Abstract
Optimal function of the serotonin system is essential for mental health and its role in psychopathologies is undisputed. Enhancing the ability to study primate serotonin neurons in culture would facilitate understanding of intracellular signaling pathways that mediate the action of drugs and other epigenetic or developmental factors impacting human mental health. We were the first group to report differentiation of the nonhuman primate rhesus monkey embryonic stem cells (ESC) line 366.4 into cultures of serotonin neurons. In this study, we optimized yield and obtained functional characteristics of the derived serotonin neurons. Sequential treatments of ESC 366.4 during expansion stage with FGF4 and SHH markedly increased the yield of serotonin neurons. These serotonin neurons propagated action potentials and expressed GABA receptors. Also for the first time we demonstrate that these ESC-derived serotonin neurons exhibit functional high affinity transporter sites, as well as high affinity 5HT1A binding sites, which are essential targets of common psychoactive drugs. Finally, to test the generality of this method, we utilized another rhesus ESC line, ORMES-22, which efficiently differentiated into serotonin neurons. Together these findings demonstrate the feasibility of our protocol to direct different primate neuronal ESC lines to serotonin neurons with physiological characteristics, which makes them a useful in vitro model system.
Keywords: Embryonic stem cells, Differentiation, Serotonin, Rhesus macaque, Serotonin reuptake transporter, 5HT1A autoreceptor
Introduction
The serotonin neural system plays a pivotal role in mood, stress sensitivity, integrative cognition and other autonomic functions. Serotonin functions through many different serotonin receptors, and it is important during development for guidance of neurogenesis, ultimate structure of the CNS, and neuronal plasticity (1, 2). Dysfunction of the serotonin system has been implicated in many psychopathologies such as depression, anxiety, schizophrenia, bipolar disorder, obsessive-compulsive disorder and eating disorders. Various drugs such as serotonin reuptake inhibitors, monoamine oxidase inhibitors, and receptor antagonists have been designed for treatment; however, these medications only partially alleviate the symptoms and have numerous undesirable side effects (3, 4). Intracellular signaling cascades, neurotransmitter synthesis, trafficking and release in primate serotonin neurons, as well as the effects of drugs on these events, have been unavailable for detailed study in vitro. The availability of a higher primate serotonin cell line would greatly facilitate study of the serotonin neural system at the molecular level in a way that would be relevant to human mental health.
Embryonic stem cells (ESC) are unique pluripotent cells that can proliferate indefinitely in an undifferentiated state and are theoretically capable of differentiating into any cell-type (5). Recently, a study showed differentiation of serotonin neurons from human ESC with ~ 50% differentiation rate (6). Our laboratory was the first group to report the utilization of non-human primate rhesus monkey ESC 366.4 to differentiate into predominantly serotonin neurons (7) in hope of providing a cell culture model for cellular and molecular studies as well as generating neurons for experimental transplantation. However, the attractive pluripotent characteristics of stem cells also present significant challenges since they do not necessarily generate a single type of neuron (i.e. a pure culture). The goal of this study was to improve our previous protocol in order to optimize yield and to obtain physiological characterization of the ESC-derived serotonin neurons. Different combinations of mitogenic signaling molecules, specifically fibroblast growth factor 4 (FGF4) with sonic hedgehog (SHH), improved the yield of the serotonin neurons. These ESC-derived serotonin neurons have neuronal membrane properties and express specific functional serotonin reuptake transporters (SERT) and 5HT1A autoreceptors, thus making them a useful in vitro model. The feasibility of our new optimized protocol was shown by using another rhesus ESC line ORMES-22, which efficiently differentiated to serotonin neurons.
Materials and Methods
ESC Culture and In Vitro Differentiation
The rhesus monkey ESC line 366.4 was obtained from Dr. James Thomson (Wisconsin National Primate Research Center). This line was derived from an in vivo flushed preimplantation embryo and has been characterized for its pluripotency including its potential to differentiate into cells of the neural lineage (8). The rhesus monkey ESC line ORMES-22 was derived by Dr. Shoukhrat Mitalipov (ART Core, Oregon National Primate Research Center) (9).
