Abstract
Targeted muscle reinnervation (TMR) is a surgical intervention to improve the control of myoelectric prostheses in high-level upper-limb amputation. This article briefly describes the procedure and presents the protocol for postoperative, preprosthetic care. We also recommend a guide to patient training using standard-of-care prosthetic devices controlled by up to four intuitive, independent, and isolated myoelectric signals. We discuss the advantages of this new control paradigm and methods for optimizing clinical outcomes for patients with high-level upper-limb amputations. This material is based on more than 6 years of experience treating patients with TMR in a research setting. Detailed results of this research are reported elsewhere.
Keywords: amputation, myoelectric control, nerve transfer, occupational therapy, preprosthetic training, prosthetic training, rehabilitation, simultaneous control, targeted muscle reinnervation, upper limb
Introduction
Controlling prostheses at the transhumeral and shoulder disarticulation levels of amputation is challenging. Several mechanical joints need to be operated, but limited inputs for control exist. A single degree of freedom (DOF) can be controlled at a time with body-powered cables. Similarly, only a single DOF can be controlled with electromyographic (EMG) signals from the remaining muscles. At the transhumeral level, no more than two myoelectric sites (biceps and triceps) are available to control three pairs of movements at the elbow, wrist, and hand. Unfortunately, the biceps and triceps are not intuitive control sources for the wrist or hand. In the case of shoulder disarticulation, sites may be located on the chest and back muscles. The chest and back muscles act on the shoulder and are not intuitive control sources for either the elbow or hand. These limitations make control of multiple joints tedious and preclude simultaneous control of several joints [1–2]. As a result, a better strategy is required to provide functional, intuitive control of myoelectric prostheses for high-level amputees.
Targeted muscle reinnervation (TMR) is a new elective surgery that increases the number of EMG control signals, thus improving the potential for enhanced prosthetic function. TMR takes advantage of intact residual nerves that previously connected to muscles distal to the amputation. The intact residual peripheral nerves are transferred to surgically denervated areas of unused musculature in the residual limb or chest (Figure 1). The arm and hand nerves are transferred to reinnervate the “target” muscle so that the nerves represent the absent limb physiologically. These new target muscle contractions correlate physiologically to the movements of the prosthetic device. The increased number of EMG signals enables simultaneous myoelectric control of the elbow and hand and frees the shoulder to control a powered wrist as well. The resulting control is more intuitive and thus requires less effort. Prosthetic movements are more efficient without the necessity of switching between functions. Initial outcome measure results have been very encouraging. All subjects with TMR have increased performance significantly [3]. In a Box and Block Test, patients moved an average of three times as many blocks with TMR control versus conventional myoelectric control [4]. Patients with TMR were 50 percent faster in a clothespin moving test and improved in the Assessment of Motor and Process Skills [5–6]. TMR is now being performed as a clinical service, not just as a research protocol. To date, TMR programs have been developed in six different centers around the world.
Figure 1.
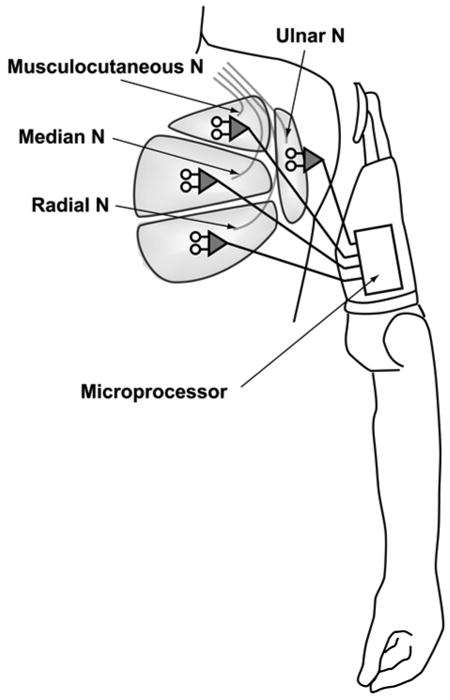
Targeted muscle reinnervation of peripheral nerves to pectoralis major in shoulder disarticulation amputation. N = nerve.
A critical aspect to successfully implementing TMR is the therapy that patients receive to effectively use their new prostheses. This article presents our therapy strategies unique to patients undergoing TMR. This approach can be incorporated into the customary occupational therapy for myoelectric prosthesis fittings [7].
