Abstract
We previously reported that synthetic or natural Toll-like receptor (TLR) 7/8 agonists present within dead cells enhanced cell-associated antigen presentation both in vitro and in vivo. Here, we investigated the immunopotency of different chemically synthesized TLR7/8 agonists, Resiquimod, Gardiquimod, CL075, and CL097, on HBsAg immunogenicity. These agonists stimulated inflammatory monocyte-derived cells to become potent antigen-presenting dendritic cells (DCs), which augmented HBsAg specific T cell proliferation after they were conditioned with HBsAg. The TLR8 agonist CL075 and the TLR7/8 dual agonist CL097 showed more potent effects than the TLR7 agonist. Compared with alum adjuvant, when HBsAg mixed with CL075 was injected intramuscularly into mice, more monocyte-derived DCs carried antigens into draining lymph nodes and spleens. Specific Abs, particularly IgG2a, were significantly increased, and more IL-5 and IFN-γ were produced by splenocytes and intrahepatic immunocytes in mice that received HBsAg mixed with CL075 and CL097. These results suggest that TLR8 agonists are good candidates to enhance recombinant HBsAg immunogenicity to induce specific humoral and cellular immune responses.
Keywords: TLR8 agonists, Monocyte-derived dendritic cells, Cellular immune responses
1. Introduction
Adjuvants are critical for purified protein vaccines in order to stimulate optimal immune responses to them. Alum, the most widely used immune adjuvant, is the only one approved by the FDA for use in humans. Recent reports indicated that alum acts directly on some antigen-presenting cells (APCs), including resident macrophages, immature dendritic cells (DCs), and circulating monocytes, to facilitate antibody productions [1]. However, the effects of alum for stimulating specific Th1 cell responses that favor CTL development are uncertain. DCs have proven to be the most potent APCs for their unique capacity to migrate from the periphery to the T cell zones of draining lymph nodes wherein they prime naïve T cells to induce specific immunity [2]. Peripheral blood monocytes constitute an important pool of migratory DCs in draining lymph nodes under inflammatory conditions. Monocyte-derived DCs that are formed de novo are essential for T cell immunity against invading pathogens [3]. In some animal models, circulating monocyte-derived DC precursors were the major APC population that induced specific CD8+T cell responses [4]. Signaling pathways of Toll-like receptors (TLRs) expressed by these cells, through recognition of various pathogen-associated molecular patterns (PAMPs), induce these cells to secrete distinct cytokines, which in turn, influence T cell differentiation. Thus, monocyte-derived DCs are important targets for the vaccines [2,5,6].
We recently surveyed the protective efficacy of the hepatitis B (HB) vaccine in a clinical study cohorts established during the 1980s in a rural area of China [7,8]. Occult HBV infection in some young adults after neonatal HB vaccination, even in the presence of anti-HBs antibodies (Xu et al., vaccine, in press) was observed. Studies of patients with self-limited HBV infections demonstrated that anti-HBV CTL responses played critical roles for terminating HBV infection [9]. A defect in specific T cell immunity against HBV antigens caused persistent chronic HBV infection [10]. Thus, new types of HB vaccines should be able to induce specific T cell responses, as well as to stimulate specific neutralizing antibodies.
Previously, we reported that human monocytes differentiated into potent APCs after they phagocytosed dead cells containing ssRNA and induced strong CD8+T cell responses to the cell-associated antigens [11]. A TLR7/8 agonist proved to be a good candidate for inducing Th1 responses. In non-human primates, it has been reported that HIV Gag protein conjugated to a TLR7/8 agonist enhanced the magnitude and quality of Th1 and CD8+ T cell responses [12,13]. However, R848, a TLR7/8 dual agonist, was not capable of augmenting immune responses against HBsAg in mice that were immunized by either intramuscular or subcutaneous routes [14]. The adjuvant effects of TLR7/8 agonists for HBsAg in humans need to be confirmed. It was recently demonstrated that TLR7 and TLR8 had distinct effects on human blood DCs and their precursors. TLR7 is expressed by pDCs [15,16], but its expression by mDCs and monocytes remains controversial [17,18]. TLR8 can be detected in monocytes and mDCs [15-18]. Using human CD34-DCs, TLR7 and TLR8 triggered different signaling pathways that played different roles in DC maturation [19]. In contrast, mice have a defect in TLR8 [20]. It would be clinically desirable to determine which type of TLR7/8 agonist is effective as an adjuvant for humans. Here, we adopted and modified our previously reported in vitro tissue engineered immunological module [11,21,22] to investigate the immunopotency of different chemically synthesized TLR7/8 agonists, Resiquimod, Gardiquimod, CL075, and CL097, on HBsAg immunogenicity.
2. Materials and methods
2.1. Reagents
Imject alum (Pierce Biochemicals) is a mixture of aluminum hydroxide and magnesium hydroxide. Recombinant HB vaccine (yeast), containing 10 μg of recombinant HBsAg and 1 mg of alum adjuvants in a 0.5 ml vial, and purified recombinant HBsAg were from Dalian Hissen Bio-pharm Inc, China. HBsAb quantification EIA kits were from Wantai Biological Pharmacy, Beijing, China. Recombinant human GM-CSF, IL-4, and CCL21 were purchased from Peprotech. Resiquimod (R848), Gardiquimod, CL075, CL097 were purchased from Invivogen. The purified anti-human BDCA1 and BDCA2 Abs were purchased from Miltenyi Biotec, Inc. (Auburn, CA). Purified anti-human CD14, PE-anti-human CD86, APC-anti-human HLA-DR, PE-anti-human CD4, PE-Cy7-anti-human CD8, anti-mouse CD11b, CD11c and their isotype controls were all purchased from eBiosciences (San Diego, CA). Purified anti-human CCR7 was purchased from R&D systems.
