Abstract
Background & Aims
Aberrant activation of Ras pathway is ubiquitous in human hepatocarcinogenesis, but the molecular mechanisms leading to Ras induction in the absence of Ras mutations remain underinvestigated. We defined the role of Ras GTPase activating proteins (GAPs) in the constitutive activity of Ras signaling during human hepatocarcinogenesis.
Methods
Mutation status of Ras genes and Ras effectors was assessed in a collection of human hepatocellular carcinoma (HCC). Levels of Ras GAPs (RASA1-4, RASAL1, nGAP, SYNGAP1, DAB2IP, and NF1) and the RASAL1 upstream inducer PITX1 were determined by real-time RT-PCR and immunoblotting. Promoter and genomic status of RASAL1, DAB2IP, NF1, and PITX1 were assessed by methylation assays and microsatellite analysis. Effects of RASAL1, DAB2IP, and PITX1 on HCC growth were evaluated by transfection and siRNA analyses of HCC cell lines.
Results
In the absence of Ras mutations, downregulation of at least one Ras GAP (RASAL1, DAB2IP, or NF1) was found in all HCC samples. Low levels of DAB2IP and PITX1 were detected mostly in a HCC subclass from patients with poor survival, indicating that these proteins control tumor aggressiveness. In HCC cells, reactivation of RASAL1, DAB2IP, and PITX1 inhibited proliferation and induced apoptosis, whereas their silencing increased proliferation and resistance to apoptosis.
Conclusions
Selective suppression of RASAL1, DAB2IP, or NF1 Ras GAPs results in unrestrained activation of Ras signaling in the presence of wild-type Ras in HCC.
Keywords: Ras GAPs, HCC, methylation
Ras proteins are molecular switches for signalling cascades modulating many aspects of cell biology [1,2]. Two distinct conformations characterize Ras proteins: an inactive GDP-bound and an active GTP-bound state, which are controlled by Ras guanine nucleotide exchange factors (GEFs) and Ras GTPase-activating proteins (GAPs) [3]. Ras GEFs trigger activation of Ras by increasing the exchange of GDP for GTP, whereas Ras GAPs enhance the intrinsic Ras GTPase activity, leading to Ras inactivation through GTP into GDP conversion [3]. Approximately 30% of human tumors express an oncogenic form of Ras genes (Ha-, K-, and N-Ras), which is locked in the active conformation as a result of being insensitive to Ras GAPs [1,2]. Besides oncogenic mutations, aberrant activation of Ras cascade may also occur in the presence of wild-type Ras genes in cancer. Indeed, Ras pathway is often deregulated in tumors through mutations in upstream inducers and downstream effectors, or via inactivation of Ras inhibitors, including DAB2, RASSF1A, and SPRY2 [4]. Suppression of Ras GAPs may constitute an additional mechanism whereby aberrant Ras activation promotes tumorigenesis [5]. Various Ras GAPs have been identified, including p120GAP or RASA1, the SynGAPs (Disabled homolog 2 Interacting Protein or DAB2IP, nGAP, and SynGAP1), neurofibromin (NF1), and the GAP1 proteins (GAP1m or RASA2, GAP1IP4BP or RASA3, Ca2+-promoted Ras inactivator or RASA4, and Ras GTPase-activating-like protein 1 or RASAL1) [3,5]. The role of Ras GAPs in carcinogenesis remains unexplored. Only NF1 was shown to be a bona fide oncosuppressor gene [5], whereas few studies were conducted on other Ras GAPs. Recently, DAB2IP downregulation was detected in human prostate cancer [6], and two genome-wide studies identified DAB2IP as a putative oncosuppressor gene in aggressive prostate adenocarcinomas [7]. In human hepatocellular carcinoma (HCC), DAB2IP is often epigenetically silenced [8], while RASAL1 is downregulated in HCC cells [9]. However, Ras GAPs status has not been comprehensively examined, and no functional studies on the role of Ras GAPs in liver cancer cells have been performed.
Here, we investigated the expression levels of Ras GAPs in a large collection of human HCC, determined the molecular mechanisms responsible for inactivation of Ras GAPs, and studied their biologic role in HCC cell lines. Our findings indicate that inactivation of RASAL1, DAB2IP, or pituitary homeobox 1 (PITX1; a RASAL1 upstream inducer) is a major oncogenic event leading to unconstrained activation of wild-type Ras in human HCC.
Materials and methods
Human tissue samples
Ten normal livers, 88 HCCs and corresponding surrounding non-tumorous livers were used. Clinicopathological features of the patients are listed in Supplementary Table 1. HCCs were divided in two groups, HCC with poor prognosis (HCCP) and HCC with better prognosis (HCCB), which were characterized by a shorter (< 3 years) or longer (> 3 years) survival following liver partial resection, respectively [10]. Tissues were kindly provided by Dr. Z. Sung (National Laboratory of Molecular Oncology, Beijing, China) and the Liver Tissue Procurement and Distribution System (Minneapolis, MN; Pittsburgh, PA; Richmond, VA), funded by NIH Contract #N01-DK-9-2310. Institutional Review Board approval was obtained at participating hospitals and the National Institutes of Health.
