days, ranging from 6 to 23 days.
The overall complication rate was 11% (four patients); two of them developed parietal infection that was successfully managed using medical treatment. The third patient presented a slight thoracic kyphosis of <20° and was treated conservatively by orthopedic treatment, and had a favorable outcome. We also noticed a bedsore in one patient, which was treated with daily dressings with a good outcome. No death was recorded in the studied series. The average follow-up period for these patients was 2 years, ranging from 1 to 4 years.
DISCUSSION
Vertebral tuberculosis represents the most common form of skeletal tuberculosis and constitutes about 50% of all cases in reported series.[16] It affects all individuals and, particularly, children. Children represent the high-risk group for being affected by the disease.[2] This disease is still endemic in developing countries and is increasingly recorded in the developed world because of human immunodeficiency virus infection.
In children, the main route of infection of spinal tuberculosis is through hematogenous spread from a primary site of infection, which is often unknown.[9] A concomitant active pulmonary disease is present in <50% of the cases.
Indeed, mycobacterium is deposited via the ending arterioles in the vertebral body adjacent to the anterior aspect of the vertebral end plate. Thus, the anterior portion of the vertebral body is most commonly involved. While the infection is developing, the cortex is disrupted and the infection may spread up and down, stripping the anterior and posterior longitudinal ligaments and the periosteum from the front and sides of the vertebral bodies. This results in loss of the periosteal blood supply and distraction of the anterolateral surface of the vertebrae.[16]
The intervertebral disc is involved late in the disease process leading to disc space narrowing and constitution of spondylodiscitis.[5] Subligamentous spread of the infection may lead to multiple levels of contiguous or skip the vertebral body involvement. Extension of the infection into the adjacent soft tissue to form paravertebral or epidural masses is commonly observed. The end may result in neurological complications such as spinal cord compression.[9] In longstanding cases, there may be multiple levels of vertebral body collapse, resulting in a gibbous deformity.
In children, vertebral destruction is more severe because most bone is cartilaginous.[13] Additionally, the angulation is mostly significant due to growth retardation of the anterior column and unrestricted growth of the posterior column.
The clinical symptoms of spinal tuberculosis in children are often insidious and include back pain, fever, paraparesis, sensory disturbance and bowel and bladder dysfunction.[14] In our context, delayed diagnosis of this pathology is generally due to the insidious course of the disease associated with the poor socioeconomic conditions of the patients.
Spinal tuberculosis in children commonly affects the dorsal spine; cervical spine involvement occurs in <5% of the patients.[3,7,8,10] In contrast, the lumbar spine was mostly affected in our study. This difference might be explained by the limited sample size.
X-ray images might be normal or may show mild osteopenia in early disease. It is estimated that bone loss of more than 50% must occur before changes are evident on X-ray images. Later on, loss of height of vertebral bodies may be observed, with indistinct end-plates, bony erosions and sequestra. The collapse of the intervertebral disc space occurs when disc involvement happens. Extension of infection into the psoas muscle causes asymmetric bulging of the psoas outline. Scalloping of the anterior vertebral body may be noticed if subligamentous spread occurs.[12]
The CT scan is useful in demonstrating bony sclerosis and destruction, especially in the posterior elements, which are difficult to assess by conventional radiography. It is also a useful technique in guiding percutaneous biopsy. However, the CT scan is inferior to the MRI exploration in the exhibition of soft tissue masses, discs involvement and spinal cord compression. In fact, the MRI detects spinal tuberculosis 4-6 months earlier compared with the conventional methods.[6] It is also the modality of choice for determining the extent of soft tissue involvement of the disease process and for assessing the response to treatments.[1] Moreover, MRI is able to give precise information regarding the level of cord compression and is thus helpful for surgical planning in patients requiring surgical decompression.
Furthermore, Gadolinium administration improves the assessment of epidural, soft tissue and subligamentous spread of the infection. Edge enhancement of these soft tissue masses has been reported to be the diagnosis of tuberculosis of the spine.[1]
In a majority of the cases, the diagnosis of spinal tuberculosis was confirmed by radiological characteristic findings concomitant with other positive findings such insidious clinical history of fever and anorexia, positive tuberculin skin test, suggestive chest radiography/chest CT scan findings and/or a positive response to antituberculous drug therapy.[1] The histological evidences of tuberculosis are obtained only in a few patients that underwent surgery or needle aspiration.[1]
In general, spinal tuberculosis can be confidently diagnosed by clinical and radiography tools. If any case does not fit into the classical clinical or radiological findings suggestive of spinal tuberculosis, surgical treatment or at least needle aspiration should be performed and adequate tissue should be obtained for histopathological examination and final diagnosis.
Definitely, the most common differential diagnosis in children is vertebral osteomyelitis.[1] The insidious onset of the disease, the smooth margins of the paraspinal mass and the rim enhancement of this mass in MRI are the main criteria for distinguishing vertebral tuberculosis from pyogenic spondylitis.
The treatment goals of spinal tuberculosis are to eradicate the infection, to ensure a good recovery from any neurological deficits and to cure the disease with minimum residual spinal deformity.
