Abstract
The liver is an organ is which several major pathogens evade immune clearance, and achieve chronicity. How do they do it? Recent research has documented multiple mechanisms by which immune responses in the liver are biased towards tolerance. In this review, the induction of local, intrahepatic tolerance is explored from the perspective of antigen presentation. Experiments support the role of liver Dendritic Cell subsets, but also of diverse subsets of unconventional antigen-presenting cells, in inducing immune suppression. The literature on this topic is controversial and sometimes contradictory, making it difficult to formulate a unified model of antigen handling and T cell priming in liver. Here I offer a critical review of the state of the art in understanding antigen presentation in the liver.
Introduction
The liver is the site of several infections of major importance, against which the immune system normally delivers either an ineffective, or a pathogenic response. In the case of Hepatitis B and Hepatitis C, immune responses occur but they are frequently ineffective. With the lack of virus elimination, chronic immune responses cause cumulative tissue damage and eventual fibrosis, leading to disruption of the liver's hemodynamics, and the loss of liver function. In contrast, malaria parasites migrate through the liver and undergo an essential part of their maturation there, yet there is no evidence of an endogenous immune response. While much of the understanding of human liver immunology is based on the study of immune responses to viral hepatitis, the world's most prevalent serious infection, malaria, is also a liver pathogen. All species of mouse and human malaria parasites undergo an obligatory developmental stage in the liver. The sporozoite, introduced by the bite of an infected mosquito, interacts sequentially with liver sinusoidal endothelial cells (66) and Kupffer cells (65). The Kupffer cells appear to be an essential “gateway” through which sporozoites penetrate the endothelial barrier, and enter hepatocytes (4). Once in hepatocytes, the parasites develop rapidly over several days, after which the host cell dies and merozoites are released, which parasitize red blood cells. The liver stage is an attractive vaccine target, and genetically modified murine malaria parasites create sterilizing immunity that appears to intercept the infection at the liver stage (57). The mechanism of action of the vaccine is not understood, but it appears to depend on Interferon (IFN)-gamma, and on CD8+ T cells (30).
In many mammalian species, the transplantation of the liver across a Major Histocompatibility Complex (MHC) difference does not result in rejection (13, 15). This stands in contrast to the consequences of transplanting kidneys, skin, pancreas or other organs, where rejection is the usual outcome. In addition, the transplanted liver is able to confer tolerance on another solid organ transplant from the same donor, arguing that the liver can induce systemic tolerance (14). This effect is not fully understood, but it has been attributed to: the effects of liver-derived APC dispersed throughout the host, also known as microchimerism (67); the effects of Kupffer cells or liver sinusoidal endothelial cells (LSEC) as antigen-presenting cells (APC), promoting tolerance (38, 70); the distinctive properties of liver-resident dendritic cells (DC) (53); and the induction of allospecific regulatory T cells of the CD4+, CD25+, Forkhead transcription factor-P3 (FoxP3)+ type (46). Despite the well-documented liver allograft tolerance in many animal models, human liver transplants are undertaken with the use of immunosuppressive drugs. In the context of a pathogen that re-infects the liver allograft, such as Hepatitis C Virus, this situation leads to rapid progression of the infection (54).
Clearly, the liver is a tissue in which immune responses are often suboptimal. How does this arise? Is the liver intrinsically predisposed towards immune tolerance, or is it simply that these pathogens are unusually adept at subverting host defense? The field of liver immunology addresses these issues by asking how far the liver has unique immunological properties. One key issue is that of antigen presentation; the liver contains dendritic cells (DC) and resident mononuclear phagocytes, but there is evidence that other hepatic cell types act as APC. In particular, there is an extensive literature on the immunological properties of LSEC (38), while an influential recent paper argues that the liver's distinctive vascular pericytes, termed stellate cells or Ito cells, are capable of antigen presentation (83). Some evidence also suggests that hepatocytes, the metabolic engines of the liver, can under certain circumstances activate naïve T cells (9). If this interpretation is correct, the analysis of immune responses to liver pathogens needs to take into account the possibility that unconventional APC play an important role, and may account for the failure of effective immunity. The purpose of this review is to critically evaluate the claims of each population of liver cells to be APC, and to ask how they may contribute both to effective and to maladaptive response to liver antigens.
The hepatic vasculature and leukocyte trafficking
The liver is a major focus of metabolic activity, where the products of digestion are processed, plasma proteins synthesized, and dangerous foreign chemicals detoxified. To serve these functions, the liver receives its blood supply from two sources: around 20% of the blood is arterial, delivered via the hepatic artery which branches off from the celiac axis; while the other 80% originates in the intestine. This portal venous blood carries to the liver a mixture of antigens from food, and bacterial products from the intestinal bacteria. In particular, the portal blood carries lipopolysaccharide endotoxin (LPS) at concentrations of up to 1 ng/ml (22, 50). Thus, in the liver, both antigen-specific lymphocyte receptors and pattern recognition receptors are exposed to their ligands.
The liver contains a diverse population of both adaptive and innate immune cells. T cells are abundant, with a bias towards CD8+ T cells, and activated T cells predominate (17, 78). Natural killer (NK) cells are abundant, and these cells similarly express activation markers (78). NK-T cells are more frequent than in the blood in humans, and more frequent than in the lymphoid organs in mice; this hold true whether these cells are defined expansively as NK1.1+ T cells in the mouse, or CD56+ T cells in the human, or CD1d-reactive cells, or narrowly defined as T cells that bind tetramers of a glycolipid, alpha-Galactosyl ceramide, associated with a CD1d molecule (6, 59). Lymphocytes with exactly these features can be eluted from the hepatic vasculature of a human liver lobe prior to transplant (78), suggesting that they are in found in the lumen of the blood vessels, and immunohistology similarly reveals individual T cells through the normal human liver parenchyma, as well as in portal tracts (75). For most of these cell populations, it's not possible to say how far these cells are long-term hepatic residents, and how far they are preferentially slowed down in the liver during their recirculation by adhesion molecules on the hepatic endothelium. The liver has the capacity to preferentially sequester activated CD8+ T cells from the circulation (55), and this effect depends in part on Intercellular Cell Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) expressed on the hepatic vasculature (31). However, in the specific case of NK-T cells, in vivo microscopy was used to identify these cells in the living liver, exploiting their expression of CXCR6 and a knock-in strategy to render them fluorescent. These cells were observed patrolling the hepatic sinusoids, both with and against the direction of blood flow (24). Activation of these cells causes them to stop patrolling, consistent with them having a defensive function (80). These cells, at least, were not passively drifting through the liver, and are likely to be long-term residents.