2.2. Preparation of primary human umbilical vein endothelial cells (HUVEC)
Primary HUVECs were prepared from fresh umbilical cords using standard laboratory protocols [21,22]. The cells were sub-cultured and passage 3 cells were used for all the experiments.
2.3. Preparation of adult human peripheral blood mononuclear cells (PBMCs)
Peripheral blood samples from healthy adult donors were collected according to guidelines approved by the Internal Review Board of the Cancer Hospital/Institute, Chinese Academy of Medical Sciences or Mount Sinai School of Medicine. PBMCs were isolated by Ficoll density gradient separation according to standard laboratory protocols. After washing, PBMCs were re-suspended in either culture medium or cryopreserved in DMSO-containing medium until future assays.
2.4. Preparation of the in vitro 3-D module to mimic vaccination sites
The module was set up based on our previously described methods [21], with slight modifications. Briefly, type I collagen (Inamed Biomaterials, Fremont, CA) mixed with 10×M199 medium and NaHCO3/HEPES buffered 0.1 N NaOH were mixed at a ratio of 8:1:5 and polymerized in a 96-well plate. In some experiments, Imject alum was added to the mixture before polymerization as shown in the diagram of Fig. 1A: A-1. HUVECs (30,000 cells per well) were added onto the polymerized type I collagen matrix and maintained in M199 medium (Lonza, Walkersville, MD) supplemented with 20% FBS until confluent (Fig. 1A: A-2). PBMCs were suspended in M199 medium and applied onto the HUVEC monolayer at 500,000 cells/ well for 1.5 h (Fig. 1A: A-3). For some experiments, to determine the migration of blood DC precursors and monocytes across the HUVECs into the sub-endothelial collagen, the non-migrating cells were collected from the top of the HUVECs after multiple washes using HBSS containing 1 mM of EGTA. Migrated cells into the sub-endothelial collagen were retrieved using collagenase D digestion. All cells were further analyzed after FACS staining. For some experiments, to determine the effects of TLR7/8 agonists on the differentiation of blood DC and monocytes, the migrated cells were continued to culture in medium with or without TLR7/8 agonists. Reverse-transmigrated (RT) DCs were carefully harvested at 48 h as reported [23] and used for the following analyses.
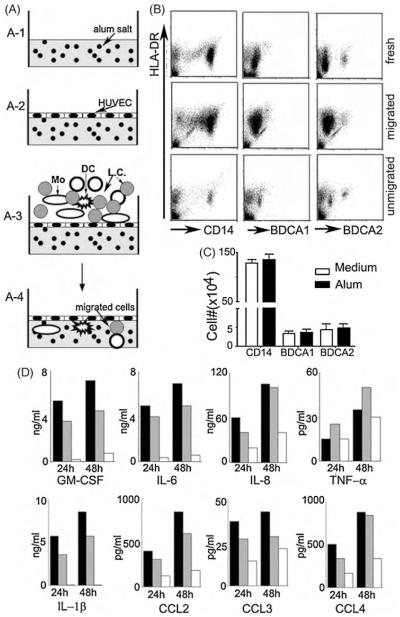
Fig. 1.
An in vitro 3-D module to mimic APC responses after vaccine delivery into tissues. (A) Schematic diagram of the 3-D module with alum incorporated into the collagen (A-1) and with primary HUVECs grown on top of the polymerized collagen (A-2). Freshly isolated PBMCs containing blood DCs (DC), monocytes (Mo) and lymphocytes (LC) were applied onto the confluent HUVEC layer for 1.5 h (A-3) and non-transmigrated cells were washed away. Transmigrated cells (A-4) were continued in culture for 2 d. (B) Migration of blood DCs versus monocytes across HUVECs into the sub-endothelial collagen. Representative analyses of 6 healthy donors for monocytes (CD14+), blood myeloid DCs (BDCA1+) and plasmacytoid DCs (BDCA2+) to determine the fraction of each cell type that had transmigrated across endothelium. (C) The number of transmigrated cells in a total of 106 PBMCs for monocytes and DCs in conditions with 50 μg/ml (filled bars) or without (empty bars, plain) alum in the collagen; n = 6. (D) Cytokines and chemokines in the cell supernatants collected 24 and 48 h after their transmigration with 50 μg/ml (filled bars) or 15 μg/ml (grey bars) alum incorporated in the collagen. Empty bars are from plain collagen.
2.5. Flow cytometry
The phenotypes of RT DCs were determined using 3-color staining as reported previously [11,22] and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
2.6. Chemotaxis assays using transwells
In the lower chamber of a transwell, 600 μl of M199 with 0.1% BSA and different doses of recombinant human CCL21 were added, as shown in the results. M199 with 0.1% BSA only was used as the spontaneous migration. The RT DCs were re-suspended in M199 with 0.1% BSA at a final concentration of 5 × 106/ml, and 100 μl/well was added into the upper chamber of the inserts. After incubation at 37 °C for 1 h and 45 min, the cells in the lower chambers were collected and counted. Migration of the RT DCs was expressed as the migration percentage by comparing the migrated and the input cell numbers.
