Abstract
Increases in visceral fat are associated with increased inflammation, dyslipidemia, insulin resistance, glucose intolerance and vascular dysfunction. We examined the effect of the potent heme oxygenase (HO)-1 inducer, cobalt protoporphyrin (CoPP), on regulation of adiposity and glucose levels in both female and male obese mice. Both lean and obese mice were administered CoPP intraperitoneally, (3mg/kg/once a week) for 6 weeks. Serum levels of adiponectin, TNFα, IL-1β and IL-6, and HO-1, PPARγ, pAKT, and pAMPK protein expression in adipocytes and vascular tissue were measured. While female obese mice continued to gain weight at a rate similar to controls, induction of HO-1 slowed the rate of weight gain in male obese mice. HO-1 induction led to lowered blood pressure levels in obese males and females mice similar to that of lean male and female mice. HO-1 induction also produced a significant decrease in the plasma levels of IL-6, TNF-α, IL-1β and fasting glucose of obese females compared to untreated female obese mice. HO-1 induction increased the number and decreased the size of adipocytes of obese animals. HO-1 induction increased adiponectin, pAKT, pAMPK, and PPARγ levels in adipocyte of obese animals. Induction of HO-1, in adipocytes was associated with an increase in adiponectin and a reduction in inflammatory cytokines. These findings offer the possibility of treating not only hypertension, but also other detrimental metabolic consequences of obesity including insulin resistance and dyslipidemia in obese populations by induction of HO-1 in adipocytes.
Keywords: adipocyte pAMPK, female obesity, heme oxygenase inducers, inflammation
Introduction
Moderate to severe obesity is associated with increased risk for cardiovascular complications and insulin resistance in humans1, 2 and animals3, 4. Cardiovascular risk is specifically associated with increased intra-abdominal fat. Women in their reproductive years have a higher BMI than males, which largely reflects increased overall subcutaneous adipose tissue or “gynoid” obesity, this is not associated with increased cardiovascular risk5. However, due to increases in visceral fat with aging, after the age of 60 the fat distribution in females more closely resembles that in males6. Increased androgen levels also often occur after the menopausal transition. These changes in visceral fat content and androgen levels correlate with both central obesity and insulin resistance and are an important determinant of cardiovascular risk7.
Heme oxygenase (HO) catalyzes the breakdown of heme, a potentially harmful pro-oxidant, into its products biliverdin and carbon monoxide, with a concomitant release of iron (reviewed in8). While HO-2 is expressed constitutively, HO-1 is inducible in response to oxidative stress and its induction has been reported to normalize vascular and renal function9–11. Further, induction of HO-1 slows weight gain, decreases levels of TNF-α and IL-6 and increases serum levels of adiponectin in obese rats and obese diabetic mice4, 9, 12. The association observed between HO-1 and adiponectin has led to the proposal of the existence of a cytoprotective HO-1/adiponectin axis4, 13.
Previously, L'Abbate et al,14 have shown that induction of HO-1 is associated with a parallel increase in the serum levels of adiponectin, which has well-documented insulin-sensitizing, antiapoptotic, antioxidative and anti-inflammatory properties. Adiponectin is an abundant protein secreted from adipocytes. Once secreted, it mediates its actions by binding to a set of receptors, such as adipoR1 and adipoR2, and also through induction of AMPK signaling pathways15, 16. In addition, increases in adiponectin play a protective role against TNF mediated endothelial activation17.
In this study, we evaluated the effect of CoPP, a potent inducer of HO-1, on visceral and subcutaneous fat distribution in both female and male obese mice. We show for the first time a resistance to weight reduction upon administration of CoPP in female obese mice but a significant decrease in inflammatory cytokines. Despite continued obesity, CoPP normalized blood pressure levels, decreased circulating cytokines, and increased insulin sensitivity in obese females. CoPP treatment of obese mice increased the number and reduced the size of adipocytes. CoPP treatment of both male and female obese mice reversed the reduction in adiponectin levels seen in obesity. This study suggests that in spite of continued obesity, HO-1 induction in female obese mice serves a protective role against obesity associated metabolic consequences via expansion of healthy smaller insulin-sensitive adipocytes.