Methods
We developed the therapy protocol during the treatment and testing of three persons with shoulder disarticulation and four with transhumeral amputations who underwent successful TMR. The therapy occurs in four phases: surgical procedure, postsurgical program before TMR fitting, diagnostic fitting, and multifunction prosthesis training.
Surgical Procedure
TMR is generally performed several months after the initial trauma when the residual limb has healed. The surgery involves two to four nerve transfers and is accomplished in a 2- to 5-hour operation. Details of the surgical technique are presented elsewhere [8–11]. The initial issues are pain and edema. Postsurgical pain is treated with standard care. The patient may experience a transient recurrence of or increase in phantom limb pain.
Postsurgical Program Before Targeted Muscle Reinnervation Fitting
Signal Strengthening
An important goal following TMR is strengthening reinnervated muscles so they generate electrical signals that can be detected by surface electrodes. Strengthening the contraction of the transferred-nerve muscles before the fitting helps the patient develop the adequate endurance needed to proceed with TMR myoelectric prosthetic training. This goal is similar to that following any amputation surgery before myoelectric control, but the exercises are different because of the redirected pathways from the brain to the host muscles. As a result, the occupational therapist (OT) must thoroughly understand peripheral nerve distribution, including which nerves are anticipated to reinnervate which muscles, to perform the appropriate strengthening exercises.
Each nerve contains numerous motor neurons. These motor neurons control numerous muscle fibers that work in conjunction to create a variety of peripheral nerve actions (Table). For example, the radial nerve innervates hand extensor muscles, wrist extensor muscles, and supination muscles. Neither the surgeon nor the OT can be certain which nerve fibers will reinnervate the host muscle; as a result, nerve fibers may disproportionately reinnervate the new target muscle, corresponding to only a few muscle actions. In light of this uncertainty, the OT must ask the patient to perform all the actions controlled by the transferred nerves. Several actions associated with motor nerve distribution may cause the muscle to contract, but evidence of the most optimal contraction can take weeks to occur. The patient performs exercises to strengthen the movement command that creates the most useful EMG signal.
Table.
Peripheral nerve actions (radial, median, and ulnar) of upper limb.
| Radial | Median | Ulnar |
|---|---|---|
| Elbow Extension | Pronation | Ulnar wrist flexion |
| Supination | Radial wrist flexion | Finger flexion at DIP (digits 4 & 5) |
| Radial Wrist Extension | Midline wrist flexion | Thumb flexion (proximal) |
| Ulnar Wrist Extension | Finger flexion at DIPs and MPs (digits 2 & 3) | Thumb abduction |
| Midline Wrist Extension | Finger flexion at PIPs | Little finger opposition |
| Index finger extension | Thumb flexion (distal) | Little finger abduction |
| Little Finger Extension | Thumb opposition | MP flexion (digits 3 & 4) |
| Thumb Extension | Thumb abduction | |
| Finger Extension | Finger abduction | |
| Finger adduction |
DIP = distal interphalangeal (joint), MP = metacarpal-phalangeal (joint), PIP = proximal interphalangeal (joint).
About 3 weeks after surgery, the OT instructs the patient to attempt moving each missing limb joint several times daily. These exercises should be brief and relaxed attempts to move the missing hand, wrist, and elbow. These attempts may promote activity in both central brain and peripheral nerve pathways to enhance reinnervation and will prepare the patient to recognize the first signs of reinnervation. The first noticeable reinnervation usually occurs at 10 to 15 weeks after surgery; a small twitch is felt or seen in the target muscles.
The strengthening program can begin as soon as reinnervation clearly exists. The patient needs to strengthen the target muscles by performing gross movement patterns using maximal contractions. Patterns should be used that incorporate the distribution of the nerve transfers as shown in the Table. If a given nerve is transferred, all actions of that nerve should be incorporated into the transfer muscle exercise program. Patterned movements should be performed bilaterally to further promote motor recruitment. Because resistance cannot be applied to a missing limb, the OT measures strength training in home exercises by increasing the duration of the contraction and number of repetitions in each set. The OT should teach the patient to recognize fatigue and encourage him or her to relax the muscles. During the reinnervation period, the OT can apply resistance to the residual humerus or scapula to strengthen the remaining shoulder muscles.