2.7. Antigen presentation
HB-vaccinated donors with anti-HBs positive were recruited for this assay. After removing the non-transmigrated cells, different types of TLR7/8 agonists were added to the medium, as indicated in the results. RT DCs were carefully recovered under sterile conditions, as reported previously [11,21,22]. These RT DCs were used as candidate APCs to be mixed with autologous T cells, which were negatively selected using a pan T cell isolation kit II from Miltenyi Biotec, Inc. (Auburn, CA) and labeled with 5 μM of CFSE. RT DCs from the different groups were mixed with labeled autologous T cells at a ratio of 1 DC to 30 T cells, then cultured for 5 days in half of the conditioned medium from the different experimental groups and half fresh medium. Cell proliferation was determined by CFSE dilution using FACS after staining with PE-anti-human CD4 and PE-Cy7-anti-human CD8 as our previous report [22].
2.8. Cytokine and chemokine analysis
The production of cytokines and chemokines was analyzed using a Beadlyte human 22-plex multi-cytokine detection system (Millipore, Billerica, MA). Human IL-12p70 was determined by using a human IL-12 immunoassay Quantikin from R&D systems.
2.9. Local recruitment of APCs after HBsAg with different adjuvants was injected intramuscularly into mice
Recombinant HBsAg only or with CL075 was conjugated to Imject alum following the manufacturer’s guidelines and mixed separately with 0.0025% of YG microbeads. Fifty μl/site of the mixtures were injected intramuscularly (IM) into the gluteal muscles of mice. At 2, 24, 48, and 72 h after injection, the injection sites, draining lymph nodes (sacral LN [1]) and spleens were removed and snap frozen for cryosectioning at 8 μm per section. After fixing with 4% PFA for 30 min, the sections were stained with DAPI and cells were observed under an immunofluoresence microscope. For some mice, tissue at the injection site was removed and digested using collagenase D to prepare single cell suspensions. After staining with bio-anti-CD11c followed by streptavidin Percp-Cy5 and PE-anti-mouse CD11b, the cells were analyzed by FACS.
2.10. Assays of humoral and cellular anti-HBs immune responses in mice
C57BL/6 mice were purchased from the Institute of Laboratory Animal Sciences, CAMS, Beijing, and maintained in pathogen-free conditions under the guidelines for the care and use of animals. The following were injected IM into mice as indicated in Fig. 6A: clinically used recombinant HB vaccine (received 2 μg of recombinant HBsAg with 200 μg of alum adjuvant per mouse); 2 μg of recombinant HBsAg conjugated to 200 μg of Imject; 2 μg of recombinant HBsAg with 5 μg of CL075 or with 5 μg of CL097 conjugated to 200 μg of Imject. Humoral responses were monitored by quantifying HBsAb concentrations in sera after live bleeding from the tail veins on wks 2, 4, 6, and 10. HBsAb subclasses (IgG1, IgG2a) of the vaccinated mice were determined as previously reported [24]. One week after the first boost, 3/8 of the mice in each group were sacrificed, and at wk 10, 2 wks after the 2nd boost, all the remaining mice were sacrificed. Immunocytes from the spleens and draining lymph nodes were isolated from individual mice using standard laboratory protocols. Intrahepatic immunocytes were isolated according to a previous report [25]. All isolated cells at 5 × 106/ml were cultured with 1 μg/ml of purified recombinant HBsAg for 3 days. Cell culture supernatants were analyzed using ELISA to quantify the amounts of IL-5 and IFN-γ (ELISA kits purchased from eBiosciences). Cytokine levels were given as means ± SDs.
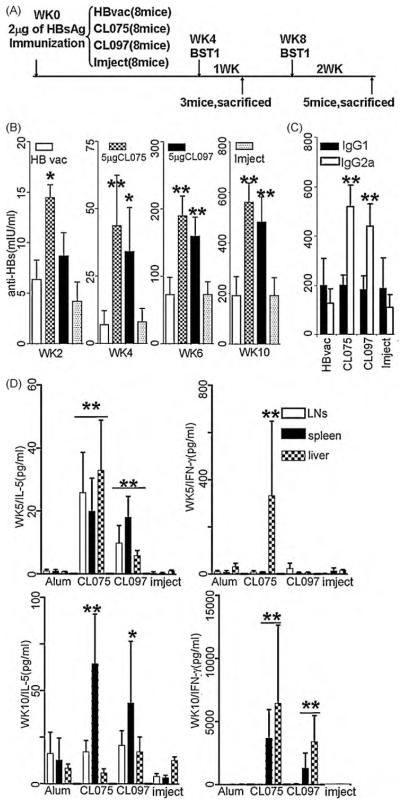
Fig. 6.
Anti-HBs antibody production and cellular responses in mice that received HBsAg with different adjuvant types. C57BL/6 mice (8 mice in each group) were immunized and boosted with HBsAg with different types of adjuvants as indicated in (A). Anti-HBs were monitored through live bleeding at wks 2, 4, 6, and 10. One wk after the first booster (wk 5) 3 of the 8 mice in each group were randomly selected and sacrificed, and 2 wks after second booster (wk 10) the remaining 5 mice in each group were sacrificed. (B) Anti-HBs humoral immune responses to injected antigens were monitored at different time points. (C) Subclasses of anti-HBs IgG in the mice at wk 10 when they were sacrificed. (D) After the mice were sacrificed at wk 5, 1 wk after the 1st booster, and at wk 10, 2 wks after the 2nd booster, their splenocytes, draining LN cells and intrahepatic immunocytes were isolated. Cells at 5 × 106/ml were cultured in the presence of 1 μg/ml of purified HBsAg for 3 days. Cell supernatants were collected and quantified for the levels of IL-5 and IFN-γ using ELISA (*P<0.05; **P<0.01).