ESC were propagated and maintained as previously described (7). Briefly, ESC were co-cultured with mitotically inactive (mitomycin C-treated; 1 mg/ml at 37°C for 30 min; Sigma-Aldrich, MO) mouse embryonic fibroblasts (MEF). ESC culture medium consisted of 85% Dulbecco’s Modified Eagle Medium (DMEM/F12) supplemented with 1% nonessential amino acids, 2 mM glutamine, 0.1 mM b-mercaptoethanol (Sigma-Aldrich, MO) and 15% fetal bovine serum (Hyclone, UT). ESC and their colonies were observed daily and passaged every 6–8 days when colonies reached 1–1.5 mm in diameter. The pluripotency of ESC was evaluated periodically by immunocytochemistry (ICC) with antibodies to Oct-4, stage specific embryonic antigens (SSEA-3 and 4) and embryonic proteoglycans (TRA-1-60 and TRA-1-81) as previously described (7).
A protocol consisting of multiple sequential steps was used to induce differentiation as follows:
Isolation of ESC colonies and formation of embryoid bodies (EBs): After ESC colonies attained a 1–1.5 mm diameter, they were isolated mechanically, triturated into intermediate sized clumps (>200 cells/clump), transferred into 60 mm dishes (BD Biosciences, MA) and cultured in ESC medium at 37°C for 7 days. Embryoid bodies were defined as Oct-4 negative, three-dimensional structures that could potentially give rise to endo-, ecto- and mesodermal cell lineages.
Selection (N1 stage): After formation of EBs, ESC medium was replaced with a selection medium composed of DMEM/Nutrient Mixture F12 (1:1) containing L-glutamine, sodium bicarbonate, pyridoxine hydrochloride, 1% ITSX (1 g/L insulin; 0.67 mg/L sodium selenite; 0.55 g/L transferrin and 0.2 g/L ethanolamine) and human plasma fibronectin (5 μg/ml). EBs were cultured in selection medium for 7 days and at this stage referred as neurospheres.
Expansion (N2 stage): At the end of the selection period, neurospheres were cultured in expansion medium composed of DMEM/Nutrient Mixture F12 (1:1), 1% N2 supplement (500 μg/ml insulin; 10,000 μg/ml transferrin; 0.63 μg/ml progesterone; 1611 μg/ml putrescine and 0.52 μg/ml selenite) and FGF4 (10 ng/ml) for 2 days supplemented with SHH (50 ng/ml) for an additional 5 days changing the medium daily.
Maturation of differentiated neural cells (N3 stage): Expanded neurospheres were gently dispersed into single cell suspension using TrypLE (Invitrogen, CA) and then plated at 90% confluency on growth factor reduced (GFR)-matrigel coated coverslips or wells depending on the application. Cells were cultured in Neurobasal medium with N2 and B27 supplement (Invitrogen, CA) up to 2 months.
Immunocytochemistry
Cells grown on coverslips were fixed with 4% paraformaldehyde for 15 min at room temperature. The cells were permeabilized with 0.2% Triton-X and 0.1% Tween-20 in phosphate-buffered saline (PBS) for 40 min, followed by incubation with blocking solution (10% normal goat serum in PBS) for 1 h. Cells were then incubated overnight at 4 °C with primary rabbit polyclonal anti-TPH2 antibody (Novus Biologicals Inc, CO) and antibody-antigen complexes were detected with either Alexa Fluor 488 secondary antibody (1:1000, Invitrogen, CA) by incubation for 1 h at room temperature. The samples were washed five times with PBS after each incubation, and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Treatment with different mitogenic factors and SHH
To improve the viability and yield of serotonin neurons, the ESCs were treated with N2 medium (see above) during the expansion stage (N2 stage) with the following:
N2 media with 10 ng/ml FGF2 (Sigma, MO) for 7 days
N2 media with 10 ng/ml FGF2 for 2 days then addition of 50 ng/ml SHH (R&D Systems, MN) for 5 days.
N2 media with 10 ng/ml FGF4 (Sigma, MO) for 7 days
N2 media with 10 ng/ml FGF4 for 2 days then addition of 50 ng/ml SHH for 5 days.