When consistent target muscle activity is palpable during gross movement exercises 3 to 5 months after surgery, the patient should discontinue the patterned program in favor of more discrete muscle actions. The patient's goal is isolating each target muscle so that the patient can activate each prosthetic function individually. Again, the patient should incorporate all actions of that nerve into the transfer muscle exercise program. Clearly, some movements contract the target muscle better than others. For example, in the muscle reinnervated by the median nerve, the patient may generate a stronger muscle contraction when trying to flex the missing wrist than when flexing the fingers. However, the relative amount of contraction caused by each movement effort may change with time and practice and as reinnervation progresses. Thus, in our example, finger-flexion effort may evolve to produce a stronger contraction in the reinnervated muscle. The patient should perform maximal contraction exercises to strengthen muscle and graded contraction exercises to promote the potential for proportional control. Some cues to help patients perform the exercises are—
Perform the exercise with the intact arm and hand. Bilateral activation can help focus the desired movements.
Isolate elbow extension. Try a push-up on a chair seat or arm with the intact limb.
Use a table mirror (or a mirror box as used to treat phantom limb pain) and watch the intact limb do the exercises while exercising the nerve transfers.
Visualize lifting, squeezing, pushing, hitchhiking, writing, turning a door knob, and wielding a hammer. The OT can encourage the patient to invent his or her own “virtual” activities and make these the cues for the movements.
Use the intact hand to palpate for the muscle contraction/relaxation. The target muscle should feel soft upon relaxation. The OT can try to localize the contractions and use this tactile feedback with the patient.
Use a table mirror to observe soft tissue movement during contractions.
Relaxation
The nerve transfer exercise program also incorporates muscle relaxation. Myoelectric prostheses often use a resting state to separate independent signals and differentiate between motor commands. The patient must be able to contract muscles selectively and avoid inadvertent cocontraction, which frequently occurs with overexertion and fatigue. Maximal relaxation is most easily detected following maximal contraction. The OT can use the exercise program to teach the patient to recognize “contracted” and “relaxed” states.
Phantom Limb Versus Muscle Contraction
The patient should distinguish between movement of the phantom limb and the nerve-transfer exercises described in the previous section. The brain sends signals through the nerves regardless of phantom limb position or mobility. These signals, in turn, cause motor units to fire. As the transferred nerve fibers grow into the target muscle, they are able to cause contractions of individual muscle fibers that are at first imperceptible. As a result, even if the patient feels as if the phantom limb is not moving, the OT should instruct him or her to perform the exercises because the reinnervated muscles will still contract and strengthen. Even after TMR, phantom limb sensation can confuse the patient. The patient must understand that phantom limb position is unrelated to prosthesis operation. Performing the exercises simultaneously with the intact limb encourages appropriate action from the patient and decreases any possible distraction from the phantom limb.
Interim Prosthetic Issues and Activities
The OT and patient address maximal independence in basic self-care with and without a prosthesis, one-handed techniques, and adaptive equipment early in the rehabilitation process. Initially, patients will find that transfer sites are paralyzed and will feel numb because of both muscle and skin denervation during surgery. Functional TMR requires 3 to 6 months. During this time, patients can use a prosthesis that does not require control from the transfer sites. This prosthesis may be a previously fit body-powered prosthesis, a two-site myoelectric prosthesis, a hybrid prosthesis or, for amputees with shoulder disarticulation, a prosthesis with touch pad control. The surgery will not affect use of body-powered prostheses; however, sockets will need to be adjusted for the altered shape of the residual limb. Similarly, a person with transhumeral amputation should be able to continue using a two-site myoelectric or hybrid prosthesis with the electrodes moved over the undisturbed heads of the biceps and triceps. For an amputee with shoulder disarticulation, an interim myoelectric prosthesis may not provide optimal control and function because the pectoral muscles are paralyzed and cannot be used as myosites until reinnervation occurs. Continued use of a prosthesis after healing, however, is recommended whenever possible. Early use training by the patient in prepositioning the terminal device and integrating the prosthesis into daily activities carries over to the TMR prosthesis and promotes functional independence. Early prosthetic fitting and training after an amputation have long been assumed to correlate with successful long-term integration of the prosthesis [12].