2.11. Statistics
Statistical analysis was by student’s t-test using Microsoft Excel.
3. Results
3.1. A three-dimensional (3-D) module for in situ testing of human APC differentiation in response to antigens with adjuvants
Local responses of newly recruited blood DC precursors to antigens is one of the key steps for priming naïve T cells [26,27].We attempted to mimic the cellular responses to inoculated vaccines as closely as possible in a human immune system by modifying a 3-D module that we previously established [21,22]. Because alum is the mostly used adjuvant in humans, we incorporated it into the collagen matrix in some cultures underlying the vascular endothelium (Fig. 1A). Freshly isolated PBMCs from healthy adults were applied to the top of the 3-D module for a 1.5-h incubation. After carefully removing non-adherent cells from the top of HUVECs, transmigrated cells were retrieved from the collagen matrix and analyzed by FACS (Fig. 1B). Most transmigrated cells were CD14+ monocytes; alum did not alter this outcome. A few blood DCs (BDCA1+) and plasmacytoid DCs (BDCA2+) were present (Fig. 1B and C). However, no significant differences were observed when alum was or was not incorporated within the matrix (Fig. 1C). In contrast, presence of alum induced the production of some cytokines and chemokines (Fig. 1D), including GM-CSF, IL-1β, and IL-6, reflecting its effects in vivo [1,28]. This model was recently demonstrated to be highly sensitive for assessing the immunopotency of TLR agonists [29].
3.2. TLR7/8 agonists stimulate newly recruited monocytes differentiation into DCs in the 3-D module
We previously reported that peripheral blood monocytes differentiated into DCs to induce potent CD8+T cell responses to cell-associated antigens after they phagocytosed apoptotic cells that containing ssRNA, the TLR7/8 agonists. Herein, we used synthetic chemical compounds that targeted human TLR7 (Gardiquimod), TLR8 (CL075) or dual agonists TLR7/8 (Resiquimodand CL097) to determine their direct effects on monocytes differentiation in the 3-D in vitro module (Fig. 2A) and compared these with cultures of purified monocytes maintained in standard tissue-culture plates (Fig. 2B). A parallel collagen matrix with incorporated alum was used as a control (Fig. 3).
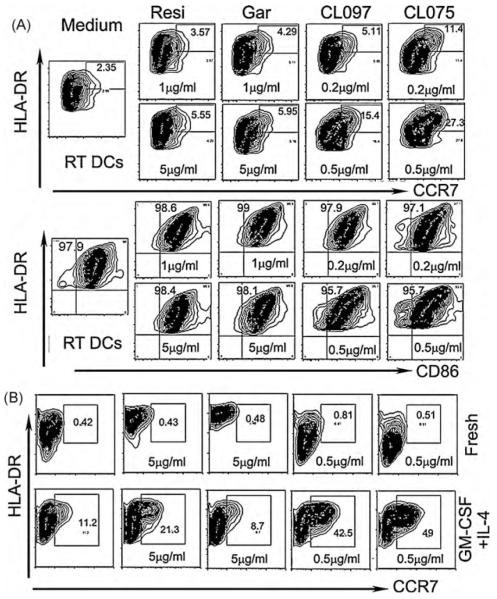
Fig. 2.
Effects of different TLR7/8 agonists on monocyte differentiation in vitro. (A) Freshly isolated unaltered monocytes were applied onto the confluent HUVEC layer for 1.5 h and then continued in culture for 2 d in the presence of 1or 5 μg/ml of Resiquimod (Resi), 1 or 5 μg/ml of Gardiquimod (Gar), 0.2 or 0.5 μg/ml of CL097 or 0.2 or 0.5 μg/ml of CL075. Medium only was used as the control. (B)Upper panel-fresh: freshly isolated unaltered monocytes were directly cultured in a culture plate in the presence of the indicated TLR7/8 agonists for 4 d; lower panel-GM-CSF + IL-4: the freshly isolated untouched monocytes were first cultured with 50 ng/ml of recombinant human GM-CSF and 34 ng/ml of recombinant human IL-4 for 5 d into immature DCs and then stimulated with the indicated TLR7/8 agonists for another 2d.
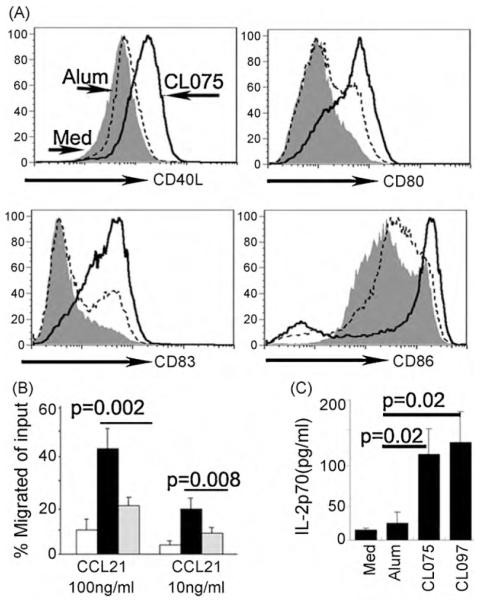
Fig. 3.
