Abstract
Classically, sleep has been considered to serve an essential restorative function for the brain. However, there are an increasing number of studies linking decreased sleep quantity and/or quality in humans to an increased obesity and diabetes risk. Reductions in sleep quantity or quality lead to an increase in hunger and appetite, which chronically could predispose an individual to obesity. Carefully controlled studies have shown that two nights of insufficient sleep is causally linked to a decrease in disposition index, the most commonly used predictor of an individual’s diabetes risk, and impairments in glucose tolerance and insulin sensitivity. Thus, sleep appears to play a critical role in modulating energy metabolism in peripheral tissues. Here we will discuss recent work implicating adipose tissue as a potential direct target of disruption of sleep quality, and explore the potential mechanistic links between sleep, adipose tissue and the global control of energy metabolism in humans.
Keywords: Adipocyte, insulin resistance, sleep quality and duration, stress
Physiological role of the adipocyte in the body
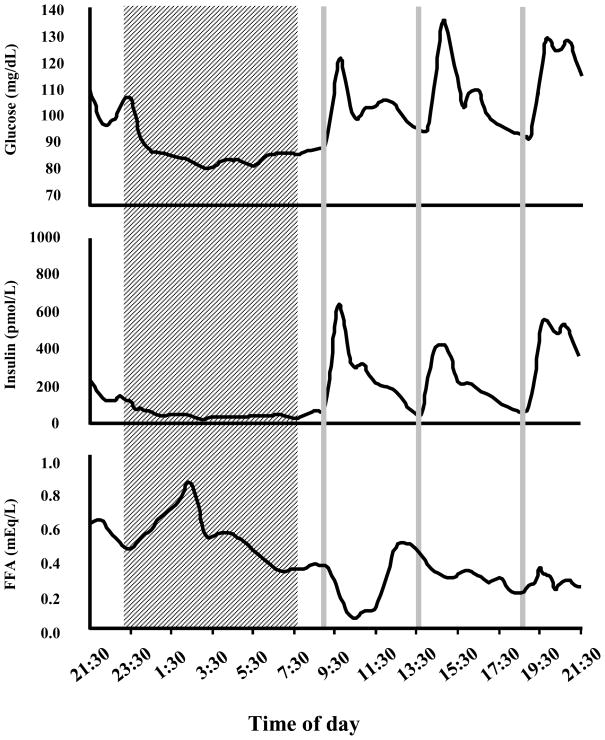
Adipose tissue is an important insulin sensitive tissue comprised of many cell types, the bulk of which are adipocytes. Commonly referred to as fat cells, adipocytes play a critical role for survival, serving as the central lipid storage depot that accounts for the vast majority of energy storage in mammals. In response to a meal and subsequent insulin signaling, adipocytes take up glucose and free fatty acids, which are stored primarily as triglycerides. The stored lipids are released for use as energy between meals, during the normal nocturnal fast during sleep, or in more precarious situations such as starvation and famine. During times of fasting, counter-regulatory hormones such as growth hormone, catecholamines and glucagon signal to adipocytes to initiate hydrolysis of stored triglycerides and the release of free fatty acids and glycerol to be metabolized primarily by skeletal muscle and liver, respectively. Figure 1 illustrates the 24-h profiles of plasma glucose, insulin and free fatty acids levels in a single subject who received three identical carbohydrate-rich meals at 9 am, 2 pm and 7 pm. Meal intake inhibits the release of free fatty acids while the overnight fast is associated with a surge of free fatty acid, relative to average daytime levels. In a state of adequate energy intake, however, the primary role of insulin signaling in the adipocyte is to indicate sufficient energy influx and inhibit the unnecessary lipolysis of triglycerides and release into the circulation.
Figure 1. 24 hour profiling of human sera glucose, insulin and free fatty acids (FFA).
For 3 consecutive nights, subject spent 8.5 hours in bed (shaded area). Starting at 9:30 pm, blood samples were frequently drawn and circulating glucose, insulin and FFA levels were determined. Gray lines indicate consumption of meals.
The two major depots of adipose tissue are subcutaneous (under the skin) and visceral (co-localized with internal organs). Increased visceral fat has been implicated as playing a role in the development of insulin resistance and diabetes and is often associated with the “apple” body shape phenotype [1]. Subcutaneous fat accumulation is more closely linked to the “pear” body type, and is considered less metabolically deleterious, and therefore a “safer” depot in which to deposit excess energy. It is generally accepted that the adipokine leptin, which regulates feeding behavior and energy expenditure, is released primarily from the subcutaneous depot in direct proportion to insulin-stimulated glucose uptake and total subcutaneous fat mass [2], [3]. Genetic deficiencies in leptin secretion and/or signaling in the hypothalamus result in profound obesity in mice and humans [4], [5].