The cells were immunostained for TPH2 with a rabbit antibody generated against human TPH2 followed by development with an anti-rabbit mouse monoclonal antibody conjugated to Alexa Fluor-488. The cultures were photographed at a low magnification (10×) using the Marianas stereological workstation (Intelligence Imaging Innovations, CO). The Marianas stereological workstation with Slidebook 4.2 was used obtain a montage and for analysis. The green labeled, TPH2 positive pixel area and the DAPI positive pixel area, indicating the total number of cells, were both reported in square microns (μ2 positive area). Two montages from two independent experiments for each treatment group (total of 4 montages) were analyzed. The TPH2 positive pixel area was divided by the DAPI positive pixel area in each montage to obtain a ratio of TPH2 labeled neurons to total cells in the differentiated culture (n=4/treatment). Differences between the treatment groups were determined by ANOVA followed by Newman-Keuls post-test using Prism Statistical software (Graph-Pad Software, Inc., San Diego, CA, USA). P<0.05 was considered statistically significant.
Electrophysiology
Whole-cell patch-clamp recordings were made with electrodes pulled to 2–4M Ohm resistance filled with an internal solution (138mM potassium methylsulfate, 10mM HEPES; 10mM KCl, 1mM MgCl2, 1mM EGTA, 0.3mM CaCl2, 4mM MgATP, 3mM NaGTP, pH 7.4). The external solution (146mM NaCl, 30mM dextrose, 5mM KCl, 5mM HEPES, 2.5mM CaCl2, 1.2mM MgCl2, pH7.35). Junction potentials were calculated (JPCalc, Molecular Devices, CA) and corrected at the beginning of the experiments. Capacitance and series resistance compensation (>80%) were corrected and data was collected with a Multiclamp amplifier (Molecular Devices) at 10kHz and filtered with a low-pass Bessel filter at 2kHz. Currents were digitized with Digidata1322 (Molecular Devices, CA), collected and analyzed using Axograph (Axograph Scientific, Australia). Action currents were elicited with a depolarizing step from −70 mV to −30 mV. Current-voltage plots were generated from a series of 10 mV steps from −100 mV to +20 mV. Drugs were diluted in appropriate buffers in concentrated stock solutions, then diluted further in external solution to final concentration.
SERT binding assay
To determine if the ES-derived serotonin neurons manifested a specific serotonin reuptake-binding site (SERT), a binding assay was conducted. The SERT binding assay was adapted from Lu et al. (10). Briefly, N3 stage serotonin neurons were pre-incubated in binding assay buffer (50mM Tris-HCl, 120mM NaCl, 5 mM KCl, pH7.4) for 15 min at room temperature. 2 μM [3H]-paroxetine (15 Ci/mmol, Perkin-Elmer Life Sciences, MA) alone or in combination with 1 μM Fluoxetine (Eli Lilly, IN) was added for 1.5 hr at room temperature. To further verify specificity, 1 μM mazindol (Sigma, MO), a noradrenaline reuptake inhibitor, was also added along with [3H]-paroxetine. Cells were collected in 100 μl of binding assay buffer and radioactivity was measured using Liquid Scintillation counter (Beckman, LS6000IC). Differences between the treatment groups were determined by ANOVA followed by Newman-Keuls post-test using Prism Statistical software (Graph-Pad Software, Inc., San Diego, CA, USA) (n=6/treatment). P<0.05 was considered statistically significant.
5HT1A binding assay
To determine if the ESC-derived serotonin neurons manifested a specific 5HT1A autoreceptor, a binding assay was conducted. The 5HT1A autoreceptor binding assay was adapted from Lu and Bethea. Briefly, N3 stage serotonin neurons were incubated in pre-incubaction buffer (170mM Tris-HCl, 4mM CaCl2, pH7.6) for 15 min at room temperature. Then, the cultures were incubated in binding assay buffer (pre-incubation buffer plus 0.01% L-ascorbic acid, 10 μM pargyline (Sigma, MO), 1 μM fluoxetine containing 2 nM [3H]-8-OH-DPAT (124.9 Ci/mmol, Perkin-Elmer Life Sciences, MA) alone, or in combination with either 2 μM cold 8-OH-DPAT or 2 μM serotonin (Sigma, MO) for 1.5 hr at room temperature. Cells were collected in 100 μl of incubation binding assay buffer and radioactivity was measured using Liquid Scintillation counter (Beckman, LS6000IC). Differences between the treatment groups were determined by ANOVA followed by Newman-Keuls post-test using Prism Statistical software (Graph-Pad Software, Inc., San Diego, CA, USA) (n=6/treatment). P<0.05 was considered statistically significant.