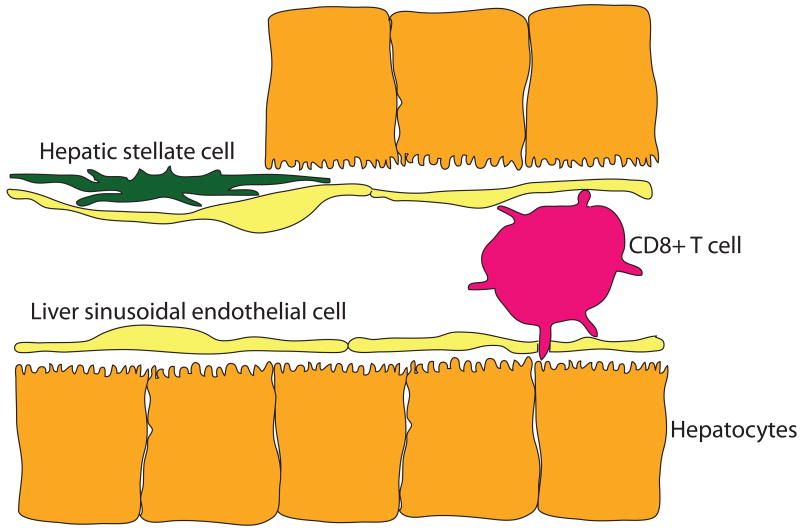
Blood percolates through the liver in thin-walled vessels termed sinusoids, the endothelium of which is penetrated by small holes (fenestrations) grouped in clusters (sieve plates). The fenestrations are large enough to permit contact between lymphocytes in the blood space, and the underlying hepatocytes (Figure 1). Electron micrographs show contact between T cell microvilli and their counterparts on the hepatocytes (82), though the physiological significance of such interaction is not clear. Certainly, these contacts are not sufficient to allow the formation of an immunological synapse, but in the living sinusoid they may act as initiators of more intimate contact. This progression has not yet been observed directly. The flow of blood is slow, due to the large cross-sectional area of the sinusoidal bed, and this is likely to facilitate interactions with both intrasinusoidal and peri-sinusoidal cells. Some electron micrographs reveal gaps in the endothelial layer, but it is likely that in the living sinusoid, the liver's resident macrophage population, Kupffer cells, occupies these gaps. We know this partly because elimination of the Kupffer cells using toxic liposomes results in gaps in the endothelial barrier, through which malaria sporozoites gain easy access to hepatocytes, bypassing their usual route through Kupffer cells (4).
Figure 1. Immunological players in the hepatic sinusoid.
The liver sinusoidal endothelial cells are penetrated by holes (fenestrations), through which a CD8+ T cell can make direct contact with an underlying hepatocyte. Between the endothelial cells and the hepatocytes is the Space of Disse, in which reside hepatic stellate cells (Ito cells), a specialized pericyte with immunological properties. These cells respond to TLR ligands and synthesize chemokines, and they may also act as antigen-presenting cells, particularly for CD1d-restricted NKT cells.
Resident and transient myeloid cells
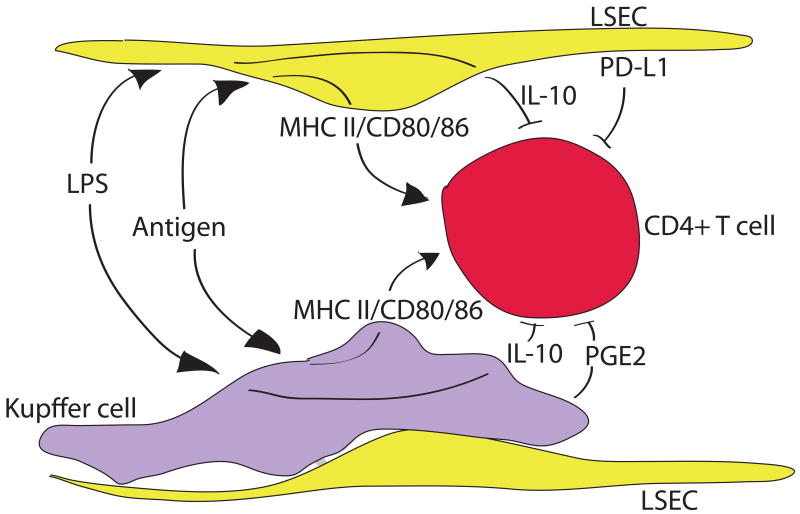
The liver's large macrophage population, also known as Kupffer cells, are unusual in that a large fraction of these cells are radio-resistant and difficult to extract from tissue, even after collagenase digestion. These sessile Kupffer cells are nevertheless phagocytic, but they do not migrate to inflammatory foci in the liver, which are formed by incoming, blood-derived cells (36). In these features, the sessile Kupffer cells resemble microglia in the brain (34). Kupffer cells have some credentials as APC, but the balance of evidence suggests they commonly promote T cell tolerance. Thus, Kupffer cells stimulated with LPS secrete the immunosuppressive cytokine, IL-10 (37), and secrete immunosuppressive prostaglandin E2 under metabolic stress (12). Kupffer cells express MHC class I and MHC class II, as well as co-stimulatory molecules at low density (88), and can induce T cell activation. However, in mixed cultures they suppressed T cell activation induced by DC, and in this study, prostaglandins were key to the immunosuppressive effect, rather than IL-10. In contrast, human Kupffer cells activated through Toll-like receptor-2 (TLR2) and TLR4 ligation synthesized IL-10, and in this case the IL-10 suppressed IL-18-dependent NK cell activation (79). The capacity of Kupffer cells to act as APC can clearly be modulated by innate signals, since both reactive oxygen species and TLR3 ligation increased the expression of MHC class II and promoted APC function (51, 88). This is particularly provocative, since TLR3 may sometimes make the critical difference between liver tolerance and immunity (44). In the case of the spirochaete bacterium Borellia burgdorferi, phagocytosis of the bacterium by Kupffer cells led to CXCR3 chemokine secretion and recruitment of iNKT cells, which contributed to containment of the infection (45). Kupffer cells can therefore switch their immunological role in two senses: from inactivators to activators of NK cells, and from tolerance-inducing APC to immunogenic APC. In both cases, their dominant action appears to depend on innate immune signals, particularly on LPS (Figure 2).