Insulin exerts its metabolic and mitogenic effects in target cells (i.e. muscle, liver and fat cells) by binding to its cell surface receptor to activate multiple signaling cascades and elicit a variety of metabolic effects in a tissue specific fashion. The three most important insulin-sensitive peripheral tissues are skeletal muscle, liver and adipose tissue. Insulin promotes glucose uptake into skeletal muscle, accounting for 50–90% of glucose clearance from peripheral circulation following a meal, while simultaneously insulin suppresses energy production and mobilization from the liver and adipose tissue. Insulin resistance in mammals is defined as a decrease in the efficacy of insulin to promote cellular responses and is the strongest risk factor for the development of diabetes. Although a variety of genetic, environmental and pharmacologic factors can induce insulin resistance in humans, excess energy accumulation in adipose tissue during the development of obesity is the most common cause of insulin resistance. Systemic insulin resistance results in decreased glucose uptake by muscle, inappropriate glucose production by liver and an increase in free fatty acid release into the bloodstream by adipose tissue. The elevation of circulating lipids due to the development of insulin resistance in adipose tissues results in ectopic fat accumulation in other cells types, which in turn further impedes insulin action and production. Thus, the localized impairment of insulin signaling in adipocytes can exert profound effects on global energy metabolism through the disruption of insulin signaling in other peripheral tissues.
Regulation of leptin secretion by sleep
The identification of leptin as a regulated secreted peptide from adipocytes was a key development in the identification of adipose tissue as an endocrine organ [5]. Despite intense research, the precise metabolic and molecular mechanisms by which leptin secretion is regulated remain incompletely understood, though insulin stimulated glucose uptake and metabolism in adipose tissue appears to play a critical role. Leptin expression and secretion are associated with insulin-stimulated glucose uptake into adipocytes [6–9]. Leptin promoter activity is also increased after treatment with insulin, but this can be blocked by the administration of a non-metabolizable glucose analogue [10]. Inhibitors of glycolysis also block leptin secretion, even in the presence of insulin [9]. Cumulatively, these results indicate that insulin stimulation, as well as glucose uptake and metabolism, are necessary for the production and secretion of leptin from the adipocyte. Although leptin levels increase in proportion to fat mass, the ability of leptin to reduce food intake can be impaired in an obese state, possibly due to a saturable transport system [11], indicating the development of leptin resistance in addition to insulin resistance in obesity.
Another layer of complexity surrounding adipokine secretion is the large circadian variation of their peripheral levels. For instance, serum leptin levels peak between midnight and early morning, and fall to a nadir around mid-day [12–15]. In a study of 6 young men studied under so-called “constant routine conditions” (i.e. continuous wakefulness under recumbent conditions and constant light exposure) with identical meals every 2 hours, a clear circadian rhythm of leptin was observed despite the absence of dark-light and sleep-wake cycle and the uniform distribution of caloric intake across the study span [16]. Nonetheless, meal timing clearly plays a role in the diurnal variation of leptin, since a well-documented study showed a marked delay in the leptin profile following a 6-hour delay of meal timing without change in the timing of the sleep period [17].
In addition to its regulation by insulin and glucose signaling, leptin has been shown to be robustly affected by changes in sleep duration. Population based studies have shown that self-reported sleep duration was positively associated with leptin levels, independently of body mass index (BMI) [18] and that leptin levels in people sleeping 5–6 hours were approximately 15–17% lower than expected based on fat mass [19]. In a laboratory setting, it has been demonstrated that 88 hours of total sleep deprivation results in a decrease in peak amplitude as well as overall mean leptin levels and this is reversed upon recovery sleep [20]. Additionally, in a study of chronic partial sleep restriction to 4 hours of sleep for 6 nights followed by 12 hours of sleep for 6 nights, mean leptin levels were 19% lower during sleep restriction [21]. These changes in leptin levels occurred despite identical caloric intake and physical activity and no change in weight [21]. The maximal leptin level during sleep restriction was on average 1.7 ng/ml lower, which is somewhat larger than the decrease in leptin reported in young adults after three days of dietary intake restricted to 70% of energy requirements [22]. Another group showed that seven days with 4 hours of sleep per night is associated with approximately 33% lower leptin levels as compared to 8.5 hours of sleep (p<.001) [23]. Just two nights of sleep restriction are enough to elicit an 18% reduction in leptin levels as compared to longer sleep times [24]. In this study, subjective hunger and appetite were also increased during short sleep, specifically, there tended to be an increase in appetite ratings for food groups that included sweets, salty foods, starchy foods in the sleep restriction condition, compared to sleep extension indicating an increase in desire for calorie-dense foods in a state of accumulating sleep debt [25].