Mutation analysis
Mutations at Ha-RAS, Ki-RAS, and N-RAS, A-RAF, B-RAF, RAF-1, and EGFR genes were assessed in the whole sample collection by direct DNA sequencing as described [11-13].
Real-time RT–PCR, methylation analyses, microsatellite analysis, imunoblotting, and immunoprecipitation
Real-time RT PCR, methylation and microsatellite analyses were performed as described (Supplementary Material) [4]. Primers for Ha-Ras, Ki-Ras, N-Ras, RASA1-4, nGAP, SYNGAP1, hDAB2IP, RASAL1, NF1, PITX1, and ribonucleic acid ribosomal 18S (RNR-18, internal control) genes were from Applied Biosystems (Foster City, CA). Primers to assess the promoter status of PITX1 were designed using the MethPrimer software [14], and those for RASAL1, DAB2IP, and NF1 genes were previously generated (Supplementary Table 3) [6,9,15]. Presence of promoter hypermethylation was defined as the amplification of the specific PCR product of the investigated gene when using methylated-specific primers. Tissue lysates were subjected to immunoblotting and immunoprecipitation as reported (Supplementary Material) [4].
Ras activation and Ras GEF assays, microvessel density (MVD)
Levels of activated Pan-, Ha-, Ki-, and N-Ras (Pan-, Ha-, Ki-, and N-Ras-GTP) were determined with the Ras Activation Assay Kit (Millipore, Billerica, MA). Ras GEF activity and MVD were determined as described (Supplementary Material) [16,17].
Cell Lines, viability, apoptosis, and vascular endothelial growth factor-α secretion assays
Human HCC cell lines were subjected to either silencing or demethylating experiments as described in Supplementary Material.
Results
Activation of wild-type Ras during human hepatocarcinogenesis
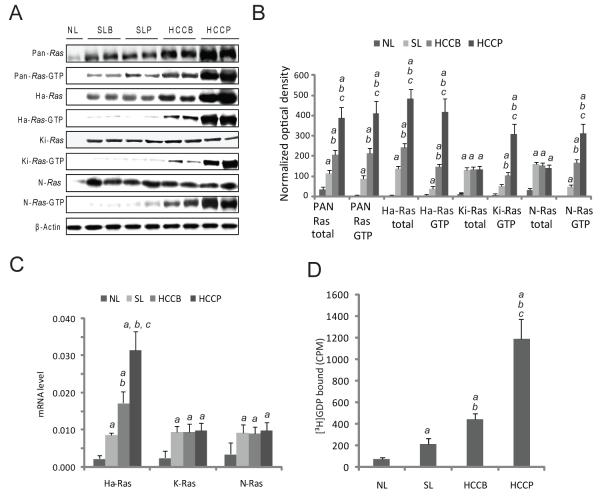
Protein and mRNA levels of Pan-, Ha-, Ki-, and N-Ras, as assessed by immunoblotting and real-time RT-PCR, were significantly higher in non-neoplastic surrounding livers as compared with normal livers, with no significant differences between the two prognostic HCC subclasses. No further upregulation of Ki-Ras and N-Ras was detected in HCC, whereas an additional increase of Pan- and Ha-Ras levels occurred in HCC, predominantly HCCP (Fig. 1A, B and C). Levels of activated (GTP-bound) Pan-, Ha-, Ki-, and N-Ras were instead progressively upregulated from normal livers to HCCP (Fig. 1A and B), which was paralleled by an increase in Ras GEF activity (Fig. 1D). This suggests that Ki- and N-Ras overactivity is not due to transcriptional induction in HCC. Thus, we tested whether mutations in Ha-, Ki-, and N-Ras, Ras upstream inducers (EGFR) or downstream effectors (A-Raf, B-Raf, and Raf-1) were responsible for Ras activation. No mutations were detected, indicating that unconstrained activation of Ras proteins is independent of gene mutations in human hepatocarcinogenesis.
Figure 1. Activation of Ras proteins in human HCC.
(A) Whole cell lysates were prepared from normal livers (NL), surrounding livers with better (SLB) or poor (SLP) prognosis, and HCC with better (HCCB) or poor (HCCP) prognosis and immunoblotted with indicated antibodies. Representative immunoblotting shown. (B) Optical densities were normalized to β-actin values and expressed in arbitrary units. Each bar represents mean ± SD. (C) Expression levels of Ha-, Ki-, and N-Ras in normal livers, HCC and corresponding non-tumorous surrounding livers by real-time RT-PCR. N-Target (NT) = 2 −ΔCt; ΔCt = RNR18-Ct target gene. Each bar represents mean ± SD. (D) Assessment of Ras GEF activity in normal livers, HCC and matching non-tumorous surrounding livers. Tissue lysates were added to the unlabeled GDP Ras-agarose complex in the presence of 10 μCi of [3H]GDP. The amount of bound Ras-[3H]GDP was determined by a scintillation counter. Addition of 2 mmol/L GTP-γ-S to the mix of [3H]GDP, tissue lysates, and unlabeled GDP Ras-agarose complex, abolished the exchange reaction. Each bar represents mean ± SD. SLB and SLP data did not show statistical differences, and are thus merged and indicated as surrounding livers (SL) in B, C, and D. Multiple comparison test (Kruskal-Wallis) p < 0.05 a, vs. NL; b vs. SL; c, vs. HCCB.