Results
Improvement in viability and yield of serotonin neurons
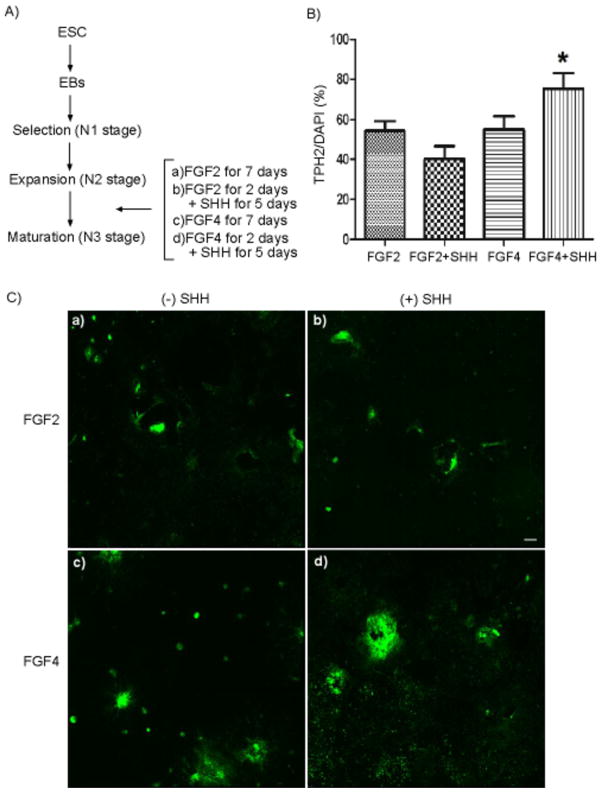
Previously, we found that exposure of neurospheres to FGF2 helped differentiate the rhesus 366.4 ESC into serotonin neurons. These cultures were extensively examined by ICC to express markers that typify serotonergic neurons of the dorsal raphe of the monkey brain including tryptophan hydroxylase (TPH), serotonin and SERT (7). In order to improve the yield of monkey serotonin neurons, we cultured neurospheres in expansion (N2) medium with different combinations of FGF2 or FGF4 plus or minus SHH after 3 days for total of 7 days (Figure 1A). Figure 1C shows low power montages of cultures that were immunolabeled for tryptophan hydroxylase 2 (TPH2), the rate-limiting enzyme for serotonin synthesis (11), and illustrate the colonies of TPH2-positive serotonin neurons. TPH2 positive pixel area was reported in square microns (μ2 positive area) along with DAPI positive pixel area in square microns (μ2 positive area). TPH2 positive neurons were expressed as a percentage of DAPI positive area. (Figure 1B). Compared to FGF2 treatment, the addition of FGF4 followed by addition of SHH gave a higher yield of serotonin positive neurons resulting in ~75 % of serotonin neurons.
Figure 1. Improvement in differentiation of ESC-derived serotonin neurons by mitogenic factors and SHH.
A) Schematic diagram of differentiation protocol. B) Percentage of TPH2 positive cells in the differentiated cultures with different combination treatments with mitogenic factors (FGF2 or FGF4) and SHH (n=4, *p<0.05). C) Montage of serotonin neurons immunolabeled for TPH2 (green). (a) FGF2 alone, (b) FGF2 with SHH, (c) FGF4 alone, (d) FGF4 with SHH. Scale bar 200μm.
