Abstract
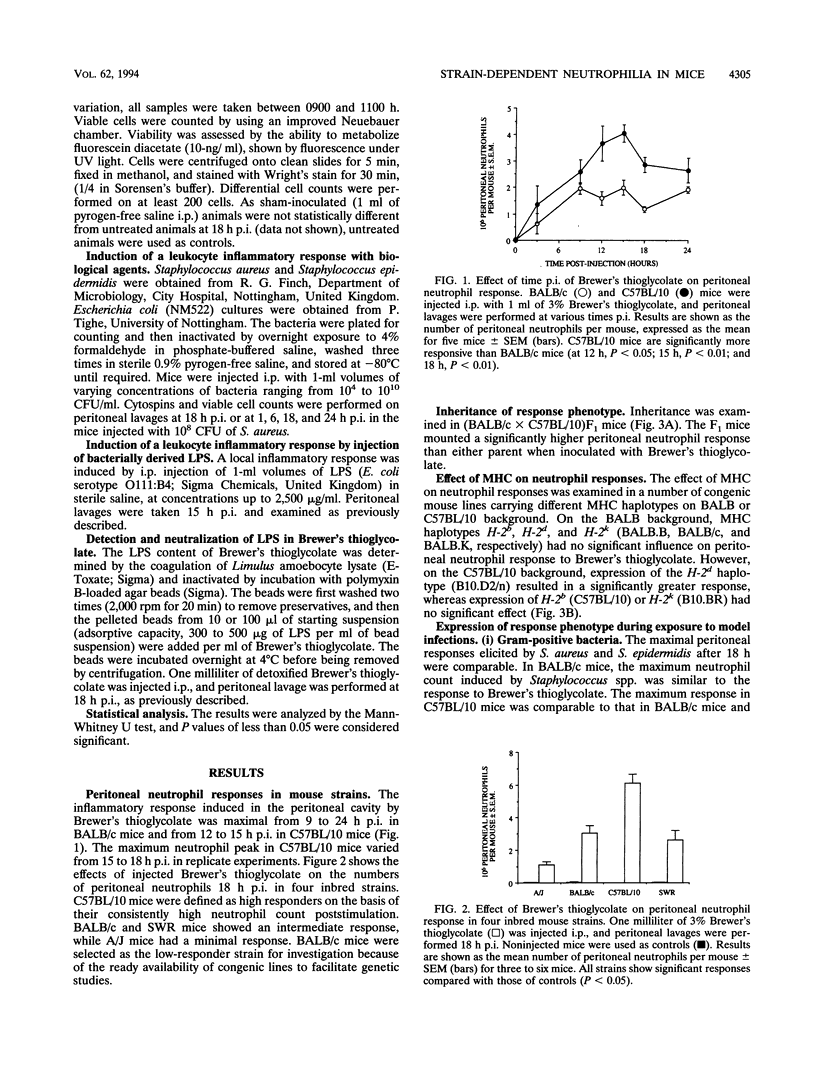
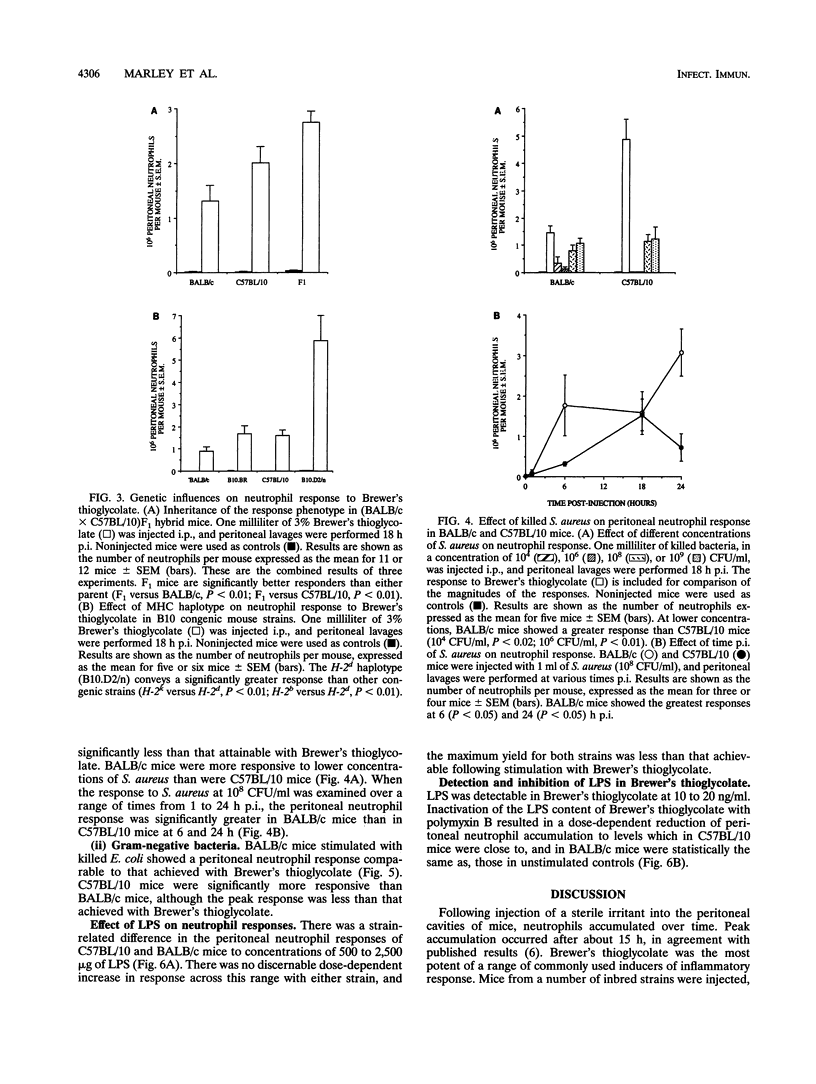
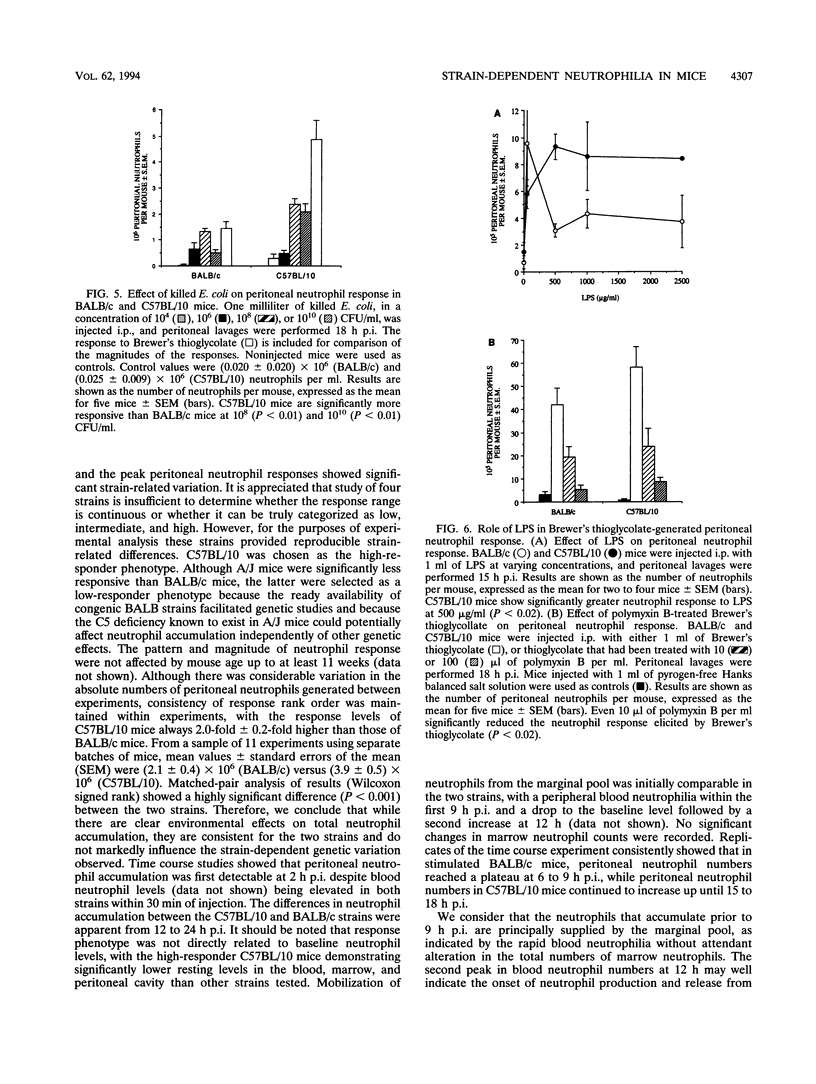
Mice from a variety of strains were injected with a sterile irritant (Brewer's thioglycolate) and killed bacteria (Staphylococcus aureus, Staphylococcus epidermidis, or Escherichia coli) to determine their effect on accumulation of neutrophils in the peritoneal cavity. Peak accumulation occurred around 15 h postinjection and showed significant strain-related variation. C57BL/10 mice were identified as having a high-responder phenotype and BALB/c mice a low-responder phenotype. Inheritance of the high-responder phenotype followed simple Mendelian genetics: (BALB/c x C57BL/10)F1 mice were found to be more responsive than either parental phenotype. Major histocompatibility complex H-2d haplotype was found to convey an augmented neutrophil response in conjunction with B10 background high-responder genes (B10.D2/n) but the H-2d haplotype per se was not the only factor in determining high responsiveness. Gram-positive and gram-negative bacteria appeared to activate different immune mechanisms. Both gram-negative bacteria and lipopolysaccharides (LPS) induced a response similar to, but less potent than, that induced by Brewer's thioglycollate. Neutralization of the LPS content of Brewer's thioglycolate abrogated the response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N. M., Behnke J. M., Manger B. R. The pattern of peripheral blood leucocyte changes in mice infected with Nematospiroides dubius. J Helminthol. 