Materials and Methods
Animal Care and CoPP Administration
Male and female obese mice (B6v-Lep obese/J) were purchased from Harlan (Chicago, IL) at the age of 7 weeks. Lean mice, (age-matched B6.V, lean, Harlan Chicago,IL) were used as control. Sex matched lean and obese mice were fed a normal laboratory animal diet and had free access to water. At 8 weeks of age after obese mice established diabetes, CoPP(3 mg/Kg/once a week) or stannous mesoporphyrin (SnMP), a potent inhibitor of HO activity, (3mg/Kg/3 times a week), were administered intraperitoneally for 6 weeks to 48 obese mice (24 males and 24 females) and 20 lean mice (10 males and 10 females). Measurements of glucose and insulin tolerance, body weight, and fasting blood glucose (BG) were made during the course of the study. Animal tissues and serum were then collected for additional studies. For evaluation of adipocyte size analysis, digital images of adipose tissue sections were captured using a light microscope (Olympus, Germany) at 20× magnification. For each group, three fields from each of five different haematoxylin-eosin stained sections per animal were analyzed. Individual adipocyte areas (μm2) within each field were determined using image analysis software (Image Pro Plus, Immagini e Computer, Milan, Italy). For the quantitative analysis, adipocyte areas were calculated in arbitrary fields, measuring fifty adipocytes for each section. Other methodological details are provided in the online Data Supplement (available at http://hyper.ahajournals.org). There was no difference in food intake in any of the treatment groups. The Animal Care and Use Committee of New York Medical College approved all experiments.
Results
Effect of induction of HO-1 on body weight, appearance, and fat content of female and male obese mice
Previously, we have shown CoPP treatment results in the prevention of weight gain in several male models of obesity including obese and db/db mice and Zucker fat rats4, 12. We extended our studies to examine the effect of CoPP on weight gain in female obese mice. CoPP-treatment prevented weight gain in male obese mice when compared to age-matched male controls (Figure S1). The prevention of body weight gain was accompanied by a reduction in visceral fat in male obese mice. However, female obese mice administered CoPP did not lose weight but continued to gain weight at the same rate as untreated female obese mice (Figure S1). This was in spite of food intake being comparable between the two groups. CoPP administration decreased subcutaneous fat content in both obese males and females (p<0.05; p<0.05, respectively). CoPP produced a decrease (p<0.05) in visceral fat in male but not in female obese mice when compared to untreated obese mice (Figure S1D).
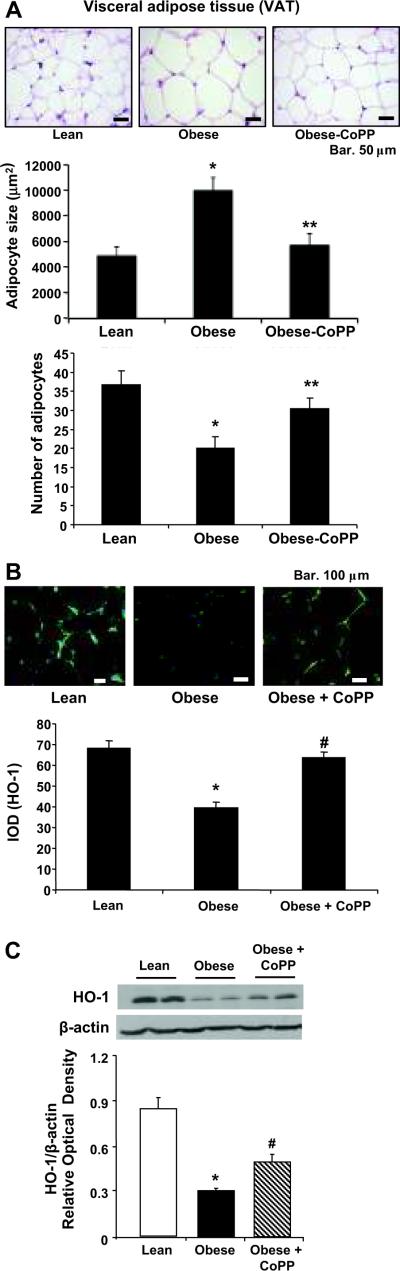
We examined adipocyte size by haematoxilin-eosin staining in both lean, obese and CoPP treated obese female mice (Figure 1A, upper panel). CoPP treatment resulted in a decrease in adipocyte size (p<0.05) compared to untreated obese animals (Figure 1A, lower left panel). We then examined the number of adipocytes in lean, obese and CoPP-treated obese female mice. The number of adipocytes (mean±SE) in lean, obese and CoPP-treated obese animals was 40.83±3.50, 18.33±1.80 and 32.00±1.67 respectively indicating that CoPP treatment of obese mice increased the number of adipocytes to levels similar to those in lean animals (Figure 1A, lower right panel). Similar results were seen in male animals.
