Abstract
Neuronal excitability can be modulated by release of intracellular calcium but the impact of calcium store depletion on intrinsic neuronal properties is unknown. In this issue of Neuron, Narayanan et al. describe an intrinsic plasticity that is depletion-induced, regionally restricted and may protect neurons from pathological alterations in calcium signaling.
The view that intracellular calcium stores are passive reservoirs is a thing of the past. In the past few years, with the discovery of the molecular components responsible for refilling the stores, including the TRP family of channels (Ambudkar et al., 2007) and the Stim/Orai complex (Cahalan, 2009), the depletion of the calcium store in the endoplasmic reticulum (ER) has taken on new significance. As more members of the calcium signaling toolkit have been identified, additional functions have been assigned to specific cellular pathways (Choe and Ehrlich, 2006; Mikoshiba, 2007; Rizzuto and Pozzan, 2006). Along with the functional assignments is the understanding that modulation of the physiological signaling underlies these processes and, when disrupted, leads to pathological situations and chronic diseases.
Much of our knowledge of ER calcium storage and depletion has been obtained from studies using non-excitable cells. Although neurons have been more difficult to study, in this issue of Neuron, Narayanan, Dougherty and Johnston elegantly examine the aftermath of ER calcium store depletion on hippocampal neurons. They found that ER calcium store depletion in CA1 pyramidal neurons of the hippocampus leads to an increase in functional h channels in the plasma membrane. The enhanced h current depended upon calcium release through inositol 1,4,5 trisphosphate receptors (InsP3Rs), calcium entry through store operated channels (SOCs), and activation of protein kinase A (PKA). Increased h channel activity resulted in a persistent, perisomatic reduction in intrinsic neuronal excitability, which was accompanied by an increase in the optimal response frequency of the neuron. The authors suggest that this form of depletion-induced intrinsic plasticity could have a neuroprotective role in situations of pathological alterations of calcium signaling.
Remarkably, despite the global inhibition of the sarcoplasmic/endoplasmic reticular calcium ATPase (SERCA), changes in the electrical responses of the neurons were predominantly confined to the soma. What is the basis for the regional difference observed in the response to calcium store depletion? The authors suggest that this can be accomplished in numerous ways. One simple explanation could be that calcium release only occurs in a limited region. This strategy would be especially useful in neurons and in polarized epithelial cells where large distances exist between subcellular regions and where extracellular stimuli are released locally. In the experiments described by Narayanan et al. (2010), the combination of bath application of the SERCA inhibitors and the uniform distribution of SERCA in hippocampal neurons (Jacob et al., 2005) supports the assumption that the ER is unloaded everywhere. That there is a similar, albeit more attenuated, response along the dendrite of the CA1 neuron provides additional support for the idea that the calcium stores are depleted in the dendrites as well as the soma.
An alternative explanation is that components of the calcium signaling tool kit can be distributed in a cellular and regionally specific manner, and that the observed specificity is provided by cooperative action or regulation of these components. The differences in distribution of calcium signaling molecules across cell types are not subtle and can have profound influences on function. An excellent example is in the distribution of the channels responsible for the unloading of the ER stores: striated muscles contain predominantly ryanodine receptors (RyR), whereas neurons contain predominantly InsP3R (Berridge et al., 2003; Rizzuto and Pozzan, 2006). The high expression of RyR and use of the T-tubule network provides a rapid and explosive increase in calcium throughout the cell that is necessary for muscle contraction. In contrast, the delay inherent in the biochemical cascade needed to produce InsP3 provides layers of control consistent with the processing and regulation of neuronal functions. Within a cell there also are clear subcellular specialties in ER channel location. The InsP3R can be juxtaposed to the plasma membrane for rapid responses to agonist stimulation, or it can be deeper in the cell for more delayed calcium signals (Delmas et al., 2002). The interactions between the plasma and ER membrane can be regulated by scaffolding proteins such as Homer which form subdomains by linking the InsP3R of the ER to metabotropic glutamate receptors of the plasma membrane (Xiao et al., 2000).