Diagnostic Fitting
When the target muscles are adequately reinnervated, patients with transhumeral amputation are expected to be able to independently and intuitively control elbow flexion (musculocutaneous nerve) and extension (proximal radial nerve) from their natural, undisturbed lateral biceps and medial triceps muscles. The distal radial and median nerve reinnervated muscles (lateral triceps and medial biceps, respectively) control hand opening and closing. An ulnar nerve transfer to the brachialis or other residual muscle can sometimes be used for hand opening (finger abduction) or closing (ulnar finger flexion). Patients with shoulder disarticulation are expected to be able to independently control the elbow and hand from four nerve-transferred myosites.
The success of any myoelectric fitting depends on the patient's ability to isolate signals. The patient must be able to relax while moving joints proximal to the amputation without eliciting an EMG signal. This is critical during movement in space while functional activities are performed. This ability of the patient to isolate signals is equally important for the TMR-controlled myoelectric fitting. Initially, signals will be weak, with limited endurance. Optimal sites may change over time as reinnervation progresses. During this period, OTs must have their patients describe as accurately as possible what they feel they are doing by demonstrating with their intact arm and hand and performing bilaterally. The OT must know, for example, what yields the strongest, most isolated “hand open” signal. The most intuitive movements that yield strong EMG signals are preferred whenever possible. As an example, extension of the index finger, little finger, thumb, wrist, or full hand (radial nerve) may produce the best, most physiologically appropriate signal to use for hand opening. Clearly, use of hand extension would be the preferred movement for controlling prosthetic hand opening if EMG signals are adequate. This uncertainty is why OTs encourage patients to exercise all potential actions. OTs ensure that the strongest possible signal at a given electrode site is available to control a given prosthesis movement.
In terms of strength and signal separation, the optimal EMG signal may not feel the most “physiological” to patients. When the OT and prosthetist have identified distinct signals sufficiently isolated for elbow and terminal device control, the prosthetist proceeds with a diagnostic socket.
Multifunction Prosthesis Training
The prosthetist fits patients with a diagnostic socket using independent myoelectric control of four functions: elbow flexion, elbow extension, hand open, and hand close. User intentions are reflected in the signal and corresponding functions of the prosthesis. Training begins with repetitive exercises of each separate function. The exercises strengthen and reinforce the physiologically appropriate EMG signals associated with TMR control and ensure optimal socket fit and electrode locations. The goal of these initial exercises is to minimize cocontractions and encourage signal separation. OTs ensure that patients continue to be able to flex or extend the elbow without the hand opening or closing. Patients perform elbow operations while the hand is partially open to observe unwanted activity. Performing hand functions while keeping the elbow and wrist in a midrange position is equally important.
For the patient, having the prosthetist available during this phase of training is helpful. Frequent adjustments to electrode gains, EMG thresholds, and electrode location in the socket are necessary. EMG signal separations are essential and can be difficult if electrodes are close or if the nerve transfer EMG signal is small compared with neighboring muscles. For example, inadequate signal separation exists if the hand closes during elbow flexion. The prosthetist may change gains and thresholds or move the electrodes farther apart. If the problem is cocontraction, the OT must work with the patient to isolate control.
The diagnostic socket is sent home with the patient as soon as the team agrees control is adequate and the patient is able to troubleshoot issues that may arise. Time between appointments should be kept short (days rather than weeks).
Exercises Using Added or Altered Components and Control Options
Special attention is given to any prosthetic component changes as they are implemented. The addition of a powered wrist rotator or change of a terminal device requires familiarization and incorporation into the prosthesis exercises for mastering control. Once the patient demonstrates consistent, independent control of the four myoelectric sites, control of any additional features may be added. If unfamiliar controls are introduced, the patient is trained to operate them without interfering with the myoelectric functions. For example, if a patient with a transhumeral amputation previously had passive wrist rotation, and now a linear transducer pull-switch is introduced for wrist rotation, the patient should learn to reach forward without activating the switch and activate the switch without unwanted elbow or hand activity.
When the patient demonstrates consistent, isolated control of each prosthetic action during exercises, he or she is encouraged to move more quickly between actions while maintaining signal separation. Ultimately, patients are encouraged to perform combined actions. Intuitive combinations include elbow flexion with hand closed or elbow extension with hand open. Opening the hand while bending the elbow is usually more challenging to the patient, because the combination is not a synergistic pattern. The pattern is not essential but presents an opportunity to practice independent and simultaneous function control.