ESC-derived serotonin neurons have basic membrane properties
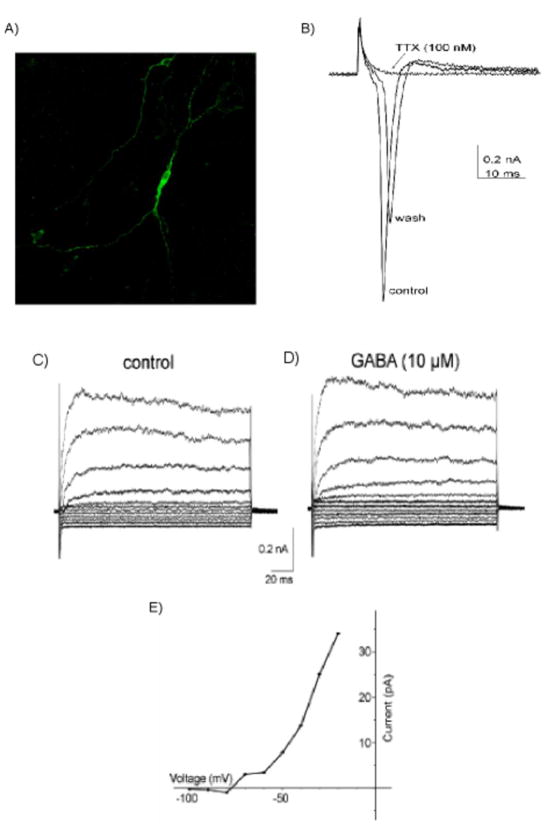
We examined whether these ESC-derived serotonin neurons have neuronal membrane properties. Figure 2A illustrates the striking neuronal morphology attained by a neuron that has been immunolabeled for TPH2. The electrophysiological recordings showed that ESC-derived serotonin neurons exhibit action potentials that are blocked by TTX (Figure 2B). Figure 2C illustrates that these cells express the GABAA receptor/channel as is common in cultured raphe cells (12). Currents elicited by a series of depolarizing voltage steps are increased in the presence of GABA (10 μM) (Figure 2D) and reverse at hyperpolarized potentials as would be expected for the GABAA-induced chloride current (Figure 2E). Together these data indicate that ESC-derived serotonin neurons have membrane properties that are consistent with a neuronal phenotype.
Figure 2. ESC-derived serotonin neurons elicit action currents.
A) Representative serotonin neuron. B) Action currents in depolarizing voltage step −70 mV to −30 mV. Voltage-dependent sodium channel implicated by absent action potential during TTX superfusion, recovers after washout. C) Currents evoked by depolarizing 10 mV voltage steps, D) presence of GABA. E) Current-voltage plot of the subtracted (GABA-control).
ESC-derived serotonin neurons have high affinity transporter and autoreceptor sites
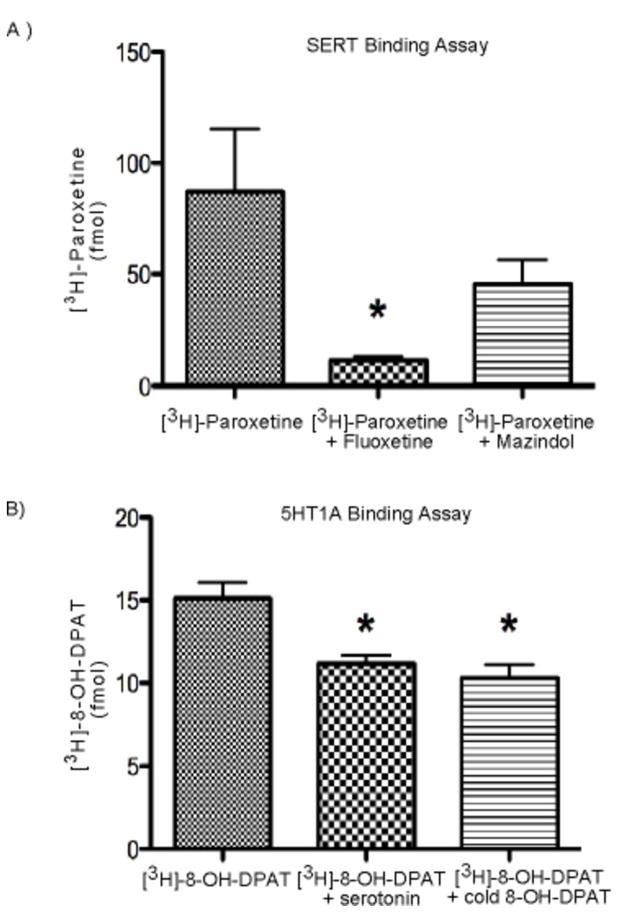
CNS serotonin neurons have a high affinity uptake mechanism that utilizes the SERT, which is one of the major targets of the class of psychoactive drugs known as selective serotonin reuptake inhibitors (SSRIs) (13). To examine whether ESC-derived serotonin neurons express SERT, [3H]-paroxetine, a common SSRI, was used in a radioligand-binding assay. ESC-derived serotonin neurons exhibited an average [3H]-paroxetine specific binding of 87.36 fmol at a substrate concentration of 2μM. To determine the specificity of [3H]-paroxetine binding, 1 μM fluoxetine, another common SSRI, was used for competition. Fluoxetine significantly displaced [3H]-paroxetine binding (Figure 3A, * p=0.03). To further verify the specificity of [3H]-paroxetine binding, mazindol, which has a high affinity for norepinephrine transporters, was incubated along with [3H]-paroxetine. Mazindol did not displace [3H]-paroxetine binding.