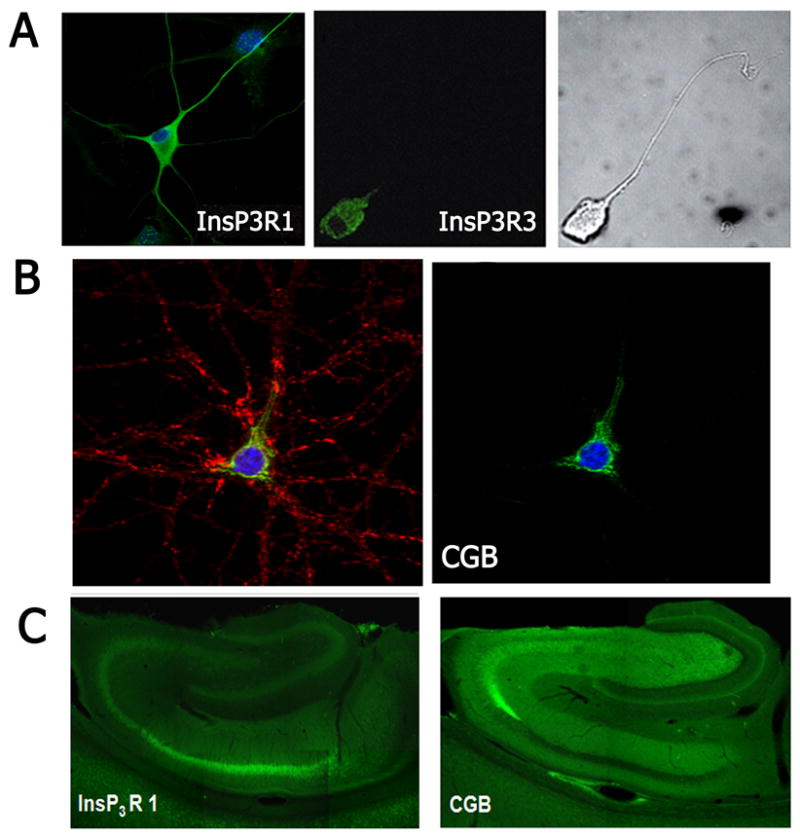
For the InsP3R, an additional aspect of its specialization is that the distribution of its isoforms is often found restricted to specific subcellular regions. Although these isoforms are highly homologous, they have distinct functional properties (Tu et al., 2005). The InsP3R type 3 is often exclusively expressed in the soma of neurons (Johenning et al., 2002). Among the isoforms, InsP3R type 3 has the lowest affinity for InsP3, and thus serves to respond to high levels of InsP3 resulting from intense stimulation. On the other hand, the InsP3R type 2, although not expressed in many neurons, is often found on the nuclear membrane where its location and high InsP3 affinity make it suitable for regulating calcium-dependent nuclear events (Leite et al., 2003). The InsP3R type I, the isoform most commonly found in neurons, is expressed throughout the cell and its exquisite sensitivity to inhibition by calcium enhances its role in calcium oscillations (Johenning et al., 2002). It is the combination of functional distinctions such as these along with restricted regional distribution that become crucial in understanding functional responses to unloading calcium stores.
The experiments by Narayanan et al. (2010) strongly suggest that distribution of calcium release channels within a cell alone cannot fully explain the regional responses to changes in cytoplasmic calcium. Regionally-specific regulation may be due to non-uniform distribution of accessory proteins that regulate calcium signaling. One particularly relevant example of such regulation is the interaction of the InsP3R type I with chromogranin B (CGB). CGB is expressed in the ER of hippocampal cells and, when bound to the luminal side of the InsP3R, significantly increases its open probability (Choe et al., 2004). Notably, CGB is expressed in the soma and proximal dendrite of pyramidal cells of the hippocampus (Jacob et al., 2005). Regions with high CGB correlate with the location for calcium signal initiation sites (Jacob et al., 2005), and interestingly, there appears to be a reciprocal relationship between the expression of CGB and the InsP3R in the hippocampus in CA1 and CA3 (Nicolay et al., 2007). The high expression of CGB might be important for compensating for low InsP3R expression, yet another mechanism for regionally-specific modulation of calcium levels
Thus, regionally specific functions in neurons can be modulated by non-uniform distribution of components of the calcium signaling tool kit within a cell. These components may be expressed in a compartment of the cell, may be clustered, may have regionally localized binding partners, or may be enzymatically modified. This list of the different types of regionally-specific regulation is far from being exhaustive. In the case of Narayanan et al. (2010), the increase in the h current quite possibly is the result of silent h channels that become functional after modification that changes their gating properties. In this case, the activation of PKA, rather than de novo protein synthesis, could be the event that provided the extra boost. Such a mechanism would be consistent with previous work demonstrating that many kinases, pumps, and calcium buffers are evenly expressed throughout neurons (eg., (Jacob et al., 2005)), but show specificity due to their activation by other regionally–specific processes. Activation of signaling subdomains can provide regional specificity and explain the observed functional differences in plasticity between somatic and dendritic regions.
In summary, the results of Naranyanan et al. (2010) reveal a new form of plasticity that may play a key role in neuroprotective responses to dysregulation of calcium signaling in pathological states. Beyond this, these findings underscore the complexities of calcium signaling within a cell and the numerous processes that contribute to fine-tuning its spatiotemporal properties. Future work will define the precise mechanisms and the regional specificity underlying the regulation of h channel activity and explore the relevance of depletion-induced intrinsic plasticity to neuroprotection.
Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006:re15. doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- Choe CU, Harrison KD, Grant W, Ehrlich BE. Functional coupling of chromogranin with the inositol 1,4,5-trisphosphate receptor shapes calcium signaling. J Biol Chem. 2004:35551–35556. doi: 10.1074/jbc.M311261200. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown D. Signaling microdomains define the specificity and sensitivity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Choe CU, Uhlen P, DeGray B, Yeckel MF, Ehrlich BE. Signaling microdomains regulate inositol 1,4,5-trisphosphate-mediated intracellular calcium transients in cultured neurons. J Neurosci. 2005;25:2853–2864. doi: 10.1523/JNEUROSCI.4313-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, Ehrlich BE, Nathanson MH. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- Nicolay NH, Hertle D, Boehmerle W, Heidrich FM, Yeckel M, Ehrlich BE. Inositol 1,4,5 trisphosphate receptor and chromogranin B are concentrated in different regions of the hippocampus. J Neurosci Res. 2007;85:2026–2036. doi: 10.1002/jnr.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005;88:1046–1055. doi: 10.1529/biophysj.104.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]