TLR7/8 agonists are more potent for stimulating transmigrated cells to differentiate into migratory DCs. (A) After transmigration into the collagen without incorporated alum, as indicated in Fig. 1A, CL075 (0.5 μg/ml) was added to the culture medium for 2 days and the RT DCs were collected and stained with the indicated antibodies (bold lines). The dashed lines indicate those cells recovered from collagen matrix with 15 μg/ml of alum incorporated and added to the culture medium. Shaded histogram indicates those cells recovered from collagen without alum or CL075. (B) Chemotaxis assays for these cells in response to CCL21. RT DCs were collected and added to the 5 μm pore size transwell (50,000) for 45 min. The transmigrated cells in the lower chamber were collected and counted. Results are the average percentages from 3 independent experiments. (C) Accumulated production of human IL-12 within 48 h by the monocyte-derived cells in the presence of alum or CL075 or CL097, n=3.
After 2 days of culture in the 3-D in vitro module, the reverse-transmigrated DCs (RT DCs) were collected, stained and analyzed by FACS, as previously reported [11,21,22]. The monocyte marker CD14 was down regulated in all conditions (not shown). Expressions of CCR7, CD86 and HLA-DR were analyzed and shown in Fig. 2A. Distinct synthetic chemical compounds that targeted TLR7, TLR8, or both TLR7/8 showed different, dose-dependent effects on monocyte differentiation into mature DCs. Among the tested TLR7/8 agonists, CL075 (TLR8 agonist) and CL097 (TLR7/8 dual agonist) showed the most potent effects on monocyte differentiation into DCs, as shown by the high percentages of CCR7+HLA-DRhi cells. However, Resiquimod and the TLR7 agonist, Gardiquimod, showed lesser effects on monocyte differentiation (Fig. 2A).
When monocytes cultured in standard tissue-culture plates were stimulated with the same agonists, no DC phenotypes were evident for up to 4 days (Fig. 2B, fresh). When the monocytes were cultured with GM-CSF + IL-4 for 5 days into immature DCs and then stimulated with the TLR7/8 agonists, both CL075 and CL097 showed potent capabilities to stimulate these cells to become HLA-DRhiCCR7+ DCs (Fig. 2B, GM-CSF + IL-4). However, no maturation marker was significantly increased after the cells were stimulated with Gardiquimod, a TLR7 agonist. These results suggest that the TLR7, TLR8 and TLR7/8 agonists have different effects on monocyte-derived DCs.
3.3. TLR8/7 agonists are more potent than alum adjuvants to stimulate inflammatory monocytes to differentiate into potent DCs
Compared with CL075, which strongly affected monocyte differentiation into mature DCs, alum showed much weaker effects (Fig. 3). The cell surface expressions of co-stimulatory molecules, CD40L, CD80, and CD86, and the maturation marker CD83 expressed on RT DCs increased significantly when CL075 was included compared with the cells from the conditions that alum was included (Fig. 3A). The percentage of CCR7+ cells, a marker associated with the ability of DCs to migrate to lymph nodes, also increased in the presence of CL075 (Fig. 2A). Its functional responsiveness to CCR7 ligands was further tested (Fig. 3B). Including CL075 in the module strongly induced responsiveness of the RT DCs to CCL21 (Fig. 3B), compared with a weaker alum-induced response. In addition, the production of IL-12, which is critical for Th1 cell differentiation, increased significantly after CL075, and CL097 was included in the medium (Fig. 3C). Thus, the TLR8 agonist CL075, and TLR7/8 dual agonist is more compelling than alum adjuvants to stimulate inflammatory monocytes to differentiate into potent, migratory DCs that favor the development of Th1 immune responses.
3.4. CL075 recruited more inflammatory monocytes into the antigen delivery sites and stimulated these cells to differentiate into migratory DCs after intramuscular injection
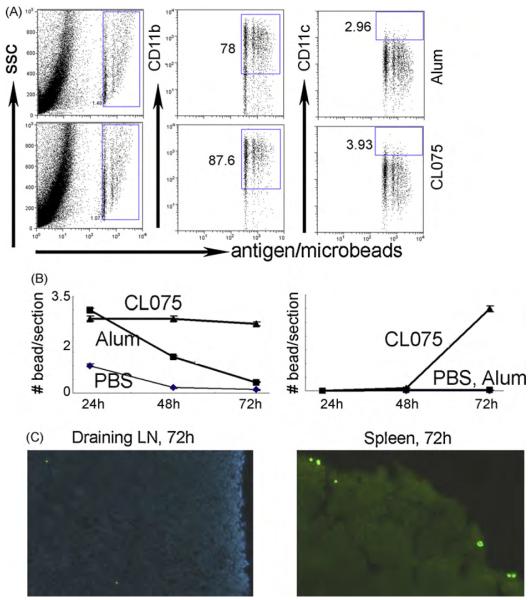
The effects of CL075 on inflammatory monocytes were further investigated in vivo. TLR8 is defective in mice and all the TLR7/8 agonists act through TLR7. We adapted an assay in which particulate antigens (fluorescent microbeads) were used to quantify the migration of APCs to draining LNs [30]. The antigens alone or with CL075 conjugated to Imject were mixed with microbeads and injected I.M. The injection sites, draining sacral LNs [1] and spleen were removed, sectioned and analyzed by immunofluorescence microscopy. Tissue sections showed that in the presence of alum or CL075, large amounts of inflammatory cells were newly recruited into the injection sites and had taken up the injected microbeads (Fig. 4A). Most of these cells were CD11b+ monocytes and a few of them were CD11Chi DCs (Fig. 4A). More CD11b cells were recruited into the injection sites in the presence of CL075 compared with alum.
Fig. 4.
