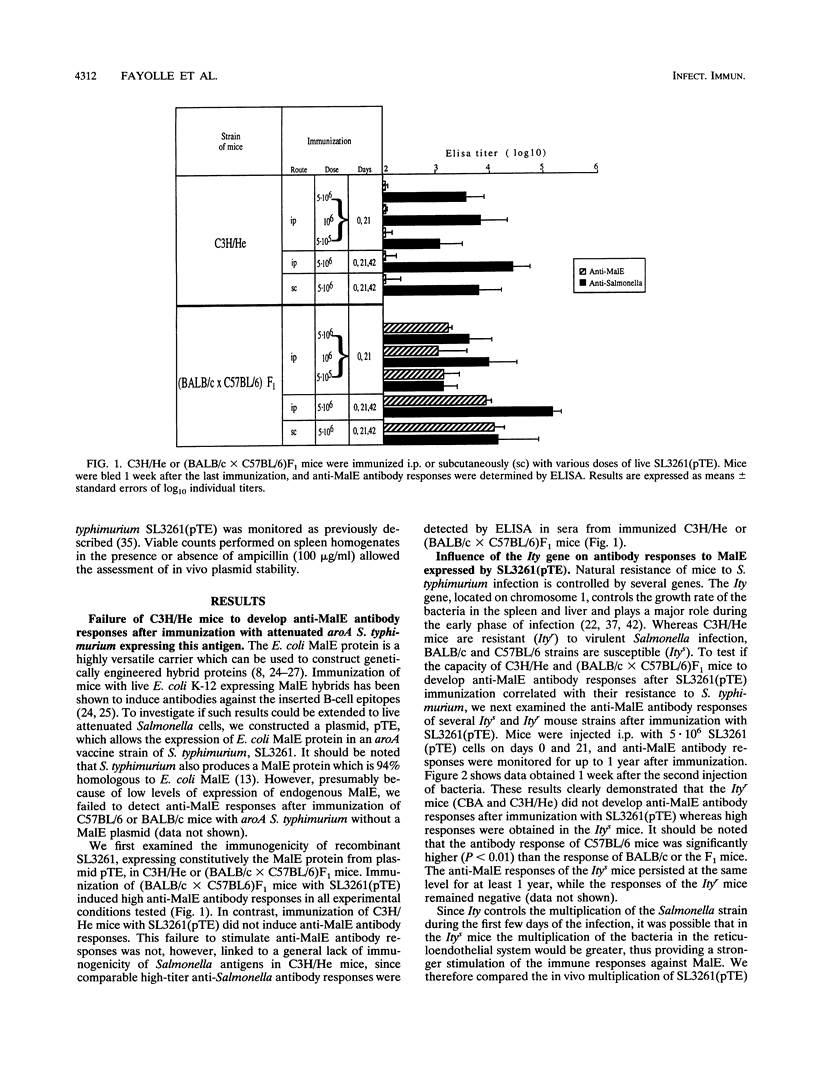
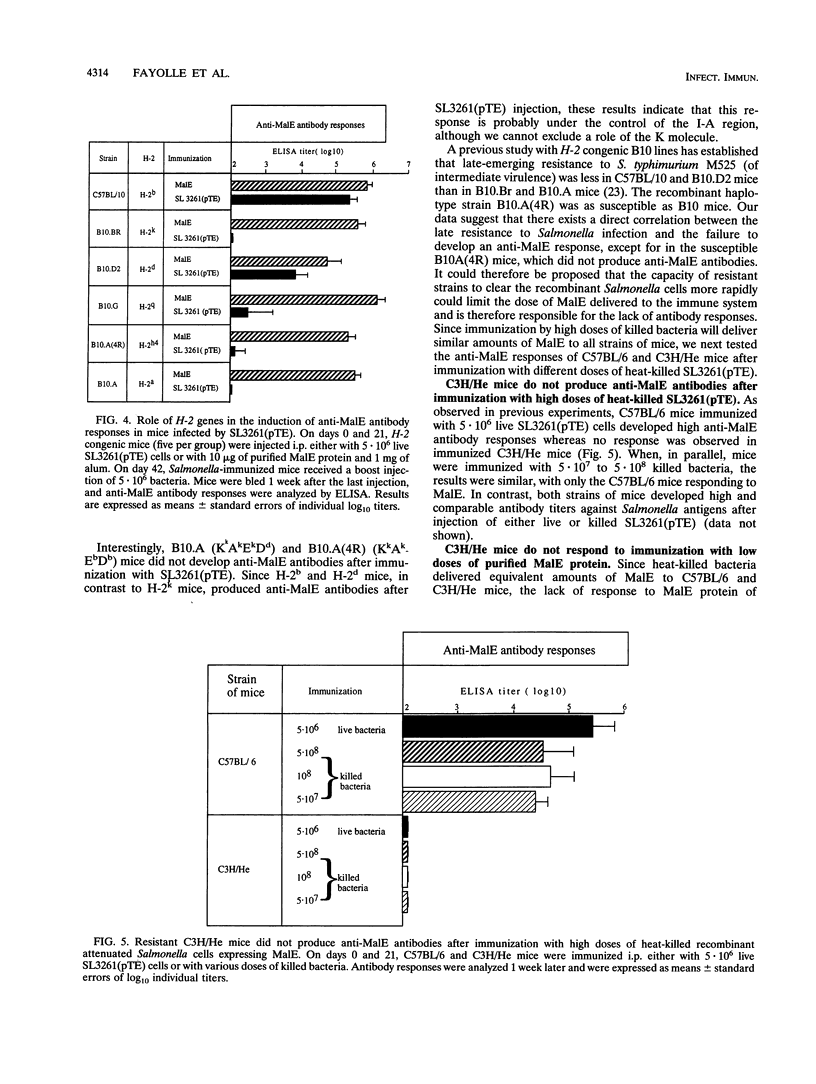
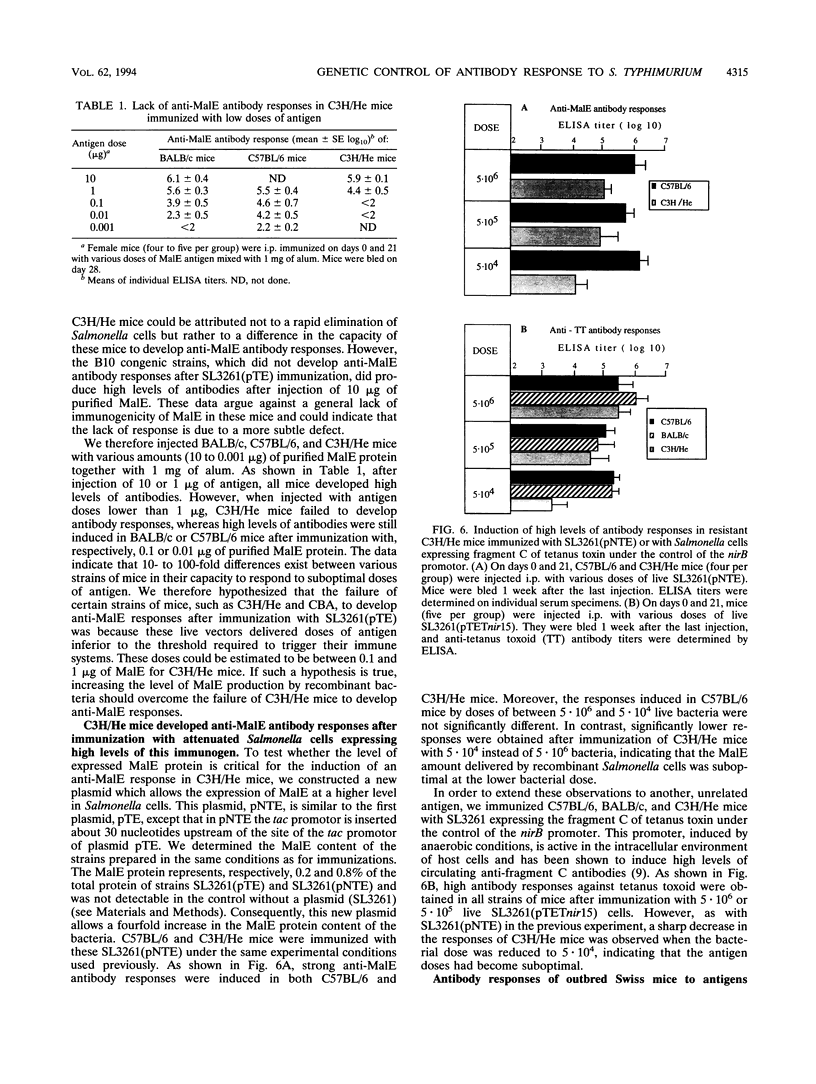
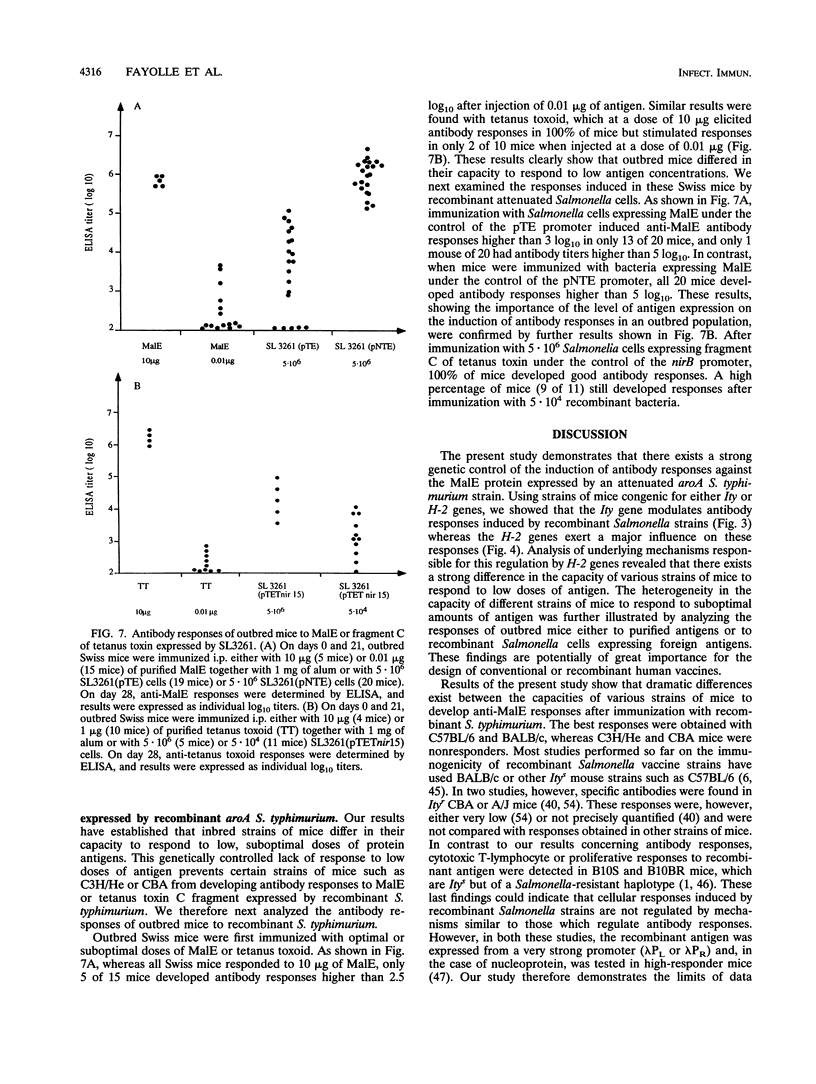
Abstract
Recombinant derivatives of nonpathogenic bacteria such as attenuated Salmonella typhi have the potential to be used for delivery of heterologous antigens to the immune system. Genetic factors may modulate the immune responses to these live attenuated organisms and could therefore modify the immunogenicity of future human vaccines. In the present study, we compared the antibody responses of Ity or H-2 congenic strains of mice to a foreign antigen expressed by the murine attenuated aroA S. typhimurium strain. Our results demonstrate that the Ity gene may modulate the antibody responses to the foreign antigen but that the major genetic influence is exerted by H-2 genes, which control the capacity of mice to respond to the antigen expressed by recombinant attenuated Salmonella cells. This genetic control is related to differences in responsiveness of different strains of mice to low doses of antigen. Increasing the amount of foreign antigen expressed by recombinant Salmonella cells overcame the genetic restriction of these responses. These findings are potentially of great importance for the design of live vaccines for humans and show that care must be taken to optimize the amount of foreign antigen delivered to the immune system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Kruskall M. S., Marcus-Bagley D., Craven D. E., Katz A. J., Brink S. J., Dienstag J. L., Awdeh Z., Yunis E. J. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med. 1989 Sep 14;321(11):708–712. doi: 10.1056/NEJM198909143211103. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Turpin A., Raynaud M. Production et purification de la toxine tétanique. Ann Inst Pasteur (Paris) 1969 May;116(5):686–712. [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Charbit A., Martineau P., Ronco J., Leclerc C., Lo-Man R., Michel V., O'Callaghan D., Hofnung M. Expression and immunogenicity of the V3 loop from the envelope of human immunodeficiency virus type 1 in an attenuated aroA strain of Salmonella typhimurium upon genetic coupling to two Escherichia coli carrier proteins. Vaccine. 1993 Sep;11(12):1221–1228. doi: 10.1016/0264-410x(93)90046-z. [DOI] [PubMed] [Google Scholar]