Frequent downregulation of DAB2IP and RASAL1 in human HCC
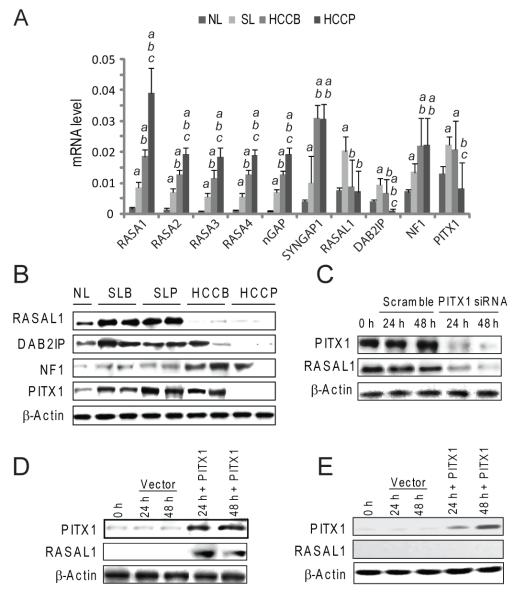
Since suppression of Ras GAPs may trigger uncontrolled activation of wild-type Ras [3,5], we investigated whether Ras GAPs expression was perturbed in human HCC by real-time RT-PCR. Based on the expression pattern, Ras GAP genes could be divided into three distinct categories (Fig. 2A). RASA1-4, nGAP, and Syngap1 genes were progressively upregulated from normal livers to HCC; NF1 expression was upregulated in most tumors and decreased in a small HCC subset (11/88, 12.5%); RASAL1 and DAB2IP mRNA were almost ubiquitously downregulated (65/88, 73.8%; and 67/88, 76.1%, respectively) in HCC when compared with normal and surrounding non-neoplastic livers. Levels of RASAL1, DAB2IP, and NF1 genes were higher in surroundings than in normal livers, while expression of DAB2IP was lowest in HCCP. Equivalent results were obtained when assessing RASAL1, DAB2IP, and NF1 levels by immunoblotting (Fig. 2B and Supplementary Fig. 1).
Figure 2. Levels of Ras GAPs in normal livers (NL), surrounding livers (SL), and HCC with better (HCCB) or poor (HCCP) prognosis, as determined by real-time RT-PCR (A) and immunoblotting (B).
(A) mRNA levels determined by real-time RT-PCR. N-Target (NT) = 2 −ΔCt; ΔCt = RNR18-Ct target gene. Each bar represents mean ± SD. Multiple comparison test (Kruskal-Wallis): P < 0.05 a, vs. NL; b vs. SL; c, vs. HCCB. (B) Representative immunoblotting shown. (C) Effect of PITX1 silencing via siRNA in HuH6 cells on RASAL1 levels. (D,E) Effect of forced overexpression of PITX1 by transient transfection on RASAL1 levels in SNU449 (D) and PLC/Alexander cell lines (E), harbouring RASAL1 unmethylated and methylated gene promoter, respectively, as assessed by imunoblotting. Following transfection, PITX1 induces RASAL1 upregulation in SNU449 but not in PLC/Alexander cells (D,E). Experiments were repeated at least 3 times in triplicate, and results shown in (C) were reproduced in Hep3B cells (not shown).
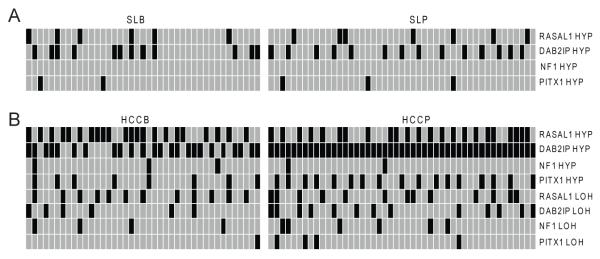
As a possible mechanism for RASAL1, DAB2IP and NF1 silencing, the promoter methylation status of these genes was investigated using methylation-specific PCR and combined bisulphite restriction analysis (Fig. 3). No hypermethylation at RASAL1, DAB2IP and NF1 promoters was observed in normal livers (not shown). Aberrant hypermethylation of RASAL1 and DAB2IP promoters occurred both in non-tumorous surrounding livers and HCC, but at significantly higher frequency in tumors (56.8% vs. 13.6%, P = 3.03 × 10−10 and 76.1% vs. 29.5%, P = 2.94 × 10−10, respectively). NF1 promoter hypermethylation occurred only in a small HCC subset (5/88, 5.7%). A significant downregulation of RASAL1, DAB2IP, and NF1 mRNA levels was detected in methylated when compared with unmethylated samples and normal livers (Supplementary Fig. 1). Strikingly, all HCCs exhibited promoter methylation of at least one of the three Ras GAPs (Fig. 3), underlining the selective pressure toward wild-type Ras unrestrained activity via Ras GAPs inactivation in hepatocarcinogenesis.
Figure 3. Frequency of promoter methylation (HYP) and loss of heterozygosity (LOH) at RASAL1, DAB2IP, NF1, and PITX1 genes in human non-tumorous surrounding livers (A) and corresponding HCC (B).