Figure 2. CD4+ T cells that are primed in the hepatic sinusoids receive mixed messages.
Pointed arrows indicate activating signals, while flat-ended arrows indicate suppressive signals. Both Kupffer cells, and liver sinusoidal endothelial cells (LSEC) take up antigen from the blood, and present it to CD4+ T cells together with co-stimulatory molecules. However, both cell types also respond to lipopolysaccharide endotoxin (LPS) via their TLR4 receptors; in both cases this leads to secretion of IL-10. The LSEC also secrete TFG-β1 and express PD-L1, while Kupffer cells secrete PGE2. Thus, the CD4+ T cell is subject to a barrage of conflicting signals.
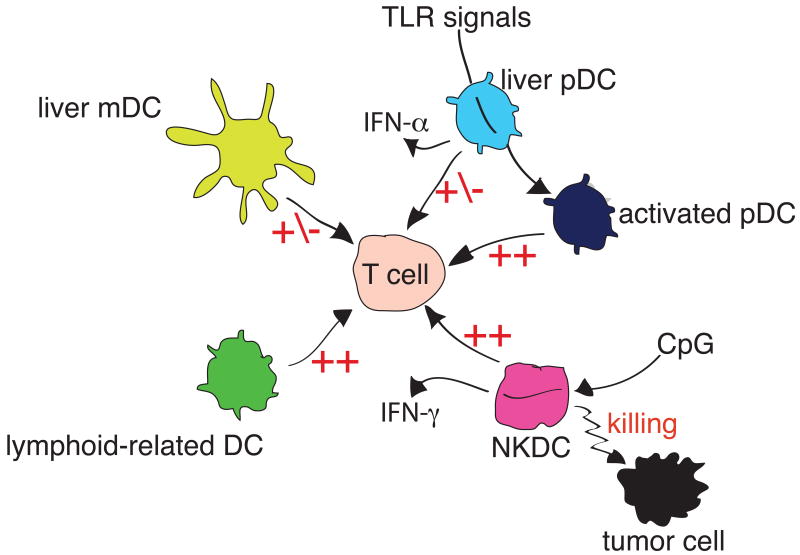
The liver contains multiple populations of DC, including classical myeloid DC (mDC) and plasmacytoid DC (pDC), as well as other populations that are more elusive (Figure 3). In the mouse, mDC are defined as CD11b+, CD11c+ and lacking in both CD8-alpha and B220. Compared to cells of similar phenotype isolated from the spleen, mouse liver mDC are less potent stimulators of T cell activation, possibly because of partial tolerance induced by constitutive exposure to endotoxin (18). In human liver, a similar population of mDC expresses CD11b, CD11c and blood DC antigen-1 (BDCA-1), and exhibits similar functions. Direct comparison of human liver-derived mDC with skin derived mDC revealed that the liver cells secreted more IL-10; in addition that promoted less proliferation, but more IL-10 secretion by T cells with which they interacted (25). This supports the model that humans hepatic mDC predispose T cells towards tolerance. Such a bias may be imposed locally, since liver stromal cells we able to bias the differentiation of hematopoietic progenitor cells towards DC with a regulatory function, based in part on the stromal cells' secretion of M-CSF (86).
Figure 3. Complex subsets of murine liver DC.
The Figure shows liver resident myeloid DC (mDC) are relatively weak APC, as are liver resident plasmacytoid DC (pDC). However, liver pDC are immature cells, which can be induced to differentiate into more powerful APC by TLR signals. Lymphoid-related (CD8α+) DC are rare cells with strong APC effects. The population termed NKDC is capable of killing tumor cells, and can present their antigens to T cells. Hepatic pDC secrete IFN-α, while liver NKDC secrete IFN-γ.
Myeloid DC are involved in trafficking through the liver, and one study shows that they migrate from the hepatic parenchyma to the portal tracts, which also contain T cells and are therefore a potential site of T cell priming (42). Such an interaction could result in the formation of portal lymphoid aggregates, as occurs for example in Propionibacterium acnes-induced liver inflammation (87), and could also occur in viral hepatitis. One issue for future investigation is the mechanism through which mDCs and the immune responses they initiate may be compartmentalized either to the parenchyma, or to portal tracts.
Murine liver pDC are B220+ and express lower levels of CD11c than mDC. As with pDC from other sources, they secrete type 1 IFN but directly-isolated cells are not highly effective APC in T cell activation (77). However, both growth factors and TLR ligation can cause these cells to mature into effective APC that can stimulate T cells (35). Therefore, their weak APC action ex vivo may reflect their differentiation state, rather than an intrinsic property of this subset. In human liver, pDC lack CD11c but express the marker BDCA-2. These cells are relatively abundant in liver, compared to other tissues (64), and in vitro they activate regulatory T cells (56), and may thus contribute to organ-specific immune tolerance.
In the mouse, liver DC also contain a population that expresses the classic DC marker CD11c, along with CD8-alpha (64), These cells have been termed “lymphoid-related DC”; unlike pDC, these cells were strong simulators of T cell proliferation (60). However, no such cells have been identified in other mammalian species, raising questions about their significance in human disease. Still more enigmatic is a subset of mouse liver cells termed “NK-DC”, based on their expression of both NK cells markers (NK-1.1) and DC markers (CD11c). These cells are cytotoxic, but also act as APC in vitro (63). Currently it is unclear whether the strongest affinity of these cells in with NK cells, or with other groups of DC. However, there is precedent for the close linkage of T lymphocyte, NK and DC maturation pathways in the thymic mDC, since these all arise from the earliest intrathymic progenitor cells (2, 74). Therefore, we could view these cells as NK cells that have acquired some DC-like properties, or as DC that retain some NK cell attributes from their precursors, or as aberrant cells that failed to commit to one or other of the lineages. Like the CD8alpha+ DC, these cells do not correspond to any subset in other mammals, so their relevance to human disease is unclear.