Thus, it is becoming increasing clear that alterations in sleep duration can directly impact leptin release from adipocytes and thus alter the neuroendocrine regulation of hunger. Thus sleep disturbances may contribute to the current obesity epidemic in industrialized countries. The current evidence suggests that even a short period of sleep restriction is associated with alterations of leptin and resulting increases in appetite ratings. If increases in hunger of the magnitude reported in several studies of sleep restriction were to translate into a commensurate increase in food intake, this would correspond to a caloric excess of 350 to 500 Kcal/day for a young normal weight sedentary adult, which would result in a high risk of clinically significant weight gain. However, these studies were conducted under controlled conditions of caloric intake, thus it cannot be determined whether the dysregulation of leptin and the observed increased subjective hunger would lead to an actual increase in food intake. The current body of evidence supports the hypothesis that due to the impact of sleep duration on leptin release from the adipocytes, there may be an increase in individual susceptibility to weight gain and obesity in the presence of sleep perturbations. Chapters in the present volume discuss both the laboratory and epidemiologic data indicating that insufficient sleep is associated with insulin resistance and an increased risk of diabetes.
Reduced sleep quantity and quality are associated with an increased risk of diabetes and obesity
The associations described above suggested that reduced sleep duration could lead to chronic alterations in leptin secretion and promote the development of obesity. Whether reduced sleep quality, independently of reduced sleep duration, could also impact adipocyte function and leptin release is unclear. However, there is a growing body of epidemiologic and laboratory evidence to indicate that sleep disturbances may result in insulin resistance and play a role in the risk of diabetes. For example, total sleep deprivation for 24 hours has been shown to result in a decrease in insulin sensitivity in healthy subjects [26]. The effects of chronic partial sleep restriction have also been examined. Six nights of 4 hours of time in bed, as compared to 6 nights of 12 hours of time in bed, resulted in decreased glucose tolerance, decreased acute insulin response to glucose and lower insulin sensitivity [27, 28]. The combination of a decrease in acute insulin response to glucose with a reduction in insulin sensitivity results in a decrease in the disposition index, a validated marker of diabetes risk. As detailed in the chapter by Spiegel et al in this issue, these findings have been confirmed and extended in a number of experimental studies.
Furthermore, decreased sleep quality, without a decrease in sleep duration, also plays an important role in maintaining metabolic health. In epidemiologic studies (reviewed by Knutson et al in this issue), self-reported poor sleep quality is associated with higher waist circumference, BMI and body fat percentage [29]. Slow wave sleep (SWS), a marker of sleep depth or intensity, is often considered to be the most physically restorative sleep stage, and the percentage of sleep spent in SWS decreases with age [30]. A 2008 study examined the impact of SWS suppression, without change in sleep duration, on glucose homeostasis in normal young subjects. In this study (reviewed in greater detail in the chapter by Aronsohn et al in this issue), the subjects received either 2 nights of 8.5 hours in bed, or 3 nights of SWS suppression achieved by acoustic stimuli, which caused individual subjects to revert to a more shallow sleep stage without experiencing a full awakening. Following suppression of SWS, as compared to undisrupted sleep, subjects had a 25% lower insulin sensitivity, a 23% decrease in glucose tolerance and a 20% worsening in disposition index [31]. This decrease in disposition index is clinically predictive of an increase in diabetes risk. These deleterious effects on diabetic risk suggest that sleep quality is as important as sleep duration with respect to maintaining glucose homeostasis.
In the most recent study evaluating the effects of reduced sleep quality on glucose metabolism, eleven healthy normal volunteers were subjected to 2 nights of sleep fragmentation by auditory and mechanical stimuli after which they underwent an intravenous glucose tolerance test. Following two nights of sleep fragmentation, insulin sensitivity was decreased by ~25% (p<0.0001). Thus both sleep duration and quality emerge as important regulators of systemic insulin sensitivity.