Normal livers did not exhibit any altered profile and, therefore, are not shown. Promoter methylation and genomic status of RASAL1, DAB2IP, NF1, and PITX1 genes was determined by methylation-specific PCR, combined bisulphite restriction analysis, and microsatellite analysis. Presence of promoter hypermethylation and/or LOH or absence of the same alterations is indicated by black and grey squares, respectively.
Subsequently, we examined the correlation between the clinicopathological data from the HCC patients and aberrant methylation status of RASAL1, DAB2IP, and NF1 promoters. Aberrant DAB2IP promoter methylation was more frequent in HCCP than HCCB (100% vs. 48.8%, P = 6.81 × 10−10), suggesting that DAB2IP inactivation contributes to HCC aggressiveness. No other clinicopathological features correlated with Ras GAPs methylation status, including patient’s age, sex, etiology, presence of cirrhosis, tumor size, Edmondson/Steiner grade, and alpha-fetoprotein levels.
Genomic status of Ras GAPs was further investigated using LOH analysis of RASAL1, DAB2IP, and NF1 loci, by comparing HCCs with respective surrounding livers. LOH rate at RASAL1, DAB2IP, and NF1 loci was 28.4%, 21.6%, and 12.5%, respectively. Noticeably, promoter hypermethylation and/or LOH for DAB2IP and NF1 genes was paralleled by DAB2IP and NF1 gene downregulation (not shown), suggesting that these molecular mechanisms are responsible for DAB2IP and NF1 silencing in human HCC. Nevertheless, 15 out of 65 (23.1%) HCCs displaying RASAL1 downregulation showed no promoter methylation or LOH, implying other mechanisms in the suppression of RASAL1.
Inhibition of PITX1 contributes to RASAL1 inactivation in human HCC
Previously, it was shown that the PITX1 gene inhibits Ras activity via RASAL1 transactivation [18]. To investigate the importance of PITX1 in modulating RASAL1 expression, we assessed the levels of PITX1 by real-time RT-PCR and immunoblotting (Fig. 2A and B and Supplementary Fig. 2). PITX1 mRNA and protein levels were induced in non-neoplastic surrounding livers when compared with normal livers, whereas PITX1 was downregulated in 37 of 88 (42%) tumors, mainly HCCP (30/37, 81.1%). Interestingly, PITX1 was overexpressed in a subset of HCCB (10/88, 11.4%) displaying RASAL1 upregulation (not shown). Furthermore, all HCCs exhibiting low RASAL1 expression independent of its promoter hypermethylation showed downregulation of PITX1, suggesting that PITX1 modulates RASAL1 expression. To substantiate our hypothesis, PITX1 was suppressed by siRNA in HuH6 and Hep3B cell lines (expressing high PITX1 levels). As expected, PITX1 inhibition induced downregulation of RASAL1 (Fig. 2C and Supplementary Fig. 3A). Likewise, PITX1 cDNA transfection into SNU449 cells (displaying low PITX1 and RASAL1 levels, but unmethylated RASAL1 promoter) triggered RASAL1 upregulation (Fig. 2D and Supplementary Fig. 3B). However, PITX1 cDNA transfection into PLC/Alexander cells (displaying low PITX1 and RASAL1 expression, the latter due to the RASAL1 promoter hypermethylation [9]) failed to restore RASAL1 expression (Fig. 2E and Supplementary Fig. 3C). Thus, PITX1 induction does not override RASAL1 epigenetic silencing. Next, we investigated the mechanisms responsible for PITX1 downregulation in HCC (Fig. 3). PITX1 promoter hypermethylation was rare (5/88, 5.7%) in non-tumorous surrounding livers, while it was found in 25 of 37 HCC (67.6%) with low PITX1 levels and at significant higher frequency in HCCP (17/25, 68%). Furthermore, 6 HCC with PITX1 promoter hypermethylation displayed LOH at the PITX1 locus (Fig. 3). The role of methylation on PITX1 silencing was substantiated in Focus and SNU389 cell lines, harbouring PITX1 promoter hypermethylation (Calvisi DF, unpublished observation). Treatment of these cell lines with the demethylating agent zebularine caused a marked up-regulation of PITX1 levels (Supplementary Fig. 4A-D), corroborating the role of promoter hypermethylation in PITX1 transcriptional repression.