Liver Sinusoidal Endothelial Cells as APC
A strong case has been made that LSEC are important APC that induce T cell tolerance, based on the isolation of these cells by centrifugal elutriation and evaluation of their purity based on their uptake of acetylated low-density lipoproteins (LDL). Thus, these cells isolated from primed mice will present ovalbumin given systemically, and cause CD8+ T cell tolerance (47). Similarly, ovalbumin given orally induces T cell tolerance, which was transferable using isolated LSEC from the antigen-fed animals (48). In such experiments, apoptotic tumor cells will also serve as an antigen donor for the induction of CD8+ T cell tolerance (7). In an allostimulation model in vitro, cultured liver cells were poor APC for both CD4+ and CD8+ T cells, but the depletion of LSEC from the stimulator cells revealed APC activity in other (albeit undefined) liver cells (61).
Several explanations have been advanced for the tolerance-inducing properties of LSEC. Two immunosuppressive products of Kupffer cells, PGE2 and IL-10, both down-regulate the APC function of LSEC. IL-10, in particular, down-regulated expression of MHC class II, CD80 and CD86, compromising the antigen-specific and co-stimulatory signals (39). During interaction with antigen-specific T cells, LSEC up-regulated B7-H1 (PD-L1), which interacts with the PD-1 receptor on activated T cells, and leads to T cell inactivation (19). Recently, LSEC were shown to have an immunoregulatory effect mediated via cross-talk, due to a contact-dependent action on DC involving the loss of IL-12, CD80 and CD86 (71). A consistent model emerges in which LSEC scavenge circulating proteins from the circulation, present them with a battery of immunosuppressive signals, and thereby help to maintain self-tolerance (Figure 2). In support of this concept, antigen presented by non-hematopieitc cells that expressed Kb under the control of the Tie-2 promoter, putative LSECs, were able to cause rapid localization of resting CD8+ T cells to the liver (81). T cells primed on LSEC did not express the classic CD25+, FoxP3-high regulatory T cell phenotype, but were nevertheless able to suppress liver injury in experimental hepatitis in mice (41). The model of LSEC-based tolerance can easily be extended to serve the need to maintain immunological silence to harmless antigenic material in the diet.
The claim that LSEC express MHC antigens and co-stimulatory molecules was challenged in a study that isolated these cells using density gradient centrifugation, followed by immunomagnetic beads to deplete essentially all CD45+ cells. The cell population isolated was evidently endothelial, since it expressed CD31, von Willebrand factor and Fcgamma receptors, but not CD11c. These cells did not, however, express MHC class II or CD86, and did not appear engage CD4+ or CD8+ T cells, based on measurements of proliferation (33). This study further showed that the uptake of acetylated LDL as not a unique feature of LSEC, but was also shared by DC, although the LSEC were faster at accumulated fluorescent acetylated LDL in vitro. So, are these cells really so different from the LSEC that were capable of cross-presentation of ovalbumin? Both cell populations were CD11c negative (33, 48) and both ultimately promoted an ineffective immune response, although in the case of the cells isolated using magnetic beads, there was no initial T cell proliferation. One key difference between the two kinds of cell isolates is the expression of CD86; LSEC isolated by magnetic bead depletion were CD86 negative, while LSEC isolated by elutriation were clearly CD86+, comparable to Kupffer cells (49). If the cells were in fact consonant populations, the magnetic bead-isolated LSEC might correspond most closely to LSEC that had been exposed to IL-10 during isolation; as already noted, such exposure causes them to down-regulate both MHC class II and co-stimulatory molecules (39). In the present state of knowledge, it seems more likely that the isolation using magnetic beads on a column could have activated IL-10 secretion from Kupffer cells and modified the phenotype and function of the LSEC, than that the extensive literature on LSEC as APC is entirely due to DC contamination.
If we accept that LSEC are authentic APC, these studies raise the issue of their uniqueness among endothelia. There is, in fact, considerable evidence both for and against antigen presentation by other vascular endothelial cells. For example, highly-purified human umbilical vein endothelial cells (HUVEC), containing fewer than 0.01% leukocytes, were competent to cause primary activation in purified primary CD4+ and CD8+ T cells (62). This study is noteworthy for the care with which cell purity was assessed; in particular, the T cells were unresponsive to anti-CD3 and PHA, indicating very effective depletion of contaminating APC. However, the finding is controversial, since a subsequent study found that HUVEC do not express CD80, and even under the influence of IFN-gamma were able to induce only tolerance in primary CD4+ T cells (52). The reason for these apparently contradictory results in unclear. An analysis of the significance of MHC class II expression on vascular endothelium versus bone marrow-derived APC found that the presence or absence of MHC class II on the endothelium was not a major influence of the speed of cardiac allograft rejection by CD4+ T cells (40). Several transgenic studies have used the endothelium-specific receptor tyrosine kinase type-2 (Tie-2) promoter to drive antigen expression in vascular endothelium. One study expressed beta-galactosidase as antigen and led to an antibody response, but it was not possible to raise an endogenous T cell response, nor to induce vascular inflammation, even after priming with a recombinant vaccinia vector (69). Similarly, Tie-2-beta-galactosidase transgenic mice were infused with antigen-specific TCR transgenic CD8+ T cells, but these were neither activated nor tolerized (10). The latter study is noteworthy because the livers of Tie-2-beta-galactosidase transgenic mice were transplanted to naïve recipients; in such transplant experiments, the antigen should have been expressed primarily on hepatic vasculature and on LSEC. However, there was no interaction with CD8+ T cells, in direct contradiction to the expectations derived from studies with ex vivo isolated LSEC.