Effects of CL075 and alum on the recruitment of inflammatory monocytes and their differentiation after intramuscular injection in mice. Recombinant HBsAg only, with CL075, or with alum was mixed with 0.0025% of 0.5 μm diameter YG microbeads and intramuscularly (I.M.) injected into mouse gluteal muscles. After removing the skin, muscle was digested and stained with anti-CD11b and CD11c (A) and analyzed by FACS. (B and C) After injection, the draining lymph nodes and spleen were removed at different time points, cryosectioned, and stained with DAPI. (B) The numbers of the microbead positive cells at different time points; (C) one of the representative tissue sections of the draining lymph nodes and spleens at 72 h after injection.
The microbead positive cells appeared in the draining LN 24 h after inoculation (Fig. 4B and C), and most of them had entered into the subcapsular area of the LNs. No major differences were observed between the groups of mice that received antigen/alum/microbeads and antigen/CL075/microbeads at this time. However, by 48 and 72 h after injection, more microbead-carrying DCs arrived in the draining LNs and most of these had entered into the inside of the LNs (Fig. 4C) in the group of mice that had received antigen/CL075/ microbeads compared to the group of mice that had received antigen/alum/microbeads. Similar results were found in their spleens (Fig. 4C). In the presence of CL075, more DC precursors were recruited into the antigen injection sites and the newly recruited monocytes developed into potential antigen-presenting DCs and carried the antigenic message into draining LN and spleen to prime immune responses.
3.5. TLR8/7 agonists are more potent than alum adjuvants to induce specific T cell proliferation in response to specific HBsAg in humans
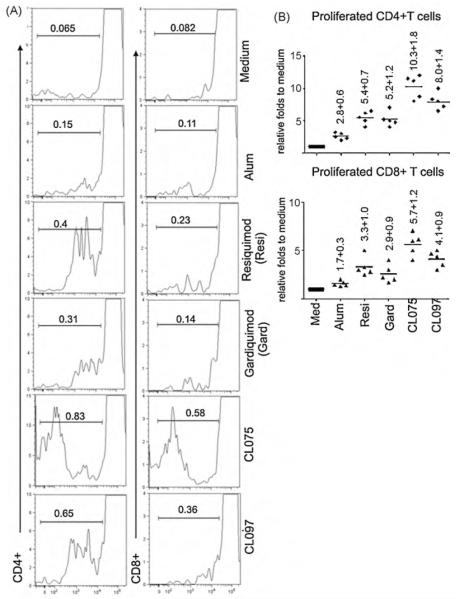
Due to the potent effects of TLR7/8 agonists on monocyte differentiation into DCs, we made a comparison of alum with TLR7/8 agonists for stimulating T cell responses in HB-vaccinated people. A total of 5 HB-vaccinated people with detectable serum HBsAb were recruited for this assay. After the monocytes transmigrated across the HUVECs in the 3-D module, 1 μg of recombinant HBsAg was included in the medium with or without different TLR7/8 agonists for 2 d. Autologous T cells that were negatively selected by depleting HLA-DR+ cells were mixed with the RT DCs, and co-cultured for another 5 d. Anti-HBs specific T cell proliferation was determined by FACS to evaluate CFSE-labeled T cell proliferation after they were stained with anti-human CD4 and CD8 (Fig. 5A). Monocyte-derived DCs that were conditioned with HBsAg in the presence of alum showed a very weak effect to stimulate both CD4+ and CD8+ T cell proliferation (Fig. 5B). Compared with medium only, proliferated CD4+ T cells stimulated by DCs conditioned from HBsAg + alum increased only by 2.8 ± 0.6 and CD8+ T cells increased by 1.7 ± 0.3 folds in the 5 HB-vaccinated adults (Fig. 5B). By comparison, monocyte-derived DCs that were conditioned with HBsAg in the presence of CL075 showed the most potent effect to stimulate both CD4+ and CD8+ T cell proliferation. Similar effects were also observed in the presence of CL097. Resiquimod and the TLR7 single agonist Gardiqumod showed weaker stimulating capacities for both CD4+ and CD8+ T cell proliferation (Fig. 5A and B).
Fig. 5.
Effects of monocyte-derived DCs after conditioning with HBsAg and different TLR7/8 agonists on autologous T cell proliferation in HB-vaccinated people. PBMCs isolated from healthy HB-vaccinated donors were applied onto the confluent HUVEC for 1.5 h and cultured for 2 d with 1 μg of recombinant HBsAg in the presence of different TLR7/8 agonists or medium only, as indicated in the figure. DCs were collected and co-cultured with CFSE-labeled autologous lymphocytes at a ratio of 1 RT DC with 30 lymphocytes for 5 days. The cells were then stained with PE-anti-human CD4 and PE-Cy7 anti-human CD8. CD4+ and CD8+ cells were gated and the proliferation of these cells was analyzed by FACS. (A) One representative of 5 volunteers. (B) Statistics for 5 volunteers. Each dot indicates one volunteer.