RASAL1, DAB2IP or PITX1 suppression promotes unrestrained Ras activity in human HCC
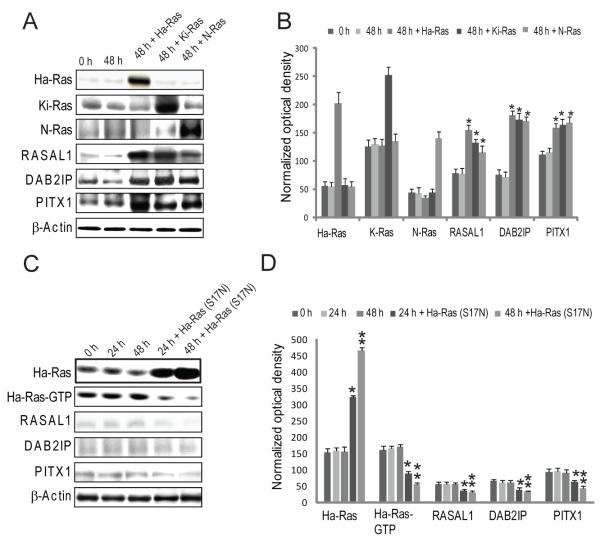
The downregulation of RASAL1, DAB2IP, and PITX1 in HCC with high levels of activated wild-type Ras suggests that either the loss of RASAL1, DAB2IP or PITX1 precludes the requirement for Ras mutation or, alternatively, that their expression is repressed by wild-type Ras. To test this hypothesis, we introduced the wild-type Ha-Ras gene into HuH6 and Hep3B cells (expressing RASAL1, DAB2IP, and PITX1). Transient transfection with Ha-Ras caused upregulation rather than downregulation of RASAL1, DAB2IP or PITX1 genes, presumably as a consequence of Ha-Ras negative regulatory loop activation (Fig. 4A and B). Similarly, transfection with a dominant-negative mutant form of Ha-Ras gene (S17N) in SNU449 cells displaying low RASAL1, DAB2IP, and PITX1 expression, did not upregulate RASAL1, DAB2IP, and PITX1 (Fig. 4C and D). Thus, inactivation of RASAL1, DAB2IP, and PITX1 triggers uncontrolled activation of wild-type Ras in HCC.
Figure 4. Expression of RASAL1, DAB2IP or PITX1 is not repressed by Ras.
(A) Wild-type Ha-Ras was overexpressed by transient transfection in HuH6 cells and effect on RASAL1, DAB2IP, and PITX1 was evaluated by immunoblotting. (B) Densitometric analysis of data showed in (A). (C) Transfection of a dominant negative mutant form of Ha-Ras (S17N) did not rescue RASAL1, DAB2IP, and PITX1 levels in PLC/Alexander cells. (D) Densitometric analysis of data showed in (C). Experiments were repeated at least 3 times in triplicate, and results shown in (A) were reproduced in Hep3B cells (not shown). (B, D) each bar represents mean ± SD. Kruskal-Wallis test p < 0.05: (B) *, different from 48 h; (D) * and ** different from 24 and 48 h, respectively.
Manipulation of DAB2IP, RASAL1, or PITX1 affects HCC cell growth in vitro
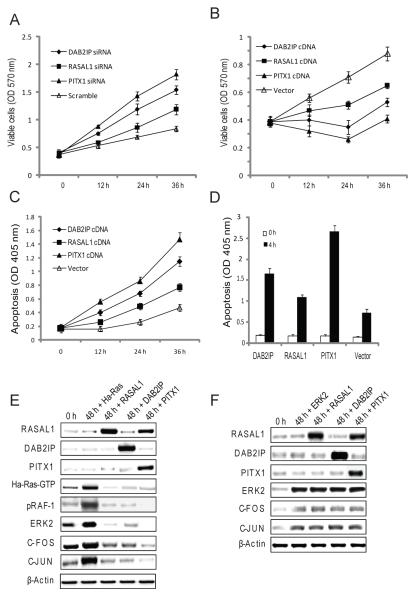
The role of DAB2IP, RASAL1, and PITX1 in HCC was investigated by their silencing (via siRNA) or overexpression in HCC cells. DAB2IP, RASAL1, or PITX1 suppression in HuH6 and Hep3B cells increased cell viability, with significant differences depending on the gene silenced. The most dramatic increase in HCC cell growth was detected when suppressing PITX1 or DAB2IP, whereas RASAL1 inhibition caused less growth promotion (Fig. 5A). Furthermore, inhibition of DAB2IP, RASAL1 or PITX1 induced a rise in Ras GEF activity regardless of the silenced gene (Supplementary Fig. 5A). Opposite results were obtained when overexpressing DAB2IP, RASAL1, or PITX1 in SNU449 HCC cells. Indeed, the strongest reduction in cell viability and increase in apoptosis were observed following transfection of PITX1 or DAB2IP (Fig. 5B and C). Combination of DAB2IP, RASAL1, or PITX1 overexpression with the apoptosis inducer Staurosporine significantly intensified HCC cells apoptosis (Fig. 5D). Ras GEF activity was equally increased following overexpression of DAB2IP, RASAL1, or PITX1 genes (Supplementary Fig. 5B).
Figure 5. Effect of manipulating RASAL1, DAB2IP, and PITX1 expression in human HCC cell lines.
(A) Silencing of RASAL1, DAB2IP or PITX1 via siRNA increased cell growth in HuH6 cells. Overexpression of RASAL1, DAB2IP or PITX1 by transient transfection induced growth restraint (B) and apoptosis (C) in SNU449 cells. The pro-apoptotic effect of RASAL1, DAB2IP, or PITX1 genes was further amplified with the use of Staurosporine (D). (E) SNU449 cells were transfected with wild-type Ha-Ras cDNA and co-transfected with RASAL1, DAB2IP, or PITX1 cDNA. Overexpression of RASAL1, DAB2IP, or PITX1 reduced the levels of activated Ha-Ras (Ha-Ras-GTP), and ERK2, c-FOS, and c-JUN effectors. (F). Overexpression of RASAL1, DAB2IP, or PITX1 in SNU449 cells by transient transfection had no effect on ERK2, c-FOS, and c-JUN expression when co-transfected with ERK2. Experiments were repeated at least 3 times in triplicate. Kruskal-Wallis test, treated vs. scrambled: (A,B,C) p < 0.05 at 36 h. Student t-test (D), 4 vs. 0 h, p < 4.69×10−9 .