These data could be reconciled in several ways. First, the LSEC could express APC activity ex vivo, but not in vivo. However, this would mean that the capacity of LSEC to cross-present oral and circulating antigens in a way that induces T cell tolerance does not explain either oral or IV induction of systemic tolerance, hardly a satisfying outcome! Secondly, it could mean that the in vitro activities of LSEC cultures are in fact due to low-level contamination by bone marrow-derived APC, such as Kupffer cells or DC. It can always be argued that any primary cell preparation is “not pure enough”; however it does not make sense to write off the APC function of LSEC in view of the expression of MHC class I, MHC class II and co-stimulatory molecules by these cells, if carefully isolated (38, 43). Finally, many of the apparent discrepancies could be resolved if LSEC present exogenous antigens, but not their own intrinsic antigens, due to unusually features of their MHC class I and class II processing pathways. There is evidence that LSEC can take up antigen using many different cell surface receptors (21). One very interesting feature of these cells is the disparity in the efficiency at antigen uptake, versus cross-presentation of a soluble antigen. A direct comparison with DCs suggested that the LSECs were much more efficient at uptake, but equivalent in terms of cross-presentation, suggesting differences in the cell biology of antigen handling in LSECs (73). This observation argues strongly against the idea that the potent APC functions of LSECs can be explained by low-level DC contaminaition.
Hepatic Stellate Cells as APC
The sinusoids of the liver are surrounded by the Space of Disse, in which resides a distinctive population of vascular pericytes termed hepatic stellate cells, or Ito cells. These cells are understood to regulate blood flow through the sinusoids, and have a key role in liver fibrosis, since they undergo trans-differentiation into myofibroblasts in response to diverse inflammatory and toxic insults, and in this role they secrete inhibitors of tissue matrix metalloproteinases, deposit collagen, and create dense fibrous tissue (23). The stellate cells recently surfaced as potential APC. Since the studies supporting the new function are few, it is challenging to synthesize the evidence for APC function in hepatic stellate cells.
In a mouse model of liver fibrogenesis induced by injection of Carbon tetrachloride, immunohistochemistry revealed the co-location of lymphocytes and stellate cells, and the isolated stellate cells expressed both MHC class II and CD11c, consistent with an APC function (58). However, this approximation is also consistent with the concept that stellate cells are important in secreting chemokines that can promote lymphocyte localization, including CXCL9 and CXCL10 (28).
The key paper supporting a role for stellate cells as APC is based on cells isolated from the mouse liver by collagenase-pronase digestion, followed by density gradient centrifugation. These cells were placed in culture, and used in experiments to test APC function. The cells expressed CD1d, a low level of MHC class II, and half of them expressed a low level of CD11c; the flow cytometric profile in Figure 1 of this paper reveals also a trace of CD11c-high cells (83). The cells were clearly shown to activate NK-T cells in the presence of alpha-Galactosyl ceramide, and to activate T cells also. The key issue here is the potential co-purification of trace numbers of CD11c-high DCs along with the stellate cells. Photomicrographs showed mainly fibroblastic cells, and individual cells stained for the presence of glial fibrillary acidic protein, consistent with stellate cells. However, while the staining for low levels of CD11c on many of the cells is consistent with other reports, the small minority of CD11c-high cells raises the possibility that co-purified DC might contribute to the APC effect. To be fair, this caveat could apply to many other studies based on liver APC purification, including our own (84).
So far, there have been relatively few subsequent studies documenting the antigen presenting activity of stellate cells. One study documented changes in chemokine receptor expression, but very little proliferation of CD8+ T cells induced by human stellate cells (20). In contrast, rat stellate cells were reported to be capable to activate an antigen-specific CD4+ T cell hybridoma (11). One paper shows that under the influence of IFN-gamma, stellate cells could engage CD4+ T cell precursors, but selectively induced the expansion of FoxP3+ T cells with regulatory T cell effects (29). This would argue for a role of stellate cells in liver tolerance, rather than as APC that promote immunity. However, the studies are still too few to allow the formulation of a consensus on stellate cell antigen-presenting capacity.
Cholangiocytes as APC?
The epithelial cells lining the bile ducts, cholangiocytes, express many molecules often linked to APC finction, and these cells are a focus of inflammation in biliary cirrhosis and sclerosing cholangitis, raising the possibility that they might initiate an immune response. Human cholangiocytes express a variety of TLRs, and at least TLR-2 and TLR-4 are functional, signaling via MyD88 and causing NF-kB activation (16). Cholangiocytes can secrete chemokines such as CXCL16 that promote adhesion of T cells (27). Freshly isolate murine cholangiocytes expressed MHC class I, class II and CD40, and after a period of culture did also expressed CD80 and CD86. However, this same study did not find APC activity in the cholangiocytes (5). This may be linked to the fact that T cells express CD154, while ligation of its counter-receptor CD40 on cholangiocytes results in their apoptosis (1). In the present state of knowledge, it seems likely that cholangiocytes can be targets of immune responses, but are unique among major liver cell populations in that no evidence supports the possibility that they activate naïve T cells.
Hepatocytes as APC
The doctrine that antigen presentation is the concern of a specialized subset of cells has given rise to the core idea that, whereas T cells respond to antigen on such specialized APC by full activation, clonal expansion and differentiation, the outcome of antigen encounter of most tissue cells in either the lack of a response, or the induction of tolerance. The mainstream doctrine requires some qualification with respect to MHC class I-restricted cytotoxic T cells; these cells must deliver their effector functions by recognizing antigenic tissue cells. From this perspective, we can approach the issue of how hepatocytes act as APC, and what are the outcomes for T cell function.
Several studies document the effects of isolated, cultured hepatocytes as APC. In a fully allogeneic liver transplant model, the early apoptosis of graft-infitrating T cells was investigated by exposing isolated, allogeneic hepatocytes to T cells; the outcome was T cell activation, followed by apoptosis (68). Similarly, the abortive activation of TCR transgenic CD8+ T cells in mice expressing the Kb alloantigen in hepatcytes led to in vitro experiments, and again the outcome was T cell activation, followed by apoptosis (9). The first feature of interest in these latter experiments is that the naïve, as well as primed T cells were activated. How are we to explain this? In the case of the fully allogeneic experiments, it could be hypothesized that the alloreactive T cells were in fact previously-activated T cells, that were cross-reactive on alloantigens. In the case of the experiments using the Kb transgenic hepatocytes, it could be argued that the responding T cells were in fact expressing dual T cell receptors, due to lack of allelic exclusion of the TCR alpha chain (26). Both of these interpretations essentially deny that the hepatocytes could activate naive CD8+ T cells. The alternative interpretation, taken by the authors, is that such activation is possible, but that the activation leads to early apoptosis, resulting in functional tolerance.