Conservative treatment, including chemotherapy and orthopedic immobilization, remains the cornerstone of the management of spinal tuberculosis in children. The American Thoracic Society and the Centers for Disease Control and Prevention clearly indicated that bone and joint tuberculosis requires a minimum of 12 months of therapy.[4] Nevertheless, some reports have recently suggested that a shorter course may be appropriate in spinal tuberculosis.[15,18]
Surgical drainage is indicated when there are large abscesses, especially in the psoas muscle. Besides, children presenting or developing neurological deficit on follow-up must undergo surgery to prevent irreversible paraplegia or worsening deformity. Almost 3% of the spinal tuberculosis in children develops severe kyphosis of more than 60°.[17] Factors increasing the risk of severe kyphotic deformity are children being below 10 years of age, involvement of three or more vertebral bodies and localization of the lesion in the thoracic spine.[17] Severe kyphosis results in spinal cord compression and cardiopulmonary dysfunction and is cosmetically unacceptable.[17]
Finally, the surgical approach is not universally standardized. Prospective studies are needed to define the surgical approach, steps, surgical stages, problems and difficulties for correcting the kyphosis of 60° or more.
CONCLUSION
Spinal tuberculosis is still a prevalent disease in developing countries, mainly in children. Complications of the disease can be devastating because of its ability to cause bone destruction, spinal deformity and paraplegia. Therefore, an early diagnosis and establishment of treatment are necessary to expect a good outcome.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/1/75459
REFERENCES
- 1.Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol. 2002;32:798–805. doi: 10.1007/s00247-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 2.Agrons GA, Markowitz RI, Kramer SS. Pulmonary tuberculosis in children. Semin Roentgenol. 1993;28:158–72. doi: 10.1016/s0037-198x(05)80105-1. [DOI] [PubMed] [Google Scholar]
- 3.Behari S, Nayak SR, Bhargava V, Banerji D, Chhabra DK, Jain VK. Craniocervical tuberculosis: Protocol of surgical management. Neurosurg. 2003;52:72–81. [PubMed] [Google Scholar]
- 4.Bass JB, Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. Treatment of tuberculosis and tuberculosis infection in adults and children: American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149:1359–74. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 5.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809–19. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 6.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76:863–9. [PubMed] [Google Scholar]
- 7.Dobson J. Tuberculosis of the spine: An analysis of the results of conservative treatment and of the factors influencing the prognosis. J Bone Joint Surg Br. 1951;33:517–31. doi: 10.1302/0301-620X.33B4.517. [DOI] [PubMed] [Google Scholar]
- 8.Fang D, Leong JC, Fang HS. Tuberculosis of the upper cervical spine. J Bone Joint Surg Br. 1983;65:47–50. doi: 10.1302/0301-620X.65B1.6822601. [DOI] [PubMed] [Google Scholar]
- 9.Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34:853–60. doi: 10.1007/s00247-004-1223-7. [DOI] [PubMed] [Google Scholar]
- 10.Hsu LC, Leong JC. Tuberculosis of the lower cervical spine (C2-C7): A report of 40 cases. J Bone Joint Surg Br. 1984;66:1–5. doi: 10.1302/0301-620X.66B1.6693464. [DOI] [PubMed] [Google Scholar]
- 11.Loke TK, Ma HT, Chan CS. Magnetic resonance imaging of tuberculous spinal infection. Australas Radiol. 1997;41:7–12. doi: 10.1111/j.1440-1673.1997.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 12.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39:329–42. doi: 10.1016/s0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 13.Mushkin AY, Kovalenko KN. Neurological complications of spinal tuberculosis in children. Int Orthop. 1999;23:210–2. doi: 10.1007/s002640050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussbaum ES, Rockswold GL, Bergman TA, Erickson DL, Seljeskog EL. Spinal tuberculosis: A diagnostic and management challenge. J Neurosurg. 1995;83:243–7. doi: 10.3171/jns.1995.83.2.0243. [DOI] [PubMed] [Google Scholar]
- 15.Rajeswari R, Balasubramanian R, Venkatesan P, Sivasubramanian S, Soundarapandian S, Shanmugasundaram TK, et al. Short-course chemotherapy in the treatment of Pott's paraplegia: Report on five years follow-up. Int J Tuberc Lung Dis. 1997;1:152–8. [PubMed] [Google Scholar]
- 16.Tuli SM. Tuberculosis of the skeletal system. 2nd ed. New dElhi: Jaypee; 1997. Epidemiology and prevalence. [Google Scholar]
- 17.Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327–31. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 18.Upadhya S, Saji J, Yau A. Duration of anti-tuberculosis chemotherapy in conjunction with radical surgery in the management of spinal tuberculosis. Spine. 1996;21:1898–903. doi: 10.1097/00007632-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 19.Ndiaye M, Sene-Diouf F, Diop AG, Sakho Y, Ndiaye MM, Ndiaye IP. Pott's spinal cord compression in the child. Dakar Med. 1999;44:49–53. [PubMed] [Google Scholar]