The effect of overexpressing DAB2IP, RASAL1, or PITX1 in SNU449 cells co-transfected with wild-type Ha-Ras was also evaluated. Forced overexpression of DAB2IP, RASAL1, or PITX1 significantly decreased the growth of Ha-Ras co-transfected cells (not shown), and the levels of activated Ha-Ras (Ras GTP), ERK2, c-FOS, and c-JUN (Fig. 5E). In striking contrast, DAB2IP, RASAL1, or PITX1 overexpression had a significantly less pronounced effect on HCC cell growth (not shown) and failed to inhibit ERK2, c-FOS, and c-JUN expression when co-transfected with ERK2 (Fig. 5F). Thus, RASAL1, DAB2IP, and PITX1 inhibit wild-type Ras growth upstream of ERK in HCC cells.
DAB2IP expression directly correlates with ASK1-driven apoptosis and inversely with activation of VEGFR2/Akt/PLC-γ cascade in human HCC
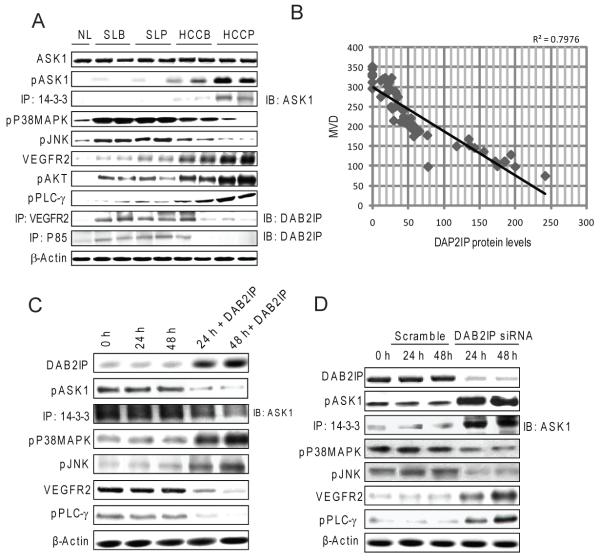
DAB2IP (or AK1-interacting protein-1, AIP1) promotes apoptosis by activating Apoptosis signal–regulating kinase 1 (ASK1), an upstream inducer of Jun-amino-terminal kinase (JNK) and p38 mitogen-activated-protein-kinase (MAPK) proteins [19]. DAB2IP activates ASK1 by allowing its release from the inactive complex with 14-3-3 [20]. Furthermore, DAB2IP suppresses growth and pro-angiogenic signals driven by vascular endothelial growth factor receptor 2 (VEGFR2)/Akt/phospholipase C-gamma (PLC-γ) axis via its binding to VEGFR2 and the p85 regulatory subunit of phosphoinositide 3-kinase (PI3K-p85) [21]. DAB2IP expression was highest in surrounding non-tumorous livers (Fig. 2A), where it was paralleled by low levels of phosphorylated ASK1, ASK1/14-3-3 complexes (signs of inactivated ASK1), and by high expression of activated (phosphorylated) JNK and p38 MAPK (Fig. 6A and Supplementary Fig. 6A). Activation of VEGFR2 cascade members, including VEGFR2, Akt, and PLC-γ, was low in surrounding non-tumorous livers, presumably due to high levels of DAB2IP bound to VEGFR2 and PI3K-p85. In contrast, DAB2IP expression was ubiquitously low in HCCP (Fig. 2A), where elevated amount of phosphorylated ASK1 and ASK1/14-3-3 and low levels of pJNK and p38 MAPK were detected (Fig. 6A and Supplementary Fig. 6A). Low levels of DAB2IP-VEGFR2 and DAB2IP-PI3K-p85 complexes were paralleled by high levels of activated VEGFR2, PLC-γ, and Akt in HCCP. A putative anti-proliferative and anti-angiogenic role of DAB2IP was further suggested by the inverse relationships between DAB2IP levels and MVD extent in HCC (Fig. 6B). Overexpression of DAB2IP into SNU449 cells resulted in ASK1 activation and suppression of the VEGFR2/Akt/PLC-γ axis, whereas opposite results where obtained when silencing DAB2IP in HuH6 cells (Fig. 6C and D, and Supplementary Fig. 6B and C). In addition, suppression of DAB2IP in HuH6 and Hep3B cells augmented VEGF-α secretion in the medium (Supplementary Fig. 7A), whereas DAB2IP overexpression in SNU449 cells dramatically inhibited VEGF-α secretion (Supplementary Fig. 7B). Altogether, these data envisage the involvement of DAB2IP in the modulation of ASK1-dependent apoptosis and VEGFR2/Akt/PLC-γ-driven angiogenesis in human HCC.
Figure 6. DAB2IP modulates the activation of the ASK1/P38MAPK/JNK and VEGFR2/AKT/PLC-γ pathways.