Figure 1A–C.
A) Morphological haematoxilin eosin staining of visceral (VAT) aorta surrounding fat harvested from lean, untreated obese, and CoPP- treated obese. Bar, 50 μM p< 0.05 compared with untreated obese mice, lower Panel, adipocyte size, and number of adipocytes VAT, *p<0.05 compared to lean or obese treated with CoPP, **; p<0.05 vs obese. n=3–4 sections per group, B) Representative images of immunostained adipocytes from each experimental group. n=3–4 sections per group. Female immunohistochemistry of HO-1 and IOD determination of HO-1 expression in visceral (VAT) aorta fat lean, untreated obese, and CoPP- treated obese.*p<0.05 (Similar results were seen in male, data not presented) and C) Effect of CoPP on HO-1 protein levels in adipocyte isolated from pooled visceral fat of female lean and obese mice. Western blot and densitometry analysis of HO-1 protein in adipocyte isolated from fat tissues of lean and obese female mice treated with CoPP. Results are the mean ± SE of the band density normalized to actin; n=4; *p<0.01 vs lean; #p<0.05 vs obese using one way ANOVA.
The induction of HO-1 was associated with a reduction in blood pressure (BP). Systolic blood pressure in obese female mice was 142 ± 6.5 mm Hg compared to obese-CoPP treated, 109 ± 8.1 mm Hg, p<0.05. The value in obese female mice treated with CoPP is similar to the blood pressure seen in lean female mice (110 ± 9.6 mm Hg). The systolic blood pressure in obese male mice was 144± 4.5 mm Hg compared to obese-CoPP treated, 104 ± 3.6 mm Hg, p<0.05.
We further examined whether CoPP affects HO-1 expression in adipocyte using immunohistochemistry and western blot analysis. Immunostaining showed increased levels of HO-1 (green staining), located on the surface of adipocytes, after CoPP treatment (p<0.05), compared with female obese mice, Figure 1B. As seen in Figure 1C, HO-1 and HO-2 levels in adipocyte isolated from lean, untreated female obese mice or female obese mice treated with CoPP. Densitometry analysis showed that HO-1 was increased significantly in female obese mice treated with CoPP, compared to non-treated female obese mice, p<0.05, which is in agreement with immunohistochemistry results. This pattern of HO expression in obesity occurs in other tissues, including aortas, kidneys and hearts of male obese mice4, 13.
Effect of CoPP on HO-1 expression and HO activity in female and male obese mice
HO-1 protein levels were increased by CoPP treatments in liver and renal tissues similar to that seen in adipocytes. Western blot analysis showed significant differences (p<0.05) in the ratio of HO-1 to actin in renal of male and female obese and lean mice (Figure S 2A). Obesity decreased HO-1 levels in both sexes when compared to age matched lean animals. In addition, HO-1 levels were significantly (p<0.05) lower in obese females compared to obese males (Figure S 2A). This reflects a less active HO system in both male and female obese animals compared to age matched lean controls. Next, we compared the effect of CoPP on male and female HO-1 gene expression in adipocytes. CoPP increased HO-1 expression in both male and female obese animals compared to untreated obese animals (Figure S 2B, p<0.001 and p<0.001, respectively). Similar results of HO-1 expression were seen in liver tissues (Result not shown).