As with the experiments discussed above in relation to Ito cell APC function, the key question here is: what was in the culture? Hepatocytes are isolated by a standard protocol involving perfusion of the liver with collagenase, followed by the making of a single-cell suspension which is then plated, typically in a specialized medium that favors hepatocyte growth, rather than the media used for lymphocyte culture. This cell isolation protocol was developed to generate cultures of hepatocytes for metabolic studies, and the contribution of LSEC, Ito cells, Kupffer cells and possibly other leukocytes is difficult to assess. Certainly, these cell isolates present the microscopic appearance of a monoculture of large cells. However, it is not customary to validate the hepatocyte culture by testing the cultures for the uptake of acetylated LDL, to identify contaminating LSEC, nor to stain for CD45, to detect leukocytes, nor to perform quantitative qRT-PCR to estimate the expression of genes characteristic of other cell types, such as F4/80 in Kupffer cells, or alpha smooth muscle actin in activated Ito cells. Therefore, the possibility remains that, in a culture of hepatocytes, the active APC were one of the other cell types we have already discussed.
Experiments in vivo have evaluated the APC functions of hepatocytes, using alloantigens driven by hepatocyte-specific promoters such as albumin, or metallothionine. These experiments support the broad consensus that hepatocytes cause primary T cell activation, but that this activation is abortive and leads to premature T cell apoptosis (3, 8, 72). However, such transgenic experiments are complicated by the possibility that the transgenic antigen could be expressed in the thymic medulla (76), potentially promoting the differentiation of regulatory T cells (32). Taking an alternative approach, investigators used an AAV vector to specifically deliver antigen to hepatocytes; this resulted in T cell activation, leading to effector function (84). However, this experiment used a very high number of antigen-specific CD8+ T cells, which may have been substituting for missing CD4+ T cell help (85). All of these experiments, taken together, fit with the idea that hepatocytes are effective primary APC for CD8+ T cells, but that the outcome of such T cell activation occurs in a context where CD4+ T cell help may be limiting, and regulatory T cells may be present; both of these factors favor an abortive immune response.
Conclusions
In the preceding discussion, I have argued that, despite some inconsistencies in the literature, immune responses in the liver follow distinctive rules, and this may be explained in part by features of the liver's APC. It is not possible to say which cell type is the most important liver APC, but many cell populations can make a strong claim. A critical analysis of current literature broadly supports the following tenets:
The liver's DCs are distinctive, and create a bias towards tolerance both because of their intrinsic properties, and because of a different representation of DC subsets.
Kupffer cells act as APC but favor immunosuppression, partly due to secretion of IL-10, and partly to secretion of PGE2.
Liver endothelial cells capture and present protein antigens, but the outcome is often immunosuppression due to their secretion of IL-10, TGF-beta1, and their expression of PD-L1. Strangely, evidence suggests the do not present their own cell-intrinsic antigens.
Hepatic stellate cells are a potent source of chemokines, and activate CD1d-reactive T cells, but whether they promote immunity or immune tolerance in CD4+ and CD8+ T cells still remains to be clarified.
There is no clear evidence that cholangiocytes are antigen-presenting cells.
Hepatocytes can cause primary CD8+ T cell activation, which may result in effector cells but does not result in a sustained response.
This summary of the state-of-the-art provides many explanations for the phenomenon of liver transplantation tolerance, and for the failure of immunity to some liver pathogens. As a real-world problem, liver allograft tolerance is of limited significance, because of the effectiveness of immunosuppressive drugs. The issue now is to determine which of these mechanisms actually contribute to defective anti-pathogen immunity, and which are innocent bystanders.
BOX 1. Central features of main liver APC.
Myeloid DC: appear to be biased towards tolerance induction.
Plasmacytoid DC: secrete type 1 IFN but relatively poor APC.
Other DC: CD8-alpha expressing “lymphoid-like” DC, and NK-DC of uncertain lineage.
Liver Sinusoidal Endothelial Cells: potent in cross-presentation of soluble and cellular antigens.
Hepatic Stellate Cells: Good APC for NK-T cells, but data contradictory for other T cells.
Cholangiocytes: express surface molecules like APC but do not activate T cells.
Hepatocytes: engage CD8+ T cells, cause abortive activation and early apoptosis.
BOX 2. “Liver tolerance”.
Originally, the failure of liver transplant recipients to reject an allograft.
Also invoked to explain tolerance to food antigens, and poor immunity to infections such as HCV.
Has been attributed to tolerance induction by liver mDC, pDC or other DC.
Has been attributed to induction of tolerance, or of T-reg cells by LSEC.
Has been attributed to abortive T cell activation by hepatocytes.
There is evidence in favor of all these possibilities.
Acknowledgments
The development of these ideas over the years has been supported principally by the NIAID and the NIDDK (grants AI063353, AI064463, AI072049 and DK075274). I also want to thank Drs Lin Huang, Wajahat Mehal, Katia Klugewitz, Ingo Klein, Sherry Wuensch, Percy Knolle, Patrick Bertolino, Angus Thomson, David Adams, Cliona O'Farrelly, Mark Orloff and Robert Pierce for many invaluable discussions on liver antigen presentation.