Linking sleep disruptions to reduced insulin sensitivity - pointing towards the adipocyte
In light of the effects of reduced sleep duration and or quality on BMI, global insulin sensitivity, glucose tolerance and leptin secretion, it is important to consider the possible changes occurring at the cellular level that could mediate these observed systemic alterations. The body of epidemiologic, experimental and clinical evidence supporting the hypothesis that short and poor sleep is causally linked to insulin resistance and correlated with diabetes risk is rapidly growing. As such, it is important to elucidate the mechanisms that link sleep and diabetes/obesity, which could ultimately lead to better prevention and therapy of these highly prevalent chronic disease states.
In several experimental studies mentioned earlier in this article, young, lean, healthy volunteers undergoing restrictions of sleep quantity and/or quality experienced a transient reduction of insulin resistance, as well as a decrease in both the mean leptin concentration and the amplitude of its circadian variation. Since adipocytes are the only cells that produce leptin, these findings position the fat cell as a likely cellular target for alterations in sleep. While leptin secretion is proportional to insulin-stimulated glucose uptake into fat cells, a reduction in leptin levels without change in caloric intake suggests the induction of insulin resistance in adipose tissue. Skeletal muscle is generally thought to be responsible for changes in systemic insulin resistance as occur following weight gain, changes on exercise levels or pharmacologic treatment but it is not known whether a reduction in systemic insulin sensitivity due to sleep disturbances also reflects the same pathway. In fact, due to the marked impact of sleep on leptin release, it is a reasonable assumption that the adipocyte may undergo a reduction of insulin signaling as well. Insulin resistance in adipose tissue resulting from sleep restriction would be predicted to decrease leptin secretion and increase lipolysis, both of which could have negative implication for global energy intake and insulin metabolic action. However, chronic sleep disruption in obese subjects with obstructive sleep apnea is associated with increased circulating leptin levels compared to age and weight matched controls [32–34], indicating that the associations between sleep quality and adipocyte function may be more complex. Regardless, the fat cell likely plays a pivotal role in the development of the sleep-elicited changes in systemic energy metabolism.
The precise molecular mechanisms by which sleep curtailment impairs insulin action in adipocytes at this stage remain hypothetical. However, several potential mechanisms that may be involved will next be considered. Proposed pathways include are increased exposure to elevated sympathetic nervous activity either via direct innervation or indirectly via elevated catecholamine levels, stimulation of the stress-responsive hypothalamo-pituitary axis with a resulting increased exposure to glucocorticoids and/or increased inflammation, all of which have been previously identified as aggravating factors in the development of adipocyte dysfunction and obesity.
Physiological mechanisms connecting sleep to the adipocyte
Sympathetic nervous system
The autonomic nervous system controls aspects of involuntary physiology, such as heart rate and respiration, as well as signals to other peripheral organs. The autonomic nervous system can be divided into the parasympathetic and sympathetic nervous systems, the latter of which is activated during times of stress. This system is activated during the “fight or flight” response. The sympathetic nervous system is also regulated by sleep-wake cycles and its activity gradually decreases during the deep sleep stages of non-REM sleep whereas during REM sleep and wakefulness, sympathetic nervous activity is increased [35]. Sleep onset itself is associated with a significant decline of circulating concentrations of catecholamines epinephrine and norepinephrine, which serve as direct readouts of sympathetic activity. Nocturnal and morning awakenings, on the other hand, are associated with increases in both hormones [36].
Chronic activation of the sympathetic nervous system has been linked to obesity and insulin resistance. However, mechanisms linking these chronic disease states with sympathetic over-activity are complex and not fully understood. Studies have shown links between increased sympathetic output and obesity in obstructive sleep apnea, hypertension and leptin resistance. Additionally, sympathetic activation leads to increased free fatty acids due to the stimulation of lipolysis by direct sympathetic innervation of the adipose tissue [37]. Regarding its effects on leptin and feeding regulation, catecholamines increase intracellular cAMP thereby inhibiting leptin mRNA expression and secretion [38]. Inhibition of leptin signaling will subsequently lead to an increase in feeding behavior, which will itself stimulate leptin production and feedback in an inhibitory manner to reduce catecholamine secretion. If sympathetic activation remains abnormally elevated, the hunger drive would remain high as well.