Effect of CoPP on cytokine levels in female and male obese mice
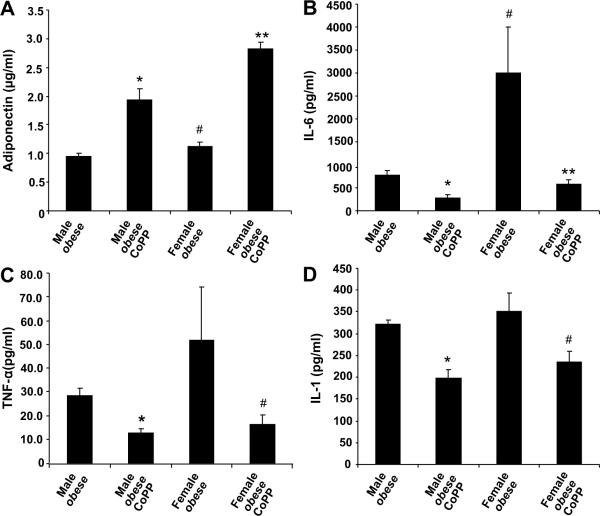
CoPP administration resulted in a significant increase in the levels of plasma adiponectin in both female (p<0.001) and male obese (p<0.001) mice (Figure 2A). Untreated female obese animals exhibited a significant (p<0.05) increase in plasma IL-6 levels when compared to age-matched male obese mice (Figure 2B). CoPP decreased plasma IL-6 levels in both female and male obese mice (p<0.05, p<0.01, respectively) when compared to untreated obese mice. Similar results were observed with plasma TNF-α and IL-1β levels (Figure 2C and 2D). These results indicate that though female obese mice exhibited elevated serum levels of inflammatory cytokines compared to male obese mice, CoPP acts with equal efficacy in both female and male obese animals in reducing inflammation while simultaneously increasing serum adiponectin levels (Figure 2).
Figure 2A–D.
A) Effect of CoPP on serum adiponectin levels in male and female obese mice. CoPP was administered once a week for 6 weeks and serum samples were obtained immediately prior to sacrifice. Results are by 2-way ANOVA, n=6–8.Levels of significance: * p<0.001 versus male obese; # p<0.05 versus male obese; **p<0.001 versus female obese. B) Effect on serum IL-6 levels in male and female obese mice. Levels of significance for IL-6: * p<0.01 versus male obese; **p<0.05 versus female obese, # p<0.05 vs male obese and C) Effect on serum TNF-α levels in obese mice. Levels of significance for TNF-α: *p<0.01 versus male obese; #p<0.05 versus female obese. D) Effect on IL-1β serum levels in male and female obese mice. Levels of significance for IL-1β: *p<0.01 versus male obese; #p<0.05 versus female obese.