- Chatfield S. N., Charles I. G., Makoff A. J., Oxer M. D., Dougan G., Pickard D., Slater D., Fairweather N. F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Biotechnology (N Y) 1992 Aug;10(8):888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- Chatfield S. N., Strugnell R. A., Dougan G. Live Salmonella as vaccines and carriers of foreign antigenic determinants. Vaccine. 1989 Dec;7(6):495–498. doi: 10.1016/0264-410x(89)90271-5. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Vanprapar N., Thisyakorn U., Olanratmanee T., Losonsky G., Levine M. M., Chearskul S. Safety and immunogenicity of Salmonella typhi Ty21a vaccine in young Thai children. Infect Immun. 1993 Mar;61(3):1149–1151. doi: 10.1128/iai.61.3.1149-1151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L., Dasgupta U., Clements J. D. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine. 1994 Jul;12(9):833–840. doi: 10.1016/0264-410x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Dahl M. K., Francoz E., Saurin W., Boos W., Manson M. D., Hofnung M. Comparison of sequences from the malB regions of Salmonella typhimurium and Enterobacter aerogenes with Escherichia coli K12: a potential new regulatory site in the interoperonic region. Mol Gen Genet. 1989 Aug;218(2):199–207. doi: 10.1007/BF00331269. [DOI] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Duplay P., Szmelcman S., Bedouelle H., Hofnung M. Silent and functional changes in the periplasmic maltose-binding protein of Escherichia coli K12. I. Transport of maltose. J Mol Biol. 1987 Apr 20;194(4):663–673. doi: 10.1016/0022-2836(87)90243-9. [DOI] [PubMed] [Google Scholar]
- Egea E., Iglesias A., Salazar M., Morimoto C., Kruskall M. S., Awdeh Z., Schlossman S. F., Alper C. A., Yunis E. J. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991 Mar 1;173(3):531–538. doi: 10.1084/jem.173.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Chatfield S. N., Makoff A. J., Strugnell R. A., Bester J., Maskell D. J., Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect Immun. 