Figure 3. ESC-derived serotonin neurons express functional transporter and autoreceptor sites.
A) SERT binding using [3H]-Paroxetine radioligand assays. A significant reduction in [3H]-Paroxetine binding in the presence of fluoxetine (n=6, * p<0.03) but not mazindol. B) 5HT1A binding using [3H]-8-OH-DPAT radioligand assays. A significant reduction in [3H]-8-OH-DPAT binding in the presence of serotonin and cold 8-OH-DPAT (n=6, * p<0.05).
The 5HT1A autoreceptor is one of the major serotonin receptors that binds serotonin in the extracellular space and decreases neuronal firing and serotonin release (14–16). Thus, it is essential that our ESC-derived serotonin neurons express functional 5HT1A autoreceptors in order for us to proceed with psychopharmaceutical studies. ESC-derived serotonin neurons were incubated with [3H]8-OH-DPAT radioligand, 5HT1A autoreceptor agonist, to measure the affinity of 5HT1A autoreceptor binding. [3H]8-OH-DPAT had an average specific binding of 15.09 fmol at a substrate concentration of 2nM. The binding was specific to 5HT1A autoreceptor as [3H]8-OH-DPAT radioligand binding was significantly displaced when incubated with either serotonin or cold 8-OH-DPAT (Figure 3B, * p<0.05). Moreover, the Bmax was similar to that previously obtained in macaque brain (17).
Altogether, the binding studies confirm that the ESC-derived serotonin neurons express functional proteins that define a neuron as serotonergic, and thus will be a useful in vitro primate model system.
Rhesus ESC line, ORMES-22, differentiates to serotonergic neurons with high efficiency
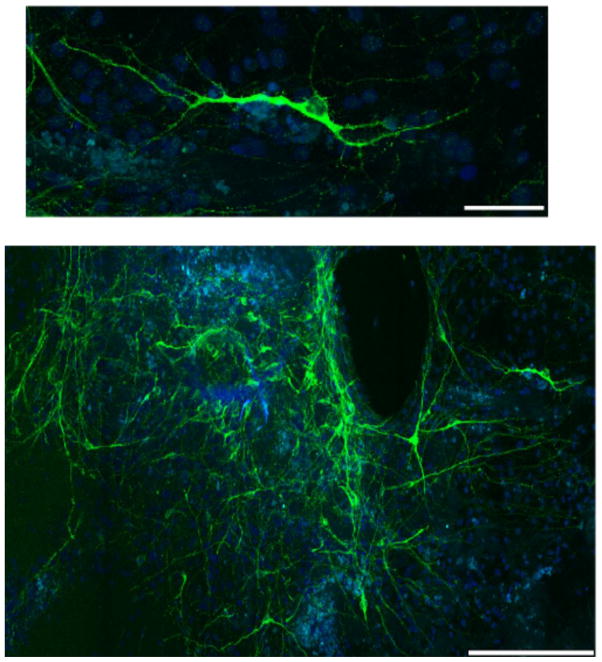
We used another rhesus ESC line, ORMES-22, to validate our optimized protocol’s efficiency to differentiate ESC into serotonin neurons. ORMES-22 is a recently established IVF (in vitro fertilized)-derived rhesus ESC line (9), which was subjected to our modified protocol. Similar to rhesus ESC line 366.4, ORMES-22 differentiated into serotonin neurons as determined by TPH2 immunocytochemistry (Figure 4). This suggests that our modified protocol can efficiently differentiate another rhesus ESC line, ORMES-22 into serotonin neurons.