The habit of operating the prosthesis sequentially is much more efficient with TMR than with control that requires switching between functions or differentiating signal strength. Beyond fast sequential control, TMR allows simultaneous operation of the hand, elbow and, possibly, wrist. Thus, the patient may benefit from cues to move more quickly to independently and simultaneously control two prosthetic actions. For example, the patient can be cued to open the hand while reaching for an object.
Special attention should be given to unwanted hand or elbow movement. Operations can be slowed down when necessary to reestablish independent, isolated control. Reaching toward an object while simultaneously opening the hand may be desirable, but reaching forward to place a full cup of water on a table requires that the hand remain closed until the intended release. While speed does not determine success, it is used to build simultaneity and support a decrease in mental load, especially during patterned movements.
Performance in One-Handed and Bimanual Activities
When the patient can demonstrate isolated control of all prosthetic functions and simultaneous myoelectric control of two actions, training may progress to activities that can be performed one-handed. This approach encourages the patient to explore potential TMR control he or she might not otherwise notice because of preexisting habits in familiar task performance. These activities can include loading a dishwasher, unloading a dryer, sorting mail, or using form boards. These tasks all have an element of repetition and some prepositioning requirements. They include all three arm functions: elbow, wrist, and hand. Additionally, the patient should engage in bimanual coordination tasks such as tying shoelaces or a necktie, folding or hanging clothes, and removing money or credit cards from a purse or wallet. The OT should have the patient identify tasks and settings (home, office, grocery store). To motivate patients to speed up performance, the OT can time and compare performance of the repeated trials. To limit unwanted movement, sometimes patients fail to use the movement capabilities of their prosthesis to achieve reach through effective positioning of the elbow. This masks issues related to control that will need to be addressed as they arise. Reeducation of body mechanics may be needed. Experience with repetitive patterned functional tasks promotes and reinforces the appropriate motor commands corresponding to the intended prosthesis actions.
The amount of time spent at each level of training is based on the individual patient and levels may overlap. We have outlined them here for training progression analysis. This hierarchy may vary and overlap depending on the patient's previous experience, prosthetic control scheme, motor skills and motor learning style, and the clinician. Figure 2 describes a timeline of the general progression for treatment, prosthetic fitting, and training following TMR.
Figure 2.
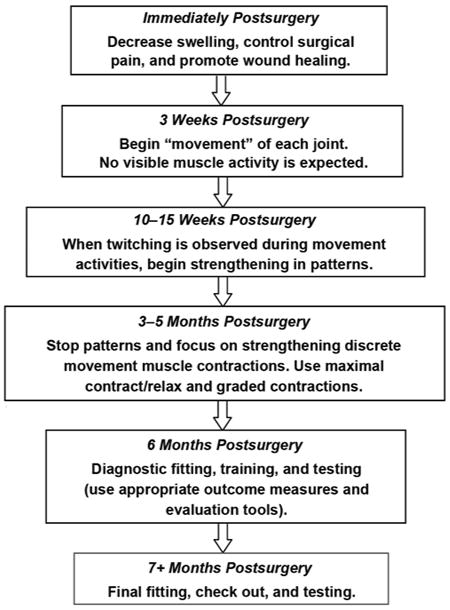
Targeted muscle reinnervation protocol outline with timeline.
Discussion
TMR is a new technique for patients with transhumeral amputations and shoulder disarticulation. By transferring residual nerves to spare muscles, more myoelectric signals can be obtained for powered prosthesis control.
TMR not only adds myoelectric control sites, but the nature of the control also contributes to easier operation of multiple joints in high-level amputations using “off-the-shelf” components. The relationship between prosthetic arm and hand movement directly correlates with the nerve signal to the missing limb redirected to remnant muscle by the peripheral nerve transfer. Additionally, less intuitive control options remain to control added DOFs as they become available (wrist rotation, wrist flexion, and humeral rotation) [13]. Marked functional improvements have been demonstrated in the laboratory and field [3]. TMR is a technique that is becoming available to patients in more places around the world.