1990 May;58(5):1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Weiss W. R., Norris K. A., Seifert H. S., Kumar S., So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990 Dec;4(12):2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Green I., Inman J. K., Benacerraf B. Genetic control of the immune response of guinea pigs to limiting doses of bovine serum albumin: relationship to the poly-L-lysine gene. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1267–1274. doi: 10.1073/pnas.66.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J. Use of Salmonella for heterologous gene expression and vaccine delivery systems. Curr Opin Biotechnol. 1993 Oct;4(5):611–615. doi: 10.1016/0958-1669(93)90085-b. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E., Harrington K. A., Joysey H. S. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J Infect Dis. 1985 Nov;152(5):1050–1056. doi: 10.1093/infdis/152.5.1050. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- LeClerc C., Martineau P., Van der Werf S., Deriaud E., Duplay P., Hofnung M. Induction of virus-neutralizing antibodies by bacteria expressing the C3 poliovirus epitope in the periplasm. The route of immunization influences the isotypic distribution and the biologic activity of the antipoliovirus antibodies. J Immunol. 1990 Apr 15;144(8):3174–3182. [PubMed] [Google Scholar]
- Leclerc C., Charbit A., Martineau P., Deriaud E., Hofnung M. The cellular location of a foreign B cell epitope expressed by recombinant bacteria determines its T cell-independent or T cell-dependent characteristics. J Immunol. 1991 Nov 15;147(10):3545–3552. [PubMed] [Google Scholar]
- Lo-Man R., Martineau P., Hofnung M., Leclerc C. Induction of T cell responses by chimeric bacterial proteins expressing several copies of a viral T cell epitope. Eur J Immunol. 1993 Nov;23(11):2998–3002. doi: 10.1002/eji.1830231141. [DOI] [PubMed] [Google Scholar]
- Martineau P., Guillet J. G., Leclerc C., Hofnung M. Expression of heterologous peptides at two permissive sites of the MalE protein: antigenicity and immunogenicity of foreign B-cell and T-cell epitopes. Gene. 1992 Apr 1;113(1):35–46. doi: 10.1016/0378-1119(92)90667-e. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). I. H-2 restriction of the murine humoral immune response to the a and d determinants of HBsAg. J Immunol. 1982 Jul;129(1):320–325. [PubMed] [Google Scholar]