Figure 4. Rhesus ESC ORMES-22 differentiates into serotonin neurons.
Confocal photomicrograph of representative differentiated serotonin neurons immunolabeled for TPH2 (green) and DAPI (blue). Scale bars, 50μm (top), 250 μm (bottom).
Discussion
The development of physiologically relevant primate serotonin neural culture system that allows direct examination of cellular and molecular signaling mechanisms is essential to our understanding and development of new psychopharmacotherapies. Human and monkey ESC differ substantially from mouse ESC in terms of morphology and surface marker expression. The differences between human and mouse ESC may result from fundamental differences in embryonic development between the species, or they may reflect a difference in the embryonic stage of origin of ESC in mouse and primate. Recently, we obtained information on the genetics of the developmental cascade throughout the differentiation stages from undifferentiated ESC to serotonin neurons (18). Most of our understanding in the developmental pathway leading to serotonin neurons comes from rodent models (19, 20); however, fetal development in rodents and primates differs significantly. We observed similarities and differences between in vivo rodent studies and rhesus ESC-derived serotonin neurons in our microarray study. Several genes thought to be critical signals for a neural and serotonin phenotype in mice such as TGF β, VEGF, Wnt and hedgehog pathways were similarly expressed during in vitro differentiation of ES-serotonin neurons. However, we also observed some differences in the ontogeny of Phox2b, Mash1, and Lmx1b gene expressions between our microarray study and in vivo mice studies (21). In the ESC-derived serotonin neurons, FEV1 appeared to be the key gene for expression of other serotonin markers. Nonetheless, the differences between in vivo rodent studies and our in vitro ESC study may point to genes that are important for the organization of the serotonin system versus the expression of the serotonin phenotype and the differences between rodents and primates.
The attractive pluripotent characteristic of stem cells also presents a significant challenge to obtain pure cultures of only one cell type. The goals of this study were to enhance our ability to differentiate rhesus ESC into serotonergic neurons at a high yield in a predictable manner and to use these cultures to obtain physiological characterization of the ESC-derived serotonin neurons. Previously, we found that exposure of neurospheres to FGF2 helped differentiate the rhesus 366.4 ESC into serotonin neurons (7). Other studies using mouse embryonic stem cells (mESC) showed sequential exposure of FGF4 then FGF8 and SHH led to a high yield of differentiated serotonin neurons (22–25). Our results corroborate the mouse studies in that the sequential combination of FGF4 and SHH improved the yield of serotonin positive neurons to ~ 75% as determined by TPH2 immunocytochemistry.
Obtaining monolayers of ESC-derived serotonin neurons with consistent well-to-well cell numbers allowed us to perform functional studies and determine whether these neurons have neuronal membrane properties, as well as serotonin reuptake transporter and autoreceptor expression in a manner similar to primate CNS serotonin neurons. The electrophysiological recordings showed that ESC-derived serotonin neurons exhibit action potentials that are blocked by TTX, and that they express GABA receptors in a fashion similar to dissociated postnatal rat serotonin neurons (12). Together these data indicate that ESC-derived serotonin neurons have membrane properties that are consistent with a neuronal phenotype.
SERT is responsible for the recycle and clearance of serotonin in the brain and is the major target of SSRIs. Therefore, in order for the ESC-derived serotonin neurons to be a good in vitro model, it is essential that they express a functional transporter. Indeed, the ESC-derived serotonin neurons exhibited a high affinity-binding site for [3H]-paroxetine that was effectively blocked with fluoxetine but not mazindol. In rats, [3H]-paroxetine exhibits an average Bmax of 120 fmol/mg protein, which is comparable to our SERT binding assay (26).