To test whether the administration of exogenous catecholamines is associated with changes in glucose regulation and metabolism in humans, a recent study infused eight healthy normal subjects with physiological levels of norepinephrine prior to an intravenous glucose tolerance test (IVGTT) [39]. Norepinephrine infusion resulted in a significant increase in blood glucose (p<0.05), as well as a 40% increase in circulating free fatty acids (p<0.01). Following the IVGTT, glucose disposal rates were also impaired after norepinephrine infusion, resulting in a 35% decrease in insulin sensitivity and overall 70% decrease in disposition index as compared to saline infusion (p<0.05). Reductions in disposition index of this magnitude indicate a drastic increase in the risk of T2DM in norepinephrine-infused subjects [39].
Sympathetic output has also been shown to be negatively affected by sleep disturbances. In a study comparing 5.5 with 8.5 hours of time in bed in 11 healthy middle-aged volunteers, a significant increase was observed in 24-hour epinephrine (p=0.04), as well as a nighttime increase in norepinephrine (p=0.04) during the short-sleep condition [40]. Furthermore, heart rate variability (HRV), which is considered to be an additional readout of cardiac sympathetic nervous system activity where a decrease in HRV indicates an increase in sympathetic drive and/or a decrease in parasympathetic activity, is also decreased during short sleep [21]. In a study of sleep restriction comparing 6 nights of 4 hours of time in bed to 6 nights of 12 hours of time in bed, 24-hour HRV tended towards decreased levels in the 4-hour condition as compared to the 12-hour condition [21]. However, daytime HRV (0900-1400) was significantly reduced in (p<0.02) [27], indicating an increase in sympathetic drive under the sleep restricted condition. If this increased sympathetic drive at the level of the heart is also present at the level of other organs, including insulin-sensitive organs, it would represent a potential mechanism for decreased insulin sensitivity.
Reductions in sleep quality without changes in duration also have demonstrable effects on sympathetic output. In a study involving 2 nights of non-specific fragmentation of sleep across all stages a shift in sympathovagal balance toward an increase in sympathetic nervous system activity was observed. Sympathetic modulation of heart rate variability was also increased by 17% (p<0.001) as determined by an increase in low frequency spectral power of the electrocardiogram. Additionally, there was a corresponding elevation in heart rate [41]. Thus, increased sympathetic activation resulted in reduced insulin sensitivity and impaired glucose tolerance. In this manner, dysfunction of the sympathetic nervous system associated with sleep deprivation or reduced sleep quality is a likely mediator of several of the effects of sleep loss and sleep disorders, including the reduction in leptin production and signaling and the reduction in global insulin sensitivity, all of which predispose an individual to obesity.
Cortisol & Sleep
The hypothalamic-pituitary-adrenal axis (HPA) is activated downstream of the sympathetic nervous system and regulates the body’s response to various physiological and psychological stressors. Activation of the HPA axis leads to the secretion of cortisol and other glucocorticoids from the adrenal cortex, as well as elevations in blood glucose levels and increases in gluconeogenesis by the liver.
Cortisol levels fluctuate with a 24 hour circadian rhythm. The nadir usually occurs in the late evening shortly after midnight, followed by a steady rise as the night progresses, reaching its peak in the morning hours to fall again during the course of the day. Sleep onset, whenever it occurs, is associated with an initial inhibition of cortisol secretion while morning awakening further increases the already high morning cortisol levels.
Glucocorticoids facilitate visceral fat accumulation (reviewed in [42]), and also induce insulin resistance and increased lipolysis in adipocytes (reviewed in [43]). Cushing’s Syndrome is characterized by chronically elevated cortisol levels, which result in abdominal obesity, as well as very large increase in the risk of insulin resistance and T2DM. Chronic stress and increased glucocorticoids levels are thus associated with weight gain, decreased insulin sensitivity and leptin resistance both markers of the metabolic syndrome.
In a study examining the effects of cortisol on insulin sensitivity in vivo, eight normal weight and eight obese women received an infusion of saline and hydrocortisone in a cross-over design. Hydrocortisone was able to significantly induce insulin resistance in obese but not in control women, suggesting that moderately elevated levels of cortisol, within the range achieved by a mild stressor, has more deleterious consequences on insulin sensitivity in obese women than in controls [44]. This has important implications for human health, as moderate sleep loss is considered a stressor and could accelerate the development or aggravate the severity of insulin resistance in the already vulnerable population of overweight or obese individuals.