Abbreviations
- IFN
Interferon
- MHC
Major Histocompatibility Complex
- LSEC
liver sinusoidal endothelial cells
- APC
antigen-presenting cells
- DC
dendritic cells
- FoxP3
Forkhead transcription factor-P3
- LPS
lipopolysaccharide endotoxin
- NK
Natural Killer
- ICAM-1
Intercellular Cell Adhesion Molecule-1
- VCAM-1
Vascular Cell Adhesion Molecule-1
- CXCR6
C-X-C chemokine receptor-6
- TLR
Toll-like receptor
- mDC
myeloid DC
- pDC
plasmacytoid DC
- BDCA
blood dendritic cell antigen
- B220
B cell isoform of CD45 of mass 220kd
- LDL
low-density lipoproteins
- HUVEC
human umbilical vein endothelial cells
- Tie-2
endothelium-specific receptor tyrosine kinase type-2
Footnotes
Financial Disclosure: the author has no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008 Feb;47(2):552–562. doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 2.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993 Apr 22;362(6422):761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 3.Arnold B. Parenchymal cells in immune and tolerance induction. Immunol Lett. 2003 Oct 31;89(2-3):225–228. doi: 10.1016/s0165-2478(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 4.Baer K, Roosevelt M, Clarkson AB, Jr, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol. 2007 Feb;9(2):397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 5.Barnes BH, Tucker RM, Wehrmann F, Mack DG, Ueno Y, Mack CL. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int. 2009 Sep;29(8):1253–1261. doi: 10.1111/j.1478-3231.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002 Apr;16(4):583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 7.Berg M, Wingender G, Djandji D, Hegenbarth S, Momburg F, Hammerling G, et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur J Immunol. 2006 Nov;36(11):2960–2970. doi: 10.1002/eji.200636033. [DOI] [PubMed] [Google Scholar]
- 8.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect’. Immunol Cell Biol. 2002 Feb;80(1):84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998 Jan;28(1):221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Bolinger B, Krebs P, Tian Y, Engeler D, Scandella E, Miller S, et al. Immunologic ignorance of vascular endothelial cells expressing minor histocompatibility antigen. Blood. 2008 May 1;111(9):4588–4595. doi: 10.1182/blood-2007-09-114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bomble M, Tacke F, Rink L, Kovalenko E, Weiskirchen R. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem Biophys Res Commun. 2010 May 28;396(2):342–347. doi: 10.1016/j.bbrc.2010.04.094. [DOI] [PubMed] [Google Scholar]
- 12.Callery MP, Mangino MJ, Flye MW. Arginine-specific suppression of mixed lymphocyte culture reactivity by Kupffer cells--a basis of portal venous tolerance. Transplantation. 1991 May;51(5):1076–1080. doi: 10.1097/00007890-199105000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Calne RY. Immunological tolerance--the liver effect. Immunol Rev. 2000 Apr;174:280–282. doi: 10.1034/j.1600-0528.2002.017419.x. [DOI] [PubMed] [Google Scholar]
- 14.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969 Aug 2;223(5205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 15.Calne RY, White HJ, Yoffa DE, Binns RM, Maginn RR, Herbertson RM, et al. Prolonged survival of liver transplants in the pig. Br Med J. 1967 Dec 16;4(5580):645–648. doi: 10.1136/bmj.4.5580.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XM, O'Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005 Dec 1;175(11):7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- 17.Crispe IN, Mehal WZ. Strange brew: T cells in the liver. Immunol Today. 1996 Nov;17(11):522–525. doi: 10.1016/s0167-5699(96)80906-6. [DOI] [PubMed] [Google Scholar]
- 18.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005 Feb 15;174(4):2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 19.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008 Jan;47(1):296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 20.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009 Jul;137(1):320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elvevold KH, Nedredal GI, Revhaug A, Smedsrod B. Scavenger properties of cultivated pig liver endothelial cells. Comp Hepatol. 2004 Aug 12;3(1):4. doi: 10.1186/1476-5926-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freudenberg MA, Freudenberg N, Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982 Feb;63(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman SL. Hepatic fibrosis-Overview. Toxicology. 2008 Jul 10; doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005 Apr;3(4):e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004 Feb;164(2):511–519. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath WR, Miller JF. Expression of two alpha chains on the surface of T cells in T cell receptor transgenic mice. J Exp Med. 1993 Nov 1;178(5):1807–1811. doi: 10.1084/jem.178.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005 Jan 15;174(2):1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 28.Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver Myofibroblasts Regulate Infiltration and Positioning of Lymphocytes in Human Liver. Gastroenterology. 2008 Oct 15; doi: 10.1053/j.gastro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Jiang G, Yang HR, Wang L, Wildey GM, Fung J, Qian S, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008 Dec 15;86(11):1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, Kappe SH, et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. J Infect Dis. 2007 Aug 15;196(4):599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004 May 1;172(9):5222–5229. doi: 10.4049/jimmunol.172.9.5222. [DOI] [PubMed] [Google Scholar]
- 32.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001 Apr;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 33.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004 Jul 1;173(1):230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997 Aug 1;90(3):986–993. [PubMed] [Google Scholar]
- 35.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007 Feb;45(2):445–454. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 36.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007 Dec 1;110(12):4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995 Feb;22(2):226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 38.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000 Apr;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 39.Knolle PA, Uhrig A, Hegenbarth S, Loser E, Schmitt E, Gerken G, et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998 Dec;114(3):427–433. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreisel D, Krasinskas AM, Krupnick AS, Gelman AE, Balsara KR, Popma SH, et al. Vascular endothelium does not activate CD4+ direct allorecognition in graft rejection. J Immunol. 2004 Sep 1;173(5):3027–3034. doi: 10.4049/jimmunol.173.5.3027. [DOI] [PubMed] [Google Scholar]
- 41.Kruse N, Neumann K, Schrage A, Derkow K, Schott E, Erben U, et al. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3- regulatory T cells suppressing autoimmune hepatitis. Hepatology. 2009 Dec;50(6):1904–1913. doi: 10.1002/hep.23191. [DOI] [PubMed] [Google Scholar]