5HT1A autoreceptor is located on the soma and dendrites of the serotonin neurons and have an ultra-short loop feedback mechanism thought to cause a delay in the onset of efficacy of antidepressant drugs (27). In depressed patients, 5HT1A autoreceptor levels are elevated and supplementation of antidepressant therapy with 5HT1A antagonists outperforms SSRIs alone (28, 29). Thus, it is essential that our ESC-derived serotonin neurons express the 5HT1A autoreceptor in order for us to proceed with psychopharmaceutical studies. The ESC-derived serotonin neurons exhibited a high affinity-binding site for [3H]-8-OH-DPAT that was blocked to a significant degree with cold 8-OH-DPAT or serotonin. Moreover, the Bmax was similar to that previously obtained in macaque brain (17). Altogether, the binding studies confirm that the ESC-derived serotonin neurons express functional proteins that define a neuron as serotonergic, and thus will be a useful in vitro primate model system.
Overall, the yield of serotonin neurons and the architecture of the cultures were significantly improved. We have also confirmed that our modified protocol is efficient in differentiation of serotonergic neurons from another rhesus ESC line, ORMES-22.
In conclusion, we are able to produce ESC-derived serotonin neurons with high efficiency in a predictable manner. This will open the door for use of these cells in high throughput pharmaceutical screening for novel drugs, and for studies on the intracellular mechanism of action of antidepressants and antipsychotics. In addition, it will further the goal of transplantation into a macaque model of mental illness for restoration of serotonin function or for transport of gene vectors that promote regeneration of in situ systems.
Acknowledgments
Supported by NIH grants: MH73564 and MH62677 to CLB, U54 contraceptive Center Grant HD 18185, S10RR024585, and RR000163 for the operation of ONPRC. We are deeply grateful to the Assisted Reproductive Technology Core (Dr. Shoukhrat Mitalipov) for the generation of the MEF and embryonic stem cell cultures and to Dr. Anda Cornea for her assistance in confocal microscopy.
Contributor Information
Yukari Tokuyama, Email: tokuyama@ohsu.edu.
Susan L. Ingram, Email: ingram@vancouver.wsu.edu.
Joy L. Woodward, Email: woodwarj@ohsu.edu.
Cynthia L. Bethea, Email: betheac@ohsu.edu.
References
- 1.Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 4.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 5.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 6.Kumar M, Kaushalya SK, Gressens P, Maiti S, Mani S. Optimized derivation and functional characterization of 5-HT neurons from human embryonic stem cells. Stem Cells Dev. 2009;18:615–627. doi: 10.1089/scd.2008.0181. [DOI] [PubMed] [Google Scholar]
- 7.Salli U, Reddy AP, Salli N, Lu NZ, Kuo HC, Pau FK, Wolf DP, Bethea CL. Serotonin neurons derived from rhesus monkey embryonic stem cells: similarities to CNS serotonin neurons. Exp Neurol. 2004;188:351–364. doi: 10.1016/j.expneurol.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitalipov S, Kuo HC, Byrne J, Clepper L, Meisner L, Johnson J, Zeier R, Wolf D. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. doi: 10.1634/stemcells.2006-0125. [DOI] [PubMed] [Google Scholar]
- 10.Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- 11.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MD. Electrophysiological and histochemical properties of postnatal rat serotonergic neurons in dissociated cell culture. Neuroscience. 1994;63:775–787. doi: 10.1016/0306-4522(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 13.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 14.Sprouse JS, Aghajanian GK. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- 15.Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- 16.Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 17.Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 18.Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 21.Bethea CL, Reddy AP, Pedersen D, Tokuyama Y. Expression profile of differentiating serotonin neurons derived from rhesus embryonic stem cells and comparison to adult serotonin neurons. Gene Expr Patterns. 2009;9:94–108. doi: 10.1016/j.gep.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 24.Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 26.Nadgir SM, Malviya M. In vivo effect of antidepressants on [3H]paroxetine binding to serotonin transporters in rat brain. Neurochem Res. 2008;33:2250–2256. doi: 10.1007/s11064-008-9703-z. [DOI] [PubMed] [Google Scholar]
- 27.Gundlah C, Hjorth S, Auerbach SB. Autoreceptor antagonists enhance the effect of the reuptake inhibitor citalopram on extracellular 5-HT: this effect persists after repeated citalopram treatment. Neuropharmacology. 1997;36:475–482. doi: 10.1016/s0028-3908(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 28.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]