Abstract
Aberrant production of IL-21 by T cells is critical for the development of type 1 diabetes (T1D) in NOD mice. The pathogenic effects of IL-21 are partly due to its ability to promote the generation of TH-17 cells. Interferon Regulatory Factor (IRF4) is a crucial regulator of IL-17 and IL-21 production. We recently found that the serine-threonine kinase ROCK2 phosphorylates IRF4 and regulates its ability to control IL-17 and IL-21 production. Here we show that NOD T cells aberrantly activate ROCK2. We furthermore demonstrate that ROCK inhibition corrects the abnormal IRF4 function in NOD T cells and diminishes their production of IL-17 and IL-21. Importantly, administration of a ROCK inhibitor to NOD mice protects against diabetes development. These studies thus support the idea that ROCK2 is inappropriately activated in NOD T cells and that ROCK kinases could represent important therapeutic targets for the treatment of T1D.
INTRODUCTION
Aberrant activation of effector CD4+ T cells has been recognized as a critical component of the pathogenesis of type 1 diabetes (T1D) [1]. Strong support for the involvement of CD4+ T cells in T1D has come from studies of the nonobese diabetic (NOD) mouse, a spontaneous mouse model of diabetes that shares many similarities with human T1D [2]. Recent studies have demonstrated that NOD mice exhibit elevated levels of IL-21, a cytokine produced by CD4+ T cells and that absence of IL-21 completely protects NOD mice from diabetes development [3; 4; 5; 6]. Interestingly, IL-21 maps to Idd3, the strongest non-MHC-linked locus for T1D [7]. Some of the protective effects of the absence of IL-21 have been ascribed to a reduction in IL-17-producing T helper cells in these mice [5]. Consistent with these findings TH-17 cells have been shown to promote diabetes and neutralization of IL-17 was shown to be beneficial in NOD mice [8; 9; 10]. Increased production of IL-17 by peripheral blood T cells has furthermore been detected in children with T1D [11]. Thus, delineating the molecular mechanisms that control the production of IL-17 and IL-21 and determining whether these mechanisms are deregulated in T1D could provide important insights into the pathogenesis of T1D and potentially lead to the development of new approaches for the treatment of T1D.
Interferon Regulatory Factor 4 (IRF4), a member of the IRF family of transcription factors, plays a unique and essential role in the production of IL-17 and IL-21 [12; 13; 14]. The expression of IRF4 is not restricted to the TH-17 lineage but is instead upregulated upon TCR stimulation irrespective of the presence of different TH polarizing conditions [15]. Consistent with this finding, IRF4 also serves a crucial function in nonpolarized effector CD4+ T cells [15]. Given the broad expression of IRF4 in activated T cells, regulatory mechanisms must therefore exist to control its function in a TH-lineage restricted manner.
We have previously shown that IRF4 can be sequestered in an inhibitory complex that prevents it from targeting the IL-17 and IL-21 promoters [13]. We have recently found that its ability to access the IL-17 and IL-21 promoters can also be regulated at a posttranslational level via phosphorylation by ROCK2, a member of the serine-threonine family of Rho kinases [16]. While ROCK2 is activated physiologically when CD4+ T cells from non-autoimmune mice are exposed to TH-17 conditions, effector CD4+ T cells from mice that spontaneously develop autoimmune arthritis or lupus aberrantly activate ROCK2 under neutral conditions [16]. Here we demonstrate that aberrant activation of ROCK2 under neutral conditions is also observed in effector CD4+ T cells from NOD mice. We further show that the abnormal activation of ROCK2 in NOD CD4+ T cells leads to deregulated IRF4 function. Importantly we demonstrate that ROCK inhibition diminishes the production of IL-17 and IL-21 by NOD CD4+ T cells in vitro and that administration of a ROCK inhibitor to NOD mice decreases IL-17 and IL-21 production in vivo and ameliorates diabetes.
MATERIALS AND METHODS
Mice
NOD mice were obtained from Jackson Laboratories. All mice used in the experiment were kept under specific pathogen-free conditions. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Columbia University.
Flow Cytometry
Single cell suspensions from thymus, spleen, and lymph nodes were isolated, stained with different fluorochrome-conjugated Abs and analyzed by FACS as previously described [16].
In vivo Fasudil studies and histopathology
Ten weeks old female NOD mice were fed Fasudil in drinking water (100mg/kg) or left untreated for 12 weeks. Animals were grouped based on blood glucose levels to ensure similar levels in the treated and untreated animals. During the course of study mice were weighed to ensure equivalent water intake by treated and untreated group. Pancreata tissue sections were stained with hematoxylin and eosin (H&E) and analyzed by light microscopy. The severity of insulitis was assessed as previously described [6].
CD4+ T cell isolation and ROCK2-kinase assays
CD4+ T cells were purified using the CD4+ isolation kit from Milteny Biotech. For cytokine analysis 1×106 cells/ml were stimulated with plate-bound anti-CD3ε and soluble anti-CD28 for 3 days and then rested in IL-2 for 4 days. Cells (1×106 cells/ml) were then restimulated by plate-bound anti-CD3ε and soluble anti-CD28 for 2 days. ROCK2 kinase activity assays were performed as described [16].