(A) Whole cell lysates were prepared from normal livers (NL), surrounding livers with better (SLB) or poor (SLP) prognosis, and HCC with better (HCCB) or poor (HCCP) prognosis and immunoblotted with indicated antibodies. Representative immunoblotting shown. (B) Inverse relationship between DAB2IP protein levels and HCC microvessel density. (C) Effect of DAB2IP induction by transient transfection on ASK1/P38MAPK/JNK and VEGFR2/AKT/PLC-γ cascades in SNU449 cells. (D) Effect of DAB2IP silencing via siRNA in HuH6 cells on ASK1/P38MAPK/JNK and VEGFR2/AKT/PLC-γ cascades. All experiments were repeated at least 3 times in triplicate, and results shown in (D) were reproduced in Hep3B cells (not shown).
Ras mutations and Ras GAPs promoter hypermethylation are mutually exclusive in breast, lung, pancreatic, and colorectal tumors
Finally, we investigated the frequency of Ras mutations and hypermethylation of RASAL1, PITX1, and DAB2IP promoters in a collection of human breast, lung, pancreatic, and colorectal tumors. No somatic mutations at Ha-Ras and N-Ras genes were detected (not shown). Mutations of Ki-Ras were rare in breast (1/25, 4%), intermediate in lung (5/30, 16.7%), and elevated in colorectal (12/30, 40%), and pancreatic (17/30, 56.7%) tumors (Supplementary Fig. 8). Promoter hypermethylation of RASAL1, PITX1, and DAB2IP genes was inversely correlated with Ki-Ras mutations, being extremely high in breast (21/25, 84%), intermediate in lung (17/30, 56.7%), and low in colorectal (8/30, 26.7%) and pancreatic (5/30, 16.7%) tumors. Strikingly, with the exception of a lung tumor showing concomitant Ki-Ras mutation and DAB2IP promoter methylation, tumors displayed either Ki-Ras mutations or promoter hypermethylation of Ras GAPs (Supplementary Fig. 7). Thus, Ki-Ras mutations and Ras GAP methylation are mutually exclusive events in various human tumors.
DISCUSSION
In this study, we provide evidence that suppression of RASAL1, DAB2IP, and NF1 Ras GAPs, which are responsible for switching off Ras signalling in normal cells, and PITX1, a RASAL1 upstream inducer, results in aberrant activation of the wild-type Ras in human HCC. This alternative mode of Ras activation may be implicated in carcinogenesis of other organs not harbouring Ras mutations. Indeed, we show that mutation of Ki-Ras gene and inactivation of Ras GAPs by promoter hypermethylation are mutually exclusive events in breast, lung, colon, and pancreatic cancer.
Since Ras GAPs negatively modulate the Ras cascade, their potential oncosuppressor role has been hypothesized [3,6]. Here, we demonstrate that re-expression of RASAL1, DAB2IP, or PITX1 strongly restrains HCC cell growth in vitro by reducing proliferation and increasing apoptosis. RASAL1 anti-neoplastic function resides in its ability to facilitate Ras GTP hydrolysis in a Ca++-dependent manner [3,5], and its expression is reduced in several human tumors [9]. Recently, RASAL1 was found to be downregulated in aggressive colorectal carcinomas but not in adenomas, suggesting a prognostic role for RASAL1 in colon cancer [22]. This finding differs from the almost ubiquitous inactivation of RASAL1 in HCC, suggesting that RASAL1 downregulation has a different impact on tumor development and progression depending on cell/tissue type. Furthermore, predominant inactivation of DAB2IP and PITX1 in HCCP supports their role in HCC progression. Accordingly, PITX1 is among the genes frequently silenced by promoter hypermethylation in moderately/poorly-differentiated HCC [23]. Overexpression of PITX1 or DAB2IP caused a stronger growth restraint than overexpression of RASAL1 although all three genes showed equal ability to reduce Ras GEF activity in HCC cell lines. This finding suggests that PITX1 and DAB2IP function may be directed toward targets other than Ras. Ongoing studies are investigating PITX1 molecular targets in human HCC. As concerns to the DAB2IP, previous data and present work suggest that its oncosuppressor role may also reside in the ability to activate the ASK1/JNK/p38 MAPK cascade and to inhibit the VEGFR2/Akt/PLC-γ axis [6,19-21].
Nonetheless, our data indicate that RASAL1-, DAB2IP- or PITX1-mediated suppression of Ras occurs upstream of ERK, implying that ERK deregulation cannot be effectively counteracted by RASAL1, DAB2IP or PITX1 overexpression. This finding, together with the observed inactivation of RAF/ERK inhibitors, including SPRY2 and NORE1A [4], substantiates the existence of a redundant group of genes regulating the Ras pathway at various steps that must be inactivated to achieve unconstrained activation of Ras cascade in hepatocarcinogenesis.
Our data also suggest that not all Ras GAPs possess anti-growth properties in liver. Although most Ras GAPs were upregulated in HCC, their induction did not compensate for the loss of RASAL1, DAB2IP or PITX1 and did not reduce Ras activation and Ras GEF levels. Furthermore, we cannot exclude that some Ras GAPs possess paradoxical growth properties in cancer. Indeed, RASA1 was found to be required for activation of the Akt pathway in normal cardiomyocytes and lung carcinoma cells [24].