Effect of CoPP on blood glucose and LDL levels in female and male obese mice
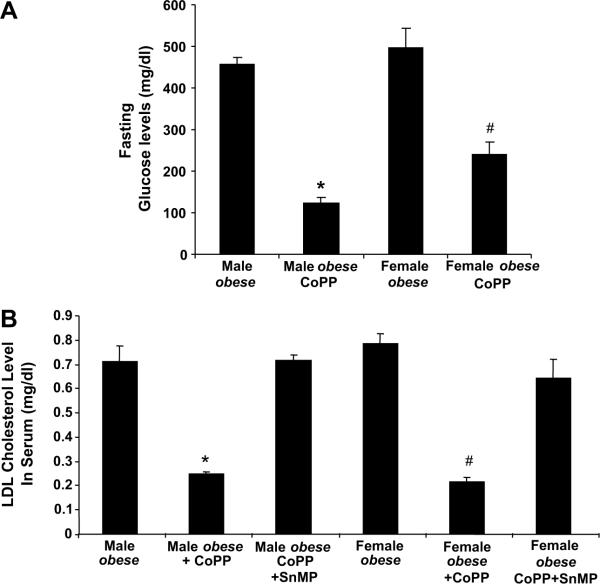
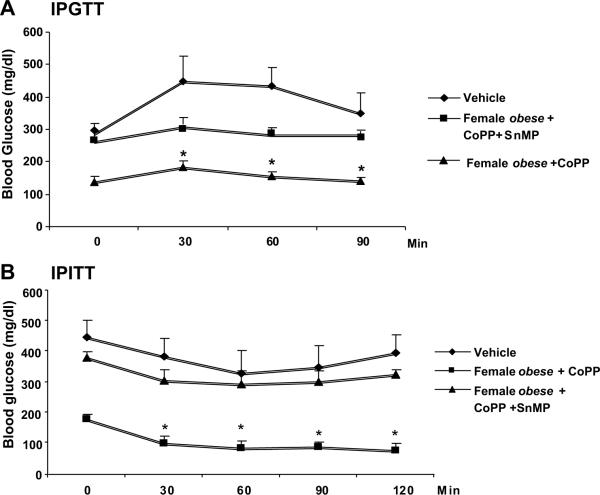
Fasting glucose levels were determined after the development of insulin resistance. CoPP produced a decrease in glucose levels in both fasting female (p<0.05) and male (p<0.001) obese mice when compared to untreated obese control animals (Figure 3A). CoPP reduced LDL levels in both male (p<0.01) and female (p<0.05) obese mice when compared to untreated obese controls (Figure 3B). Treatment with SnMP, increased LDL levels. In separate experiments two weeks apart, glucose levels and insulin sensitivity were determined after development of insulin resistance (Fig. 4A and B). Blood glucose levels in female obese mice were elevated (p<0.01) 30 min after glucose administration and remained elevated. In CoPP-treated female obese mice, blood glucose levels decreased significantly 60–120 min after glucose administration (p<0.01). Insulin administration to CoPP-treated female obese mice produced a decrease in glucose but not in the vehicle-treated female obese mice (p<0.01).
Figure 3A–B.
A) Effect of CoPP treatment on glucose levels in male and female obese mice, *p<0.001 versus male obese; #p<0.05 versus female obese. B) Effect of CoPP and SnMP treatment on LDL cholesterol levels in male and female obese mice. CoPP (administered once a week) and SnMP were administered three times/week for 6 weeks, and serum samples were obtained immediately before mice were sacrificed. Results are by 2-way ANOVA. n=6–8.Levels of significance: *p<0.01 versus male obese; #p<0.05 versus female obese.
Figure 4A–B.
Effect of HO-1 expression on glucose tolerance and insulin sensitivity. Intraperitoneal glucose tolerance (IPGTT); A) and intraperitoneal insulin sensitivity (IPITT; B) The results means ± SE, n= 6–8 mice per group. Levels of significance: *p<0.01 versus female obese.