Increased cortisol levels have been implicated in the link between short sleep and increased obesity risk. Because chronic stress has been related to the development of the metabolic syndrome, the hypothesis that sleep loss is a form of chronic stress, the effects of which may accumulate over time, has been proposed. Additionally, in a study of nearly 3,000 participants, short sleep duration and sleep disturbances were associated with increased evening cortisol secretion [45]. To further explore the connection between short sleep and increased cortisol, a controlled laboratory study compared cortisol levels in of young healthy volunteers during baseline sleep (n=9), partial (n=7) and total sleep deprivation (n=17) over the course of 32 hours. Both partial sleep restriction and total sleep deprivation resulted in significant increases in cortisol during the evening and early part of the second night in the study, as compared to the first [46]. A similar finding was obtained in a study of 12 healthy volunteers restricted to 4 hours of time in bed per night for 6 nights, followed by 12 hours of time in bed per night for 6 nights. Both a shorter quiescent cortisol period (p=0.03), as well as an increase in afternoon and early evening cortisol was observed in the short sleep condition, as compared to the long sleep condition (p=0.0001) [27]. Increased cortisol has also been observed in a recent study of 2 nights of sleep fragmentation with no change in sleep duration. In this study, morning cortisol levels were increased by 13% following fragmentation (p<0.015) [41]. These increases in cortisol imply that sleep loss and sleep disturbances may, in fact, constitute a chronic stressor with effects persisting during the waking period. Thus, increased glucocorticoids signaling, specifically in the adipocyte, may constitute a mechanistic link between the chronic impairment of sleep duration and/or quality and induction of insulin resistance in adipose tissue.
The Chicken vs. The Egg: Impact of dysregulation of adipocyte function on sleep and circadian rhythms
Increased stress responses via the coordinated activation of the sympathetic nervous system and the Hypothalamic-Pituitary-Adrenal axis are both possible contributors to the observed increase in risk of T2DM and obesity in the face of acute and chronic reductions in sleep duration and/or sleep quality. The mechanisms by which these processes may affect obesity and T2DM risk may be linked to a dysregulation at the cellular level of the adipocyte. We can also ask if the reverse is true: whether alterations in adipocyte function could lead to alterations and/or disruptions in sleep. If sleep and the functional integrity of the adipocyte are related, even indirectly, perhaps changes in the cellular function of the adipocyte could manifest as changes in sleep physiology.
To determine whether sleep is affected by changes in leptin secretion, sleep parameters were recorded and quantified in normal rats undergoing exogenous leptin administration over a 9-h period. In normally fed rats, leptin administration was associated with a 30% decrease in the duration of rapid eye movement sleep (p<0.005) and a 13% increase in the duration of slow wave sleep (SWS) (p<0.03), suggesting that not only can sleep duration affect leptin levels, but the converse is also true in that leptin levels can affect sleep parameters [47]. This hypothesis that decreased leptin signaling could lead to impairments in sleep-wake regulation has been further examined in a study of mice lacking the leptin peptide. In this study, male ob/ob mice that lack endogenous leptin were shown to have severely disrupted sleep architecture, exhibiting a significant increase in arousal over wild-type mice (p<0.001), as well as frequent shorter bouts of sleep, indicating an inability to successfully consolidate sleep cycles [48]. When leptin is given back to these mice by exogenous leptin administration, total sleep time is increased and arousals are decreased [49], suggesting that not only can sleep affect leptin levels, but leptin can also play a role in the regulation and maintenance of normal sleep. Another model of impaired leptin signaling is the db/db mouse, in which a mutation exists in the leptin receptor, though the peptide itself is present in circulation. Sleep patterns were recorded in this model and sleep fragmentation was increased as compared to wild-type mice [50]. These results indicate that impaired leptin signaling has deleterious effects on sleep regulation and that deficient leptin signaling, however it may arise, disrupts the regulation of sleep architecture and circadian organization of sleep-wake states.
An additional circulating factor secreted by adipocytes that has been implicated in disruptions of circadian rhythm is free fatty acids. In the canine model of obesity, high fat feeding leads to an increase in nocturnal free fatty acids and insulin resistance [51]. In a mouse model using a similar high fat diet, behavioral and molecular circadian rhythms were disrupted. In this study, mice were fed a regular chow diet or high fat diet and maintained in constant darkness for 2 weeks showed a normal free-running rhythm of activity throughout the study period. Mice being fed a high-fat diet showed an increase in the free-running period, as well as altered locomotion and feeding rhythm, suggesting a dysregulation of circadian rhythm in the face of 45% dietary fat as compared to the 16% fat diet received in the regular chow [52]. Perhaps the most interesting result from this study was the finding that high fat feeding can robustly affect molecular circadian clock components in peripheral organs. Transcripts of the proteins encoding CLOCK, BMAL1 and PER2 were measured in hypothalamus, liver and fat tissues. No difference in diurnal rhythmicity of these genes were observed in the hypothalamus between the two diets, however, the diurnal rhythms of Clock and Per2 expression were severely attenuated in fat tissue following high fat feeding, and to a lesser extent in liver, suggesting that high fat feeding generates both tissue-specific and gene-specific changes in expression levels of circadian clock genes [52, 53].