In summary, we provide evidence that inactivation of RASAL1, DAB2IP and PITX1 putative oncosuppressors has a causative role in promoting unrestrained activation of wild-type Ras in human HCC (Supplementary Figure 9). Our results emphasize the importance of Ras pathway in hepatocarcinogenesis and speak in favour of therapeutic approaches aimed at reactivating RASAL1, DAB2IP and PITX1 and/or inhibiting Ras in human HCC.
Supplementary Material
Acknowledgments
Funding:
Supported by funds from the Intramural Research Program of National Cancer Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations used in this paper:
- AIP1
ASK1-interacting protein 1
- ASK1
apoptosis signal–regulating kinase 1
- CAPRI
Ca2+-promoted Ras inactivator
- DAB2IP
disabled homolog 2 interacting protein
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- HCC
hepatocellular carcinoma
- HCCB
hepatocellular carcinoma with better prognosis
- HCCP
hepatocellular carcinoma with poor prognosis
- JUNK
Jun-amino-terminal kinase
- MAPK
mitogen activated protein kinase
- NF1
neurofibromin
- PITX1
pituitary homeobox 1
- PLCγ
phospholipase C-gamma
- PP2A
protein phosphatase 2A
- RASAL1
Ras GTPase-activating-like protein 1
- VEGFR2
vascular endothelial growth factor receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The Authors state the absence of any conflict of interest to disclose.
References
- 1.Barbacid M. Ras oncogenes: their role in neoplasias. Eur J Clin Invest. 1990;20:225–235. doi: 10.1111/j.1365-2362.1990.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 2.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwashita S, Song SY. Ras GAPs: a crucial regulator of extracellular stimuli for homeostasis of cellular functions. Mol Biosyst. 2008;4:213–222. doi: 10.1039/b716357f. [DOI] [PubMed] [Google Scholar]
- 4.Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, et al. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713–2722. doi: 10.1172/JCI31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernards A, Settleman J. GAPs in growth factor signalling. Growth Factors. 2005;23:143–149. doi: 10.1080/08977190500130480. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Tseng CP, Pong RC, Chen H, McConnell JD, Navone N, et al. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J Biol Chem. 2002;277:12622–12631. doi: 10.1074/jbc.M110568200. [DOI] [PubMed] [Google Scholar]
- 7.Duggan D, Zheng SL, Knowlton M, Benitez D, Dimitrov L, Wiklund F, et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst. 2007;99:1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 8.Qiu GH, Xie H, Wheelhouse N, Harrison D, Chen GG, Salto-Tellez M, et al. Differential expression of hDAB2IPA and hDAB2IPB in normal tissues and promoter methylation of hDAB2IPA in hepatocellular carcinoma. J Hepatol. 2007;46:655–663. doi: 10.1016/j.jhep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Wang X, Ying J, Wong AH, Cui Y, Srivastava G, et al. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci U S A. 2007;104:12353–12358. doi: 10.1073/pnas.0700153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Kahn SM, Guillem JG, Lu SH, Weinstein IB. Rapid detection of Ras oncogenes in human tumors: applications to colon, esophageal, and gastric cancer. Oncogene. 1989;4:923–928. [PubMed] [Google Scholar]
- 12.Fransen K, Klintenas M, Osterstrom A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 14.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 15.Rübben A, Bausch B, Nikkels A. Somatic deletion of the NF1 gene in a neurofibromatosis type 1-associated malignant melanoma demonstrated by digital PCR. Mol Cancer. 2006;5:36. doi: 10.1186/1476-4598-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li BQ, Kaplan D, Kung H, Kamata T. Nerve growth factor stimulation of the Ras-Guanine Nucleotide Exchange Factor and GAP activities. Science. 1992;256:1456–1459. doi: 10.1126/science.1604323. [DOI] [PubMed] [Google Scholar]
- 17.Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoid-like vessels in HCC: its clinical and prognostic significance. Hepatology. 1997;26:1216–1223. doi: 10.1053/jhep.1997.v26.pm0009362365. [DOI] [PubMed] [Google Scholar]
- 18.Kolfschoten IG, van Leeuwen B, Berns K, Mullenders J, Beijersbergen RL, Bernards R, et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, et al. AIP1 recruits phosphatase PP2A to ASK1 in tumor necrosis factor-induced ASK1-JNK activation. Circ Res. 2008;102:840–848. doi: 10.1161/CIRCRESAHA.107.168153. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. AIP1 mediates TNF-alpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, et al. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta M, Seto M, Ijichi H, Miyabayashi K, Kudo Y, Mohri D, et al. Decreased expression of the Ras-GTPase activating protein RASAL1 is associated with colorectal tumor progression. Gastroenterology. 2008;136:206–216. doi: 10.1053/j.gastro.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 23.Gao W, Kondo Y, Shen L, Shimizu Y, Sano T, Yamao K, et al. Variable DNA methylation patterns associated with progression of disease in hepatocellular carcinomas. Carcinogenesis. 2008;29:1901–1910. doi: 10.1093/carcin/bgn170. [DOI] [PubMed] [Google Scholar]
- 24.Pamonsinlapatham P, Hadj-Slimane R, Lepelletier Y, Allain B, Toccafondi M, Garbay C, et al. P120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie. 2009;91:320–328. doi: 10.1016/j.biochi.2008.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.