Conclusions
TMR is a new concept for upper-limb prosthesis operation and requires a new approach to patient training. In this article, we presented the occupational therapy protocol developed while treating an initial series of seven patients over the last 6 years. Every patient is unique and requires individually customized therapy; however, we have highlighted many core principles and practices in this protocol. The most important factor for the success of patients with TMR is that each team member must understand the fundamental principles of TMR. Knowledge of peripheral nerve distribution is essential, as well as the specifics of the surgery and possible outcomes for each patient. Full realization of the advantages of TMR depends on clear and frequent communication between the involved physicians, prosthetist, OT, and patient.
Acknowledgments
Funding/Support: This material was based on work supported in part by the National Institutes of Health (NIH) National Institute of Child and Human Development (grant 5 R01 HD043137-05), NIH National Institute of Diabetes and Digestive and Kidney Diseases (contract N01-HD-5-3402), and the Defense Advanced Research Project Agency Phase I and Phase II (contract 908090).
Abbreviations
- DOF
degree of freedom
- EMG
electromyographic
- NIH
National Institutes of Health
- OT
occupational therapist
- TMR
targeted muscle reinnervation
Footnotes
Author Contributions:
Study concept and design: K. A. Stubblefield, T. A. Kuiken.
Acquisition, analysis, and interpretation of data: K. A. Stubblefield.
Drafting of manuscript: K. A. Stubblefield.
Critical revision of manuscript for important intellectual content: T. A. Kuiken, R. D. Lipschutz, L. A. Miller.
Statistical analysis: K. A. Stubblefield.
Obtained funding: T. A. Kuiken.
Administrative, technical, or material support: T. A. Kuiken.
Study supervision: T. A. Kuiken.
Financial Disclosures: The authors have declared that no competing interests exist. No author had any paid consultancy or any other conflict of interest with this article.
References
- 1.Williams TW. Control of powered upper extremity prostheses. In: Meier RH, Atkins DJ, editors. Functional restoration of adults and children with upper extremity amputation. New York (NY): Demos Medical Publishing, Inc; 2004. pp. 207–24. [Google Scholar]
- 2.Childress DS, Weir RF. Control of limb prostheses. In: Smith DG, Michael JW, Bowker JH, editors. Atlas of amputations and limb deficiencies: Surgical, prosthetic, and rehabilitation principles. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2004. pp. 173–96. [Google Scholar]
- 3.Miller LA, Stubblefield KA, Lipschutz RD, Lock BA, Kuiken TA. Improved myoelectric prosthesis control using targeted reinnervation surgery: A case series. IEEE Trans Neural Syst Rehabil Eng. 2008;16(1):46–50. doi: 10.1109/TNSRE.2007.911817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–91. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 5.Fisher AG. The assessment of IADL motor skills: An application of many-faceted Rasch analysis. Am J Occup Ther. 1993;47(4):319–29. doi: 10.5014/ajot.47.4.319. [DOI] [PubMed] [Google Scholar]
- 6.Fisher AG. Assessment of motor and process skills. Vol 1: Development standardization and administration manual. 5th. Fort Collins (CO): Three Star Press; 2003. [Google Scholar]
- 7.Atkins DJ. Functional skills training with body-powered and externally powered prostheses. In: Meier RH, Atkins DJ, editors. Functional restoration of adults and children with upper extremity amputation. New York (NY): Demos Medical Publishing, Inc; 2004. pp. 139–58. [Google Scholar]
- 8.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28(3):245–53. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 9.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118(7):1573–78. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 10.Lipschutz RD, Kuiken TA, Miller LA, Dumanian GA, Stubblefield KA. Shoulder disarticulation externally powered prosthetic fitting following targeted muscle reinnervation for improved myoelectric control. J Prosthet Orthot. 2006;18(2):28–34. doi: 10.1097/00008526-200604000-000. [DOI] [PubMed] [Google Scholar]
- 11.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: A case study. Lancet. 2007;369(9559):371–80. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 12.Lake C. Effects of prosthetic training on upper-extremity prosthesis use. J Prosthet Orthot. 1997;9(1):3–9. [Google Scholar]
- 13.Miller LA, Lipschutz RD, Stubblefield KA, Lock BA, Huang H, Williams TW, 3rd, Weir RF, Kuiken TA. Control of a six degree of freedom prosthetic arm after targeted reinnervation surgery. Arch Phys Med Rehabil. 2008;89(11):2057–65. doi: 10.1016/j.apmr.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