- Moser I., Hohmann A., Schmidt G., Rowley D. Salmonellosis in mice: studies on oral immunization with live avirulent vaccines. Med Microbiol Immunol. 1980;168(2):119–128. doi: 10.1007/BF02121760. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L., Pla M. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect Immun. 1988 Sep;56(9):2407–2411. doi: 10.1128/iai.56.9.2407-2411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Taylor B. A., Rosenstreich D. L. Genetic control of natural resistance to Salmonella typhimurium in mice during the late phase of infection. J Immunol. 1984 Dec;133(6):3313–3318. [PubMed] [Google Scholar]
- O'Callaghan D., Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990 Aug;223(1):156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Martineau P., Fayolle C., Leclerc C., Guillet J. G., Charbit A., Hofnung M. Immune responses to hybrid maltose-binding proteins. Vaccine. 1993;11(2):140–142. doi: 10.1016/0264-410x(93)90009-m. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald I. P., Lantier F., Moutier R., Bertrand M. F., Skamene E. Intraperitoneal infection with Salmonella abortusovis is partially controlled by a gene closely linked with the Ity gene. Clin Exp Immunol. 1992 Mar;87(3):373–378. doi: 10.1111/j.1365-2249.1992.tb03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A., Sandström G., Tärnvik A. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect Immun. 1992 Jul;60(7):2855–2862. doi: 10.1128/iai.60.7.2855-2862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead W. W. Genetics and resistance to tuberculosis. Could resistance be enhanced by genetic engineering? Ann Intern Med. 1992 Jun 1;116(11):937–941. doi: 10.7326/0003-4819-116-11-937. [DOI] [PubMed] [Google Scholar]
- Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: Ity gene is expressed in vivo by 24 hours after infection. J Immunol. 1983 Dec;131(6):3014–3020. [PubMed] [Google Scholar]
- Szmelcman S., Clément J. M., Jehanno M., Schwartz O., Montagnier L., Hofnung M. Export and one-step purification from Escherichia coli of a MalE-CD4 hybrid protein that neutralizes HIV in vitro. J Acquir Immune Defic Syndr. 1990;3(9):859–872. [PubMed] [Google Scholar]