Effect of Obesity on Protein Expression Levels of pAKT, pAMPK, and PPARγ levels in female and male obese mice
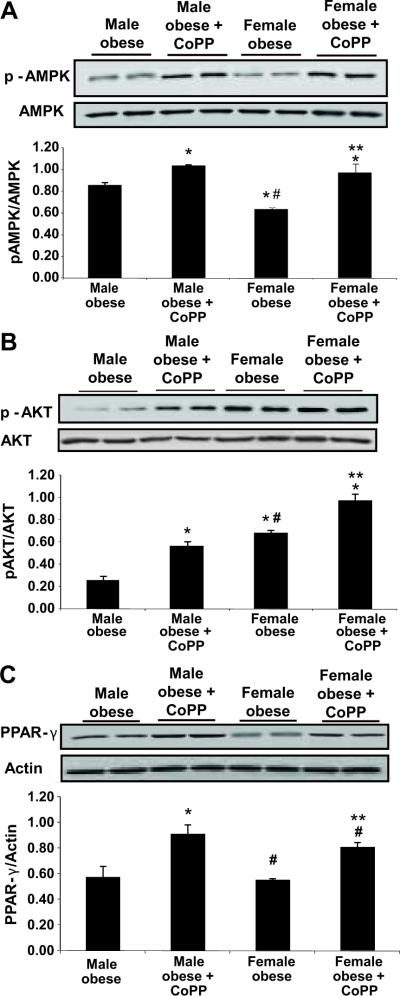
Western blot analysis of adipocytes harvested from fat tissues, showed significant differences in basal protein expression levels of pAKT and pAMPK in untreated female obese mice compared to untreated obese male mice. pAMPK levels were higher in obese females compared to obese males (Figure 5A, p< 0.05). This was also the case for pAKT protein levels, where increased levels of pAKT were seen in obese females compared to obese males (Figure 5B, p<0.05). CoPP treatment increased pAMPK and pAKT levels in bothe obese females and obese males. In addition, CoPP administration increased PPARγ levels, in both male (p<0.001) and female (p<0.05) obese mice (Figures 5C).
Figure 5A–C.
A) Effect of CoPP treatment on pAMPK in the adipocyte of male and female fat tissue obtained from obese mice. Adipocyte harvested from fat tissues samples were subjected to Western blotting for the determination of pAMPKα protein expression and densitometry analysis of pAMPKα/AMPK ratio. N=6. Levels of significance: * p < 0.05 versus male obese; # p< 0.05 versus obese male, ***p<0.05 vs female obese. B) Effect of CoPP treatment on pAKT in the adipocyte of male and female obese mice. Adipocyte samples were subjected to Western blotting for the determination of pAKT/AKT protein expression and densitometry analysis of p ratio. Levels of significance: * p < 0.05 versus male obese; *** p < 0.05 versus female obese;
# p< 0.05 versus obese male. C) Effect of CoPP treatment on PPARγ expression in the adipocyte of male and female obese mice. Adipocyte samples were subjected to Western blotting for the determination of PPARγ protein expression and densitometry analysis of p ratio. Levels of significance: * p < 0.001 vs male obese; **p < 0.05 vs male obese; #p<0.05 versus female obese.
Discussion
In the current study, we show for the first time that induction of HO-1 regulates adiposity in both male and female animals via an increase in adipocyte HO-1 protein levels. A second novel finding is that induction of HO-1 was associated not only with a decrease in adipocyte cell size but with an increase in adipocyte cell number. Further, induction of HO-1 affects visceral and subcutaneous fat distribution and metabolic function in male obese mice differently than in female obese mice. Despite continued obesity, upregulation of HO-1 induced major improvements in the metabolic profile of female obese mice exhibiting symptoms of Type 2 diabetes including: high plasma levels of proinflammatory cytokines, hyperglycemia, dyslipidemia, and low adiponectin levels. CoPP treatment resulted in increased serum adiponectin levels and decreased blood pressure. Adiponectin is exclusively secreted from adipose tissue, and its expression is higher in subcutaneous rather than in visceral adipose tissue. Increased adiponectin levels reduce adipocyte size and increase adipocyte number12, resulting in smaller, more insulin sensitive adipocytes. Adiponectin has recently attracted much attention because it has insulin-sensitizing properties that enhance fatty acid oxidation, liver insulin action, and glucose uptake and positively affect serum triglyceride levels18–21. Levels of circulating adiponectin are inversely correlated with plasma levels of oxidized LDL in patients with Type 2 diabetes and coronary artery disease, which suggests that low adiponectin levels are associated with an increased oxidative state in the arterial wall22. Thus, increases in adiponectin mediated by upregulation of HO-1 may account for improved insulin sensitivity and reduced levels of LDL and inflammatory cytokines (TNF-α, IL-1β, and IL-6 levels) in both male and female mice.