3.6. Humoral and cellular anti-HBsAg responses were enhanced in the presence of TLR7/8 agonists
The effects of TLR7/TLR8 agonists for inducing humoral and cellular responses in vivo were also tested in mice and were compared with the current clinically used HB vaccine. Currently, the HB vaccine used in China consists of 10 μg of recombinant HBsAg conjugated with 1 mg of alum. Based on calculations comparing the human body and an experimental mouse, HB vaccine containing 2 μg of recombinant HBsAg with 200 μg of alum was injected IM into mice. In addition, 2 μg of HBsAg only or mixed with 5 μg of CL075 or with 5 μg of CL097 conjugated with 200 μg of Imject were injected in the same way, as indicated in Fig. 6A. The mice showed similar responses when they received the clinical HB vaccine and recombinant HBsAg conjugated to Imject (Fig. 6). HBsAb responses were significantly enhanced in the presence of TLR8 and TLR7/8 agonists (Fig. 6B). Four wks after the primary immunization, the average titer of HBsAb in the mice that received recombinant HBsAg with 5 μg of CL075 was significantly higher than that of the mice that received the same amount of HB vaccine and the antigen with 200 μg of Imject. However, the effect of CL097 (human TLR7/8 dual agonist) was not obvious at this time point. After the mice were boosted, the increase of specific antibodies was more evident in the mice that received antigen with CL075 and CL097. At wk 10 when the mice were sacrificed, we determined the IgG subclasses (Fig. 6C). The mice that received CL075 and CL097 were mainly IgG2a, reflecting a Th1 type immune response.
Protection against some viral infections requires both humoral and cellular immunity [31]. Because HBV replicates within hepatocytes only, we isolated the immunocytes, including T cells, from the mouse livers as well as the spleens and drainingLNs and co-cultured these cells with 1 μg/ml of recombinant HBsAg for 3 d (Fig. 6C). IL-5 is an important T cell product for supporting humoral immune responses. It was noted that 1 wk after the 1st boost, the immunocytes in draining LNs, spleens and livers from the mice that received HBsAg/CL075 generated higher amounts of IL-5 (WK5/IL-5) compared to the mice that received HBsAg/alum. A similar effect of HBsAg/CL097 was seen, although weaker than that of the mice with HBsAg/CL075. More importantly, IFN-γ was only detectable in the intrahepatic immunocytes of the mice that received HBsAg/CL075. Two wks after the 2nd booster (wk 10), IFN-γ production by splenocytes and liver cells was abundant in the mice that received HBsAg/CL075 or HBsAg/CL097, but was not observed for the mice that received HBsAg/alum. Production of IL-5 by the splenocytes of the mice that received HBsAg/CL075 or HBsAg/CL097 was also in greater amounts than for the cells of mice that received HBsAg/alum. Specific humoral and cellular immunity to the inoculated antigens was significantly enhanced in the presence of the TLR7/8 agonists CL075 and CL097.
4. Discussion
After vaccine inoculation, in addition to tissue resident APCs, circulating DC precursors, particularly blood monocytes, comprise the important pools of antigen-responding cells under inflammatory situations [3,4,6,32]. Thus, it would be an important approach to enhance the specific immunity to target antigens by mixing them with suitable adjuvants. In this report, we adopted and modified the 3-D module that we previously established [11,21,22] to track the cell responses and their differentiation, including committed blood DC precursors and inflammatory monocytes. We demonstrated that a TLR8 agonist, CL075, and a TLR7/8 dual agonist, CL097, but not the TLR7 agonist Gardiqumond, stimulated monocyte-derived cells into potent DCs in the in vitro tissue engineered immunological module. Compared to alum adjuvants, HBsAg specific T cell proliferation was significantly enhanced in adults when the monocyte-derived DCs were conditioned with HBsAg mixed with these agonists. After the antigens mixed with CL075 were injected via the I.M. route, more inflammatory monocytes were recruited and differentiated into migratory DCs. Both the humoral and cellular immune responses to recombinant HBsAg were enhanced when the TLR8 or TLR7/8 agonists were given with the purified protein antigens. Thus, molecules that target the TLR8 signaling of the newly recruited DCs and/or their precursors might be effective adjuvants to enhance the humoral and cellular immune responses against recombinant HBsAg.
Previously, some investigators reported that the TLR7/8 agonist Resiquimond failed to induce specific anti-HBsAg immunity in a murine system [14]. However, in non-human primates, it has been reported that HIV Gag protein conjugated to a TLR7/8 agonist enhanced the magnitude and quality of Th1 and CD8+ T cell responses [12,13]. Due to the differences between human and murine immune systems, it would be clinically beneficial to mimic the human immune responses to the inoculated vaccines as closely as possible in vitro. By using the 3-D in vitro tissue engineered immunological module we demonstrated that TLR8 agonist CL075 and TLR7/8 dual agonist CL097 (a highly water soluble derivate of R848) showed more potent effects than alum on monocyte differentiation into mature DCs. However, the TLR7 agonist, Gardiquimod, showed less of an effect on monocyte differentiation into DCs. It has been reported that a TLR8 agonist, but not a TLR7 agonist, was responsible for IL-12 induction [33]. Indeed, our results demonstrated that more IL-12 was produced by the monocyte-derived cells in the presence of CL075 and CL097 than that of alum. IL-12 has been proven to be a key cytokine for Th1 differentiation. Recently, it was also found that IL-12 produced by activated monocyte-derived DCs specifically induced the differentiation of IL-21-producing T follicular helper-like cells that regulated antibody production [34]. Furthermore, the results from the mouse model in a certain extent confirmed the findings from the in vitro module. This module is capable of properly elucidating the effects of TLR7/8 agonists on monocytes and monocyte-derived cells in vitro. These data suggest that monocyte-derived DCs activated by TLR8 agonists will improve specific antibody production as well the cellular immune responses to specific antigens. Inclusion of TLR7/8 agonists into the recombinant HB vaccines might improve their immunogenecity in vivo.