1985 Mar;59(1):83–93. doi: 10.1017/s0022149x00034532. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Griffin J. D. Regulation of the production and function of granulocytes and monocytes. Semin Hematol. 1988 Jul;25(3):173–188. [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Dual regulation of anti-bacterial resistance and inflammatory neutrophil and macrophage accumulation by L3T4+ and Lyt 2+ Listeria-immune T cells. Immunology. 1987 Feb;60(2):287–293. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985 May;134(5):3449–3454. [PubMed] [Google Scholar]
- Fletcher J., Haynes A. P., Crouch S. M. Acquired abnormalities of polymorphonuclear neutrophil function. Blood Rev. 1990 Jun;4(2):103–110. doi: 10.1016/0268-960x(90)90033-o. [DOI] [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Gervaix A., Suter S. Neutrophiles: médiateurs de la destruction bronchique chez les patients souffrant de mucoviscidose. Pathol Biol (Paris) 1991 Jun;39(6):598–599. [PubMed] [Google Scholar]
- Hartmann D., Entringer M. A., Vasil M., Robinson W. A. The effect of bacterial infection on granulopoiesis. Proc Soc Exp Biol Med. 1981 May;167(1):6–11. doi: 10.3181/00379727-167-41115. [DOI] [PubMed] [Google Scholar]
- Kissling K., Karbe E., Freitas E. K. In vitro phagocytic activity of neutrophils of various cattle breeds with and without Trypanosoma congolense infection. Tropenmed Parasitol. 1982 Sep;33(3):158–160. [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Synthesis by mouse peritoneal cells of G-CSF, the differentiation inducer for myeloid leukemia cells: stimulation by endotoxin, M-CSF and multi-CSF. Leuk Res. 1985;9(1):35–50. doi: 10.1016/0145-2126(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Myers M. J., Pullen J. K., Ghildyal N., Eustis-Turf E., Schook L. B. Regulation of IL-1 and TNF-alpha expression during the differentiation of bone marrow derived macrophage. J Immunol. 1989 Jan 1;142(1):153–160. [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper A. G., Lewis P. Genetic neutropenia in people of African origin. Lancet. 1971 Nov;2(7732):1021–1023. doi: 10.1016/s0140-6736(71)90335-7. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Stolc V. Genetic control of blood neutrophil concentration in the rat. J Immunogenet. 1988 Oct-Dec;15(5-6):345–351. doi: 10.1111/j.1744-313x.1988.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Gans P. J., McCarroll L. A. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983 Feb;130(2):800–807. [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]
- Young A. M., Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]