In light of the findings that diet can affect circadian gene rhythmicity, it is important to understand what effects circadian misalignment can have on physiological outcomes, aside from the effects a misalignment may have on sleep quality and quantity. For example, the clock mutant mouse has a homozygous mutation in the CLOCK transcription factor, a key component of the molecular circadian clock. These mutants have a reduced diurnal rhythm, are hyperphagic and obese, and develop a metabolic syndrome of hyperleptinemia, hyperlipidemia, hyperglycemia, and hypoinsulinemia [54]. Interestingly, it is well established that shift workers are at an increased risk of the development of metabolic syndrome, cardiovascular disease and obesity, suggesting that misalignment of both sleep and feeding may be detrimental to overall health, in conjunction with the negative effects of short sleep duration [55–60]. Thus, there exists a complex interplay between central and peripheral circadian rhythms, sleep cycles and energy metabolism and insulin sensitivity that is only beginning to be appreciated.
Summary
Though sleep has historically been considered only necessary for the brain, recent work has established a link between disruptions in sleep quality and/or duration and a corresponding reduction in systemic insulin sensitivity. A molecular basis for the alterations in insulin signaling in peripheral tissue has not been elucidated but clearly must occur to account for the impact of sleep disturbances on energy metabolism, diabetes and obesity risk. Sleep may also play an essential role in preserving the function of potentially all cell types, in addition to insulin sensitive tissues. From these studies, it is clear that an intense examination of the adipocyte (and other peripheral tissues) in response to sleep alterations should be undertaken. Additionally, research investigating potential signals arising from the periphery that signal in the brain to impact sleep quality and duration is warranted. The adipocyte plays a critical role in a growing number of metabolic and endocrine pathways, and as suggested in this review, may also serve as a direct cellular target of sleep. Clearly more work is required to understand the molecular and metabolic mechanisms by which alterations in sleep can impact adipocyte physiology. These links may also have important clinical implications and perturbation of the sleep-adipocyte axis potentially may be a central contributor to the growing obesity epidemic.
Research Agenda.
The connections between alterations in sleep duration or quality on insulin sensitivity in adipose tissue requires further research.
The interplay between induction of insulin resistance in adipocytes, reduced leptin secretion and increased hunger following sleep curtailment should be investigated.
Identification of the potential molecular and metabolic alterations in adipose tissue that in turn could negatively impact sleep is a priority.
More research is needed to understand the association between chronic disruption of sleep quality and/or duration and the increased risk of developing obesity and diabetes in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Josiane Broussard, Email: josiane.broussard@usc.edu, Department of Physiology and Biophysics, Keck School of Medicine, University of Southern California, 1333 San Pablo Street, MMR 626, Los Angeles, CA 90089, Tel: 323-442-1919; Fax: 323-442-1918
Matthew J. Brady, Email: mbrady@medicine.bsd.uchicago.edu, Department of Medicine, Committee on Molecular Metabolism and Nutrition, University of Chicago, 5841 S. Maryland Ave, MC1027, Chicago, IL 60637, Tel: 773-702-2346; Fax: 773-834-0486
References
- 1.Tran TT, et al. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahima RS, et al. Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology. 2000;21(3):263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296(6):E1230–8. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooqi IS, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414(6859):34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 6.Havel PJ, et al. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274(5 Pt 2):R1482–91. doi: 10.1152/ajpregu.1998.274.5.R1482. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno T, et al. Effects of nutritional status and aging on leptin gene expression in mice: importance of glucose. Horm Metab Res. 1996;28(12):679–84. doi: 10.1055/s-2007-979877. [DOI] [PubMed] [Google Scholar]
- 8.Wellhoener P, et al. Glucose metabolism rather than insulin is a main determinant of leptin secretion in humans. J Clin Endocrinol Metab. 2000;85(3):1267–71. doi: 10.1210/jcem.85.3.6483. [DOI] [PubMed] [Google Scholar]
- 9.Mueller WM, et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139(2):551–8. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Aliaga MJ, Stanhope KL, Havel PJ. Transcriptional regulation of the leptin promoter by insulin-stimulated glucose metabolism in 3t3-l1 adipocytes. Biochem Biophys Res Commun. 2001;283(3):544–8. doi: 10.1006/bbrc.2001.4822. [DOI] [PubMed] [Google Scholar]