Females continued to gain weight in spite of the metabolic improvements. One plausible explanation for this anomaly is the direct effects of HO-1 on adiponectin mediating clonal expansion of pre-adipocytes. This supports the concept that expansion of adipogenesis leads to an increased number of adipocytes of smaller cell size; smaller adipocytes are considered to be healthy, insulin sensitive adipocyte cells that are capable of producing adiponectin23. This hypothesis is supported by the increase in the number of smaller adipocytes seen in CoPP-treated female obese animals without affecting weight gain when compared to female obese animals. Similar results for the presence were seen in males indicating that this effect is not sex specific.
Upregulation of HO-1 was also associated with increased levels of adipocyte pAKT, and pAMPK and PPARγ levels. Previous studies have indicated that insulin resistance and impaired PI3K/pAKT signaling can lead to the development of endothelial dysfunction24. In the current study, increased HO-1 expression was associated with increases in both AKT and AMPK phosphorylation; these actions may protect renal arterioles from insulin mediated endothelial damage. By this mechanism, increased levels of HO-1 limit oxidative stress and facilitate activation of an adiponectin-pAMPK-pAKT pathway and increased insulin sensitivity. Induction of adiponectin and activation of the pAMPK-AKT pathway has been shown to provide vascular protection25, 26. A reduction in AMPK and AKT levels may also explain why inhibition of HO activity in CoPP-treated obese mice increased inflammatory cytokine levels while decreasing adiponectin. The action of CoPP in increasing pAKT, pAMPK and PPARγ is associated with improved glucose tolerance and decreased insulin resistant. Insulin resistance is an independent factor for the development of both endothelial24 and vascular dysfunction27, 28. CoPP treated improved vascular function as manifest by increases in both insulin sensitivity and pAKT and pAMPK levels. Others have shown that increased phosphorylation of insulin receptors and vascular function may be a response to the increase in pAMPK and pAKT crosstalk29–31. Further activation of pAMPK and pAKT increase glucose transport, fatty acid oxidation and mitochondrial function32–34. pAKT and AMPK act as fuel sensors in the regulation of energy balance and the resultant the crosstalk of AMPK-AKT has been shown to regulate NO bioavailability and vascular function30, 35, 36. Furthermore, activated AMPK alone has been suggested as therapeutic target to ameliorate endothelial dysfunction37–39.
The novel effect of CoPP on the HO-1-adiponectin-pAKT-pAMPK-module i.e., an increase in HO-1, increases in adiponectin and the subsequent increase in AKT-AMPK crosstalk and signaling pathway provide a beneficial mechanistic basis for CoPP mediated vascular protection. Thus CoPP appears capable of reprogramming adipocytes resulting in the expression of a new phenotype containing adipocytes of reduced cell size, increased number and restored insulin sensitivity. Although CoPP caused induction of HO-1 in various tissues, it is HO-1 induction in adipocytes that may be crucial for reversal of vascular dysfunction. HO-1 upregulation in adipocytes increases the release of adiponectin, with subsequent improvement in insulin sensitivity and a marked decrease in inflammatory cytokines. Therefore, targeting adipocytes with an HO-1 gene, we might be able to address obesity mediated metabolic derangements and restore vascular function.
Perspectives
We have demonstrated that HO-1 induction in adipocyte stem cells not only ameliorates obesity associated metabolic consequences including hypertension independent of body weight, but improves glucose tolerance in both male and female obese mice. The ability of HO-1 to cause an increase in adipocyte cell number and expansion of healthy adipocytes in obese mice and reduce inflammatory cytokines appears to be primarily responsible for these effects. This appears to involve HO-1, adiponectin and the pAKT/pAMPK signaling pathway acting in unison. These novel findings underscore the importance of targeting HO-1 to attenuate hypertension, insulin resistance, dyslipidemia, and subsequent cardiovascular risk within obese populations.
Supplementary Material
Acknowledgements
Angela Burgess and Ming Li contributed equally to this work
Source of Funding This work was supported by National Institutes of Health grants DK-068134, HL-55601, and HL-34300 (NGA). This research was also supported by the Grant Agency of Charles University, GAUK 25754054007, MSM0021620806, 1M6837805002 (PM) and by a grant from the Beatrice Renfield Foundation (AK).