Our results demonstrated that phenotypes of monocyte-derived cells after stimulation by both alum (data not shown) and TLR7/8 agonists (Fig. 2) were different in tissue-culture plate vs. the 3-D module. Previously, we had demonstrated that the presence of endothelial cells has a critical role in the generation of DCs in the system [21], interaction of monocytes with HUVECs in the 3-D culture systems facilitate the monocyte differentiation into potent DCs [22]. Some cytokines for monocyte differentiation and maturation were only presented in the 3-D rather than the tissue-culture plate [22,29]. Recently, it was demonstrated that non-monocytic cell population in the PBMCs also contribute to TLR induced DC generation and non-DC activation [29] in the 3-D module. After vaccine inoculation, the differentiation and function of APCs are affected by the local environment in response to the antigen with suitable adjuvants. The responses of the monocyte-derived cells in the 3-D module might be better in reflecting the effect than that of them in the tissue-culture plate [29].
Animal studies have demonstrated that adjuvants’ effects for CpG were more efficient if they were chemically coupled to the antigens, which suggested that simultaneous activation of APCs by both antigen and adjuvants was required for their optimal effects [35]. We previously demonstrated that physical associations of TLR7/8 agonists with target antigens were necessary for their antigen presentation [11]. Thus, recruiting DC precursors to the sites of vaccination and promoting their differentiation into migratory DCs will affect the immune responses to the vaccinated antigens. DCs deliver appropriate signals to T cells to orchestrate their tolerance or activation and differentiation.
Th1 cells play a critical role, via IFN-γ production, in mediating intracellular killing against a variety of infectious pathogens. To date, no appropriate HBV infection animal model has been available. However, clinical observations have demonstrated that specific T cell immunity against HBV antigens played a critical role in terminating HBV infection [10]. The results presented here demonstrated that anti-HBsAg immunity was polarized to Th1-type immune responses in the presence of TLR8 or TLR7/8 agonists, which was quite different from the immunity induced by alum adjuvants. More importantly, IFN-γ was produced in large amounts by the intrahepatic immunocytes after the mice had received the antigen with CL075. Because HBV only replicates within hepatocytes, these TLR8 or TLR7/8 dual agonists are promising candidate adjuvants for enhancing HBsAg immunogencity in humans.
Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China, 30872301. The authors are grateful to Dr. Zongtang Sun at Cancer Institute of CAMS for his critical review and valuable comments to the manuscripts.
References
- [1].Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008 April 14;205(4):869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008 September 19;29(3):319–24. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [3].Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007 April;26(4):519–31. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- [4].Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006 February;24(2):191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [5].van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006 January;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- [6].Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008 May–June;86(4):320–4. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- [7].Sun Z, Zhu Y, Stjernsward J, Hilleman M, Collins R, Zhen Y, et al. Design and compliance of HBV vaccination trial on newborns to prevent hepatocellular carcinoma and 5-year results of its pilot study. Cancer Detect Prev. 1991;15:313–8. [PubMed] [Google Scholar]
- [8].Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol. 2002 July;67(3):447–50. doi: 10.1002/jmv.10094. [DOI] [PubMed] [Google Scholar]
- [9].Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci U S A. 2010 January 12;107(2):798–802. doi: 10.1073/pnas.0913498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- [11].Qu C, Nguyen V, Merad M, Randolph G. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol. 2009 March 15;182(6):3650–9. doi: 10.4049/jimmunol.0801532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005 October 18;102(42):15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006 May 15;203(5):1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005 November 1;23(45):5263–70. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [15].Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001 November;31(11):3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [16].Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001 September 17;194(6):863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001 October;31(10):3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [18].Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002 June 3;195(11):1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Larange A, Antonios D, Pallardy M, Kerdine-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. 2009 April;85(4):673–83. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- [20].Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002 June;3(6):499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- [21].Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998 October 16;282(5388):480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- [22].Qu C, Moran TM, Randolph GJ. Autocrine type I IFN and contact with endothelium promote the presentation of influenza A virus by monocyte-derived APC. J Immunol. 2003 January 15;170(2):1010–8. doi: 10.4049/jimmunol.170.2.1010. [DOI] [PubMed] [Google Scholar]
- [23].Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med. 1996;183(2):451–62. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amemiya K, Meyers JL, Rogers TE, Fast RL, Bassett AD, Worsham PL, et al. CpG oligodeoxynucleotides augment the murine immune response to the Yersinia pestis F1-V vaccine in bubonic and pneumonic models of plague. Vaccine. 2009 April 6;27(16):2220–9. doi: 10.1016/j.vaccine.2009.02.016. [DOI] [PubMed] [Google Scholar]
- [25].Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009 July;50(1):261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- [26].Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4(Suppl 3):S127–32. doi: 10.1186/ar567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sallusto F, Reiner SL. Sliding doors in the immune response. Nat Immunol. 2005 January;6(1):10–2. doi: 10.1038/ni0105-10. [DOI] [PubMed] [Google Scholar]
- [28].Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008 April 15;180(8):5402–12. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- [29].Ma Y, Poisson L, Sanchez-Schmitz G, Pawar S, Qu C, Randolph GJ, et al. Assessing the immunopotency of Toll-like receptor agonists in an in vitro tissue-engineered immunological model. Immunology. 2010 Mar 17; doi: 10.1111/j.1365-2567.2009.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004 November 15;200(10):1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008 April;8(4):247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- [32].Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008 November 24;205(12):2839–50. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005 February 1;174(3):1259–68. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- [34].Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009 July 17;31(1):158–69. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mullen GE, Aebig JA, Dobrescu G, Rausch K, Lambert L, Long CA, et al. Enhanced antibody production in mice to the malaria antigen AMA1 by CPG 7909 requires physical association of CpG and antigen. Vaccine. 2007 July 20;25(29):5343–7. doi: 10.1016/j.vaccine.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]