- 11.Caro JF, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348(9021):159–61. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 12.Sinha MK, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saladin R, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377(6549):527–9. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 14.Ahren B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol Scand. 2000;169(4):325–31. doi: 10.1046/j.1365-201x.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- 15.Saad MF, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83(2):453–9. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 16.Shea SA, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeller DA, et al. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100(7):1882–7. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taheri S, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput JP, et al. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 20.Mullington JM, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15(9):851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 22.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85(8):2685–91. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 23.Guilleminault C, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4(3):177–84. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, et al. Cross-Cultural Comparison of Sleep Duration: Belgium, Italy and the US. Journal of Sleep Research. 2004;13(Suppl 1):402. [Google Scholar]
- 25.Spiegel K, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Ortiz M, et al. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab. 2000;13(2):80–3. [PubMed] [Google Scholar]
- 27.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 28.Leproult R, Van Cauter E. Role of Sleep and Sleep Loss in Hormonal Release and Metabolism. Endocr Dev. 17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennings JR, et al. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 30.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Jama. 2000;284(7):861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 31.Tasali E, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ip MS, et al. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118(3):580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 33.Ulukavak Ciftci T, et al. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72(4):395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 34.Ozturk L, et al. The association of the severity of obstructive sleep apnea with plasma leptin levels. Archives of Otolaryngology -- Head & Neck Surgery. 2003;129(5):538–40. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 35.Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci U S A. 2008;105(4):1107–8. doi: 10.1073/pnas.0711635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin M, et al. Effects of Sleep and Sleep Deprivation on Catecholamine and Interleukin-2 Levels in Humans: Clinical Implications. Journal of Clinical Endocrinology and Metabolism. 1999;84(6):1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 37.Hucking K, et al. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111(2):257–64. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slieker LJ, et al. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem. 1996;271(10):5301–4. doi: 10.1074/jbc.271.10.5301. [DOI] [PubMed] [Google Scholar]
- 39.Marangou AG, et al. Hormonal effects of norepinephrine on acute glucose disposal in humans: a minimal model analysis. Metabolism. 1988;37(9):885–91. doi: 10.1016/0026-0495(88)90124-2. [DOI] [PubMed] [Google Scholar]
- 40.Nedeltcheva AV, et al. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2009;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberge C, et al. Adrenocortical dysregulation as a major player in insulin resistance and onset of obesity. Am J Physiol Endocrinol Metab. 2007;293(6):E1465–78. doi: 10.1152/ajpendo.00516.2007. [DOI] [PubMed] [Google Scholar]
- 43.Dallman MF, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14(4):303–47. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 44.Darmon P, et al. Insulin resistance induced by hydrocortisone is increased in patients with abdominal obesity. Am J Physiol Endocrinol Metab. 2006;291(5):E995–E1002. doi: 10.1152/ajpendo.00654.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kumari M, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94(12):4801–9. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leproult R, et al. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 47.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8(3):197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 48.Laposky AD, et al. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 49.Shelton J, et al. Effect of subchronic administration of leptin on sleep/wake parameters in ob/ob mice. Sleep. 2006;29:LBA 4. [Google Scholar]
- 50.Laposky AD, et al. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2059–66. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SP, et al. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292(6):E1590–8. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 52.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18(1):4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenkanen L, et al. Shift work, occupation and coronary heart disease over 6 years of follow-up in the Helsinki Heart Study. Scand J Work Environ Health. 1997;23(4):257–65. doi: 10.5271/sjweh.218. [DOI] [PubMed] [Google Scholar]
- 56.Sookoian S, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–92. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 57.Nagaya T, et al. Markers of insulin resistance in day and shift workers aged 30–59 years. Int Arch Occup Environ Health. 2002;75(8):562–8. doi: 10.1007/s00420-002-0370-0. [DOI] [PubMed] [Google Scholar]
- 58.Knutsson A, et al. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58(11):747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17(4):489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]