Footnotes
Conflict of Interest Disclosure The authors declare no competing financial interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsson B. Obesity, fat distribution and cardiovascular disease. Int J Obes. 1991;15(Suppl 2):53–57. [PubMed] [Google Scholar]
- 2.Kannel WB, D'Agostino RB, Cobb JL. Effect of weight on cardiovascular disease. Am J Clin Nutr. 1996;63:419S–422S. doi: 10.1093/ajcn/87.6.1602. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3(Suppl 5):645S–647S. doi: 10.1002/j.1550-8528.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 6.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 7.Mesch VR, Siseles NO, Maidana PN, Boero LE, Sayegh F, Prada M, Royer M, Schreier L, Benencia HJ, Berg GA. Androgens in relationship to cardiovascular risk factors in the menopausal transition. Climacteric. 2008;11:509–517. doi: 10.1080/13697130802416640. [DOI] [PubMed] [Google Scholar]
- 8.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 9.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, LAbbate A, Kappas A, Abraham NG. Heme Oxygenase-1 Induction Remodels Adipose Tissue and Improves Insulin Sensitivity in Obesity-Induced Diabetic Rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vera T, Kelsen S, Stec DE. Kidney specific induction of HO-1 prevents angiotensin II hypertension. Hypertension. 2008;52:660–665. doi: 10.1161/HYPERTENSIONAHA.108.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1472–R1478. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Burgess AP, Li M, Tsenovoy PL, Addabbo F, McClung JA, Puri N, Abraham NG. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 13.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L'Abbate A, Neglia D, Vecoli C, Novelli M, Ottaviano V, Baldi S, Barsacchi R, Paolicchi A, Masiello P, Drummond GS, McClung JA, Abraham NG. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–H3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- 15.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodella LF, Peterson SJ, Drummond G, Rezzani R, Falck JR, Abraham NG. Heme oxygenase-derived carbon monoxide restores adiponectin levels and vascular function in type 1 diabetes. Drug Metabolism Letters. 2008;2:290–300. doi: 10.2174/187231208786734058. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 20.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 21.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 22.Vinatier D, Dufour P, Tordjeman-Rizzi N, Prolongeau JF, pret-Moser S, Monnier JC. Immunological aspects of ovarian function: role of the cytokines. Eur J Obstet Gynecol Reprod Biol. 1995;63:155–168. doi: 10.1016/0301-2115(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulhak AA, Jung C, Ostenson CG, Lundberg JO, Sjoquist PO, Pernow J. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol. 2009;296:H719–H727. doi: 10.1152/ajpheart.00394.2008. [DOI] [PubMed] [Google Scholar]
- 25.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008;93:158–163. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 28.Duncan E, Crossey P, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat V, Wheatcroft S, Shah AM, Kearney M. The effect of endothelium specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming I, Schulz C, Fichtlscherer B, Kemp BE, Fisslthaler B, Busse R. AMP-activated protein kinase (AMPK) regulates the insulin-induced activation of the nitric oxide synthase in human platelets. Thromb Haemost. 2003;90:863–871. doi: 10.1160/TH03-04-0228. [DOI] [PubMed] [Google Scholar]
- 30.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 31.Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Van OE. Insulin signalling downstream of protein kinase B is potentiated by 5'AMP-activated protein kinase in rat hearts in vivo. Diabetologia. 2005;48:2591–2601. doi: 10.1007/s00125-005-0016-3. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 33.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 34.Di Noia MA, Van DS, Palmieri F, Yang LM, Quan S, Goodman AI, Abraham NG. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem. 2006;281:15687–15693. doi: 10.1074/jbc.M510595200. [DOI] [PubMed] [Google Scholar]
- 35.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci U S A. 2005;102:128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murabito JM, Keyes MJ, Guo CY, Keaney JF, Jr., Vasan RS, D'Agostino RB, Sr., Benjamin EJ. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis. 2008;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.