- 42.Kudo S, Matsuno K, Ezaki T, Ogawa M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J Exp Med. 1997 Feb 17;185(4):777–784. doi: 10.1084/jem.185.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lalor PF, Lai WK, Curbishley SM, Shetty S, Adams DH. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol. 2006 Sep 14;12(34):5429–5439. doi: 10.3748/wjg.v12.i34.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006 Sep;116(9):2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010 Apr;11(4):295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008 Aug;8(8):1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 47.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000 Dec;6(12):1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 48.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005 Oct;35(10):2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 49.Lohse AW, Knolle PA, Bilo K, Uhrig A, Waldmann C, Ibe M, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996 Apr;110(4):1175–1181. doi: 10.1053/gast.1996.v110.pm8613007. [DOI] [PubMed] [Google Scholar]
- 50.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988 Mar-Apr;8(2):232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 51.Maemura K, Zheng Q, Wada T, Ozaki M, Takao S, Aikou T, et al. Reactive oxygen species are essential mediators in antigen presentation by Kupffer cells. Immunol Cell Biol. 2005 Aug;83(4):336–343. doi: 10.1111/j.1440-1711.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- 52.Marelli-Berg FM, Hargreaves RE, Carmichael P, Dorling A, Lombardi G, Lechler RI. Major histocompatibility complex class II-expressing endothelial cells induce allospecific nonresponsiveness in naive T cells. J Exp Med. 1996 Apr 1;183(4):1603–1612. doi: 10.1084/jem.183.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005 Feb;5(2):314–322. doi: 10.1111/j.1600-6143.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 54.McCaughan GW, Zekry A. Impact of immunosuppression on immunopathogenesis of liver damage in hepatitis C virus-infected recipients following liver transplantation. Liver Transpl. 2003 Nov;9(11):S21–27. doi: 10.1053/jlts.2003.50269. [DOI] [PubMed] [Google Scholar]
- 55.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999 Sep 15;163(6):3202–3210. [PubMed] [Google Scholar]
- 56.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004 Oct 1;173(7):4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 57.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005 Jan 13;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 58.Muhanna N, Horani A, Doron S, Safadi R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clin Exp Immunol. 2007 May;148(2):338–347. doi: 10.1111/j.1365-2249.2007.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998 Jan;28(1):84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 60.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000 Jul 15;165(2):795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 61.Onoe T, Ohdan H, Tokita D, Shishida M, Tanaka Y, Hara H, et al. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. J Immunol. 2005 Jul 1;175(1):139–146. doi: 10.4049/jimmunol.175.1.139. [DOI] [PubMed] [Google Scholar]
- 62.Page CS, Holloway N, Smith H, Yacoub M, Rose ML. Alloproliferative responses of purified CD4+ and CD8+ T cells to endothelial cells in the absence of contaminating accessory cells. Transplantation. 1994 Jun 15;57(11):1628–1637. [PubMed] [Google Scholar]
- 63.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005 Mar 1;174(5):2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 64.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004 Jan 15;172(2):1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 65.Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001 May;33(5):1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 66.Pradel G, Garapaty S, Frevert U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol Microbiol. 2002 Aug;45(3):637–651. doi: 10.1046/j.1365-2958.2002.03057.x. [DOI] [PubMed] [Google Scholar]
- 67.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994 Apr;19(4):916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian S, Wang Z, Lee Y, Chiang Y, Bonham C, Fung J, et al. Hepatocyte-induced apoptosis of activated T cells, a mechanism of liver transplant tolerance, is related to the expression of ICAM-1 and hepatic lectin. Transplant Proc. 2001 Feb-Mar;33(1-2):226. doi: 10.1016/s0041-1345(00)01985-0. [DOI] [PubMed] [Google Scholar]
- 69.Rothermel AL, Wang Y, Schechner J, Mook-Kanamori B, Aird WC, Pober JS, et al. Endothelial cells present antigens in vivo. BMC Immunol. 2004 Mar 16;5:5. doi: 10.1186/1471-2172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato K, Yabuki K, Haba T, Maekawa T. Role of Kupffer cells in the induction of tolerance after liver transplantation. J Surg Res. 1996 Jul 1;63(2):433–438. doi: 10.1006/jsre.1996.0288. [DOI] [PubMed] [Google Scholar]
- 71.Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur J Immunol. 2008 Apr;38(4):957–967. doi: 10.1002/eji.200738060. [DOI] [PubMed] [Google Scholar]
- 72.Schonrich G, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, et al. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int Immunol. 1992 May;4(5):581–590. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- 73.Schurich A, Bottcher JP, Burgdorf S, Penzler P, Hegenbarth S, Kern M, et al. Distinct kinetics and dynamics of cross-presentation in liver sinusoidal endothelial cells compared to dendritic cells. Hepatology. 2009 Sep;50(3):909–919. doi: 10.1002/hep.23075. [DOI] [PubMed] [Google Scholar]
- 74.Shen HQ, Lu M, Ikawa T, Masuda K, Ohmura K, Minato N, et al. T/NK bipotent progenitors in the thymus retain the potential to generate dendritic cells. J Immunol. 2003 Oct 1;171(7):3401–3406. doi: 10.4049/jimmunol.171.7.3401. [DOI] [PubMed] [Google Scholar]
- 75.Smith F, Golden-Mason L, Deignan T, Norris S, Nolan N, Traynor O, et al. Localization of T and B lymphocytes in histologically normal adult human donor liver. Hepatogastroenterology. 2003 Sep-Oct;50(53):1311–1315. [PubMed] [Google Scholar]
- 76.Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997 Sep;9(9):1355–1365. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- 77.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J Hepatol. 2008 Dec;49(6):1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu Z, Bozorgzadeh A, Crispe IN, Orloff MS. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007 Jul;149(1):186–193. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008 Jan 21;205(1):233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008 Feb 15;180(4):2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 81.von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. 2009 May;49(5):1664–1672. doi: 10.1002/hep.22795. [DOI] [PubMed] [Google Scholar]
- 82.Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006 Nov;44(5):1182–1190. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
- 83.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007 Jan;26(1):117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Wuensch SA, Pierce RH, Crispe IN. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J Immunol. 2006 Aug 1;177(3):1689–1697. doi: 10.4049/jimmunol.177.3.1689. [DOI] [PubMed] [Google Scholar]
- 85.Wuensch SA, Spahn J, Crispe IN. Direct, help-independent priming of CD8+ T cells by adeno-associated virus-transduced hepatocytes. Hepatology. 2010 Sep;52(3):1068–1077. doi: 10.1002/hep.23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008 Oct 15;112(8):3175–3185. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001 Jan 1;193(1):35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008 Sep;48(3):978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]