- Tacket C. O., Forrest B., Morona R., Attridge S. R., LaBrooy J., Tall B. D., Reymann M., Rowley D., Levine M. M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990 Jun;58(6):1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkka E., Muotiala A., Karvonen M., Saukkonen-Laitinen K., Sarvas M. Antibody production to a meningococcal outer membrane protein cloned into liv Salmonella typhimurium aroA vaccine strain. Microb Pathog. 1989 May;6(5):327–335. doi: 10.1016/0882-4010(89)90074-0. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Gao X. M., Hughes-Jenkins C. M., Lipscombe M., O'Callaghan D., Dougan G., Liew F. Y. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. III. Delivery of recombinant nucleoprotein to the immune system using attenuated Salmonella typhimurium as a live carrier. Immunology. 1990 Aug;70(4):540–546. [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Russell S. M., Dougan G., O'Callaghan D., Jones I., Brownlee G., Liew F. Y. Antiviral immunity induced by recombinant nucleoprotein of influenza A virus. I. Characteristics and cross-reactivity of T cell responses. J Immunol. 1988 Dec 1;141(11):3980–3987. [PubMed] [Google Scholar]
- Van de Verg L., Herrington D. A., Murphy J. R., Wasserman S. S., Formal S. B., Levine M. M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990 Jun;58(6):2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz N. M., Levine B. B. Immune responses of inbred mice to repeated low doses of antigen: relationship to histocompatibility (H-2) type. Science. 1970 May 15;168(3933):852–854. doi: 10.1126/science.168.3933.852. [DOI] [PubMed] [Google Scholar]
- Vaz N. M., Phillips-Quagliata J. M., Levine B. B., Vaz E. M. H-2-linked genetic control of immune responsiveness to ovalbumin and ovomucoid. J Exp Med. 1971 Nov 1;134(5):1335–1348. doi: 10.1084/jem.134.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz N. M., Vaz E. M., Levine B. B. Relationship between histocompatibility (H-2) genotype and immune responsiveness to low doses of ovalbumin in the mouse. J Immunol. 1970 Jun;104(6):1572–1574. [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]