Statistical analyses
Results were expressed as mean ± SD. Differences between groups were calculated for statistical significance using the two tailed unpaired Student's t test and Log Rank test where indicated. P<0.05 was considered as significant.
RESULTS AND DISCUSSION
ROCK2 is aberrantly activated in NOD CD4+ T cells
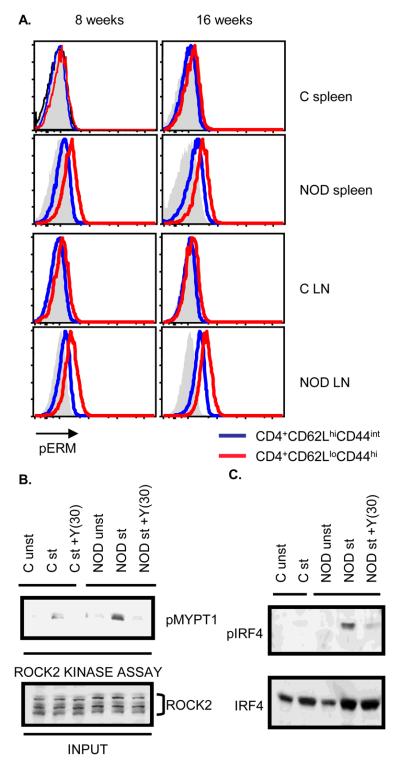
We have previously determined that ROCK2 is aberrantly activated in CD4+ T cells from two distinct mouse models of spontaneous autoimmunity [16]. We thus hypothesized that aberrant ROCK2 activation in CD4+ T cells may be a common pathogenic mechanism in the development of autoimmunity and set out to investigate whether ROCK2 activation is also deregulated in CD4+ T cells from NOD mice. Since phosphorylation of ERM proteins (pERM) can be utilized as a gauge of ROCK activity [16], we first employed intracellular FACS to evaluate the phosphorylation status of ERM proteins in CD4+ T cells from NOD mice. Enhanced pERM could be detected in NOD CD4+ T cells mice as compared to CD4+ T cells from control mice (Fig. 1A). Enhanced pERM expression was primarily observed in the effector/memory (CD62LloCD44hi) CD4+ subset (Fig. 1A) but not in B220+ or CD11b+ cells (data not shown). To confirm that ROCK2 is abnormally activated in NOD CD4+ T cells, we performed a ROCK2 kinase assay in which the activity of ROCK2 is measured by the ability of immunoprecipitated ROCK2 to phosphorylate recombinant MYPT1, a known ROCK substrate [17] (Fig. 1B). ROCK2 immunoprecipitates from stimulated NOD CD4+ T cells exhibited an increased ability to phosphorylate MYPT1 as compared to stimulated control CD4+ T cells. This effect was inhibited by Y27632, a well-known ROCK inhibitor [18]. These data thus indicate that ROCK2 is aberrantly activated in NOD CD4+ T cells.
Figure 1.
Enhanced ROCK2 activation and IRF4 phosphorylation in NOD CD4+ T cells. A. Increased levels of phosphorylated Ezrin/Radixin/Moesin (pERM) proteins in NOD CD4+ T cells. The levels of pERM in CD4+CD44intCD62Lhi (blue line) and CD4+CD44hiCD62Llo cells (red line) from either Balb/c or NOD mice were detected by intracellular staining with an anti-phospho-ERM antibody and analyzed by FACS. Solid histograms indicate isotype control. Results are demonstrated as overlaid histograms after gating on the appropriate populations. Data are representative of two independent experiments. B. Purified CD4+ T cells from Balb/c (C) or NOD mice were either left unstimulated or restimulated with αCD3 and αCD28 mAbs for 48 hours in the presence or absence of 30μM of Y27632 (Y(30)). ROCK2 kinase activity was assayed by incubating the immunoprecipitated ROCK2 with purified recombinant MYPT1 (rMYPT1) as ROCK2 substrate. Phosphorylated rMYPT1 (pMYPT1) was detected using an anti-phospho-MYPT1 antibody (upper panel). Lower panel shows the total ROCK2 levels in the input samples. C. CD4+ T cells from Balb/c (C) or NOD mice were stimulated as described in Figure 1B. Nuclear extracts were analyzed by Western blotting using an antibody that recognizes phosphorylated IRF4 (pIRF4) (upper panel). The blot was later reprobed with an antibody against total IRF4 (lower panel). The data are representative of three independent experiments.
Given that ROCK2 phosphorylates IRF4 in T cells [16], we next investigated the phoshorylation status of IRF4 in NOD CD4+ T cells. CD4+ T cells from NOD mice were cultured under neutral conditions, nuclear extracts isolated and subjected to Western blotting with an antibody, which specifically recognizes the ROCK2-phosphorylated form of IRF4 (pIRF4) [16]. Only a low-level of pIRF4 could be detected in stimulated CD4+ T cells from control mice while much higher levels of pIRF4 were instead detected in CD4+ T cells from NOD mice (Fig. 1C). As expected, the enhanced levels of pIRF4 in NOD CD4+ T cells could be blocked by the addition of the ROCK inhibitor Y27632. Thus, the aberrant activation of ROCK2 observed in NOD CD4+ T cells results in increased IRF4 phosphorylation.
ROCK inhibition diminishes the production of IL-17 and IL-21 by NOD CD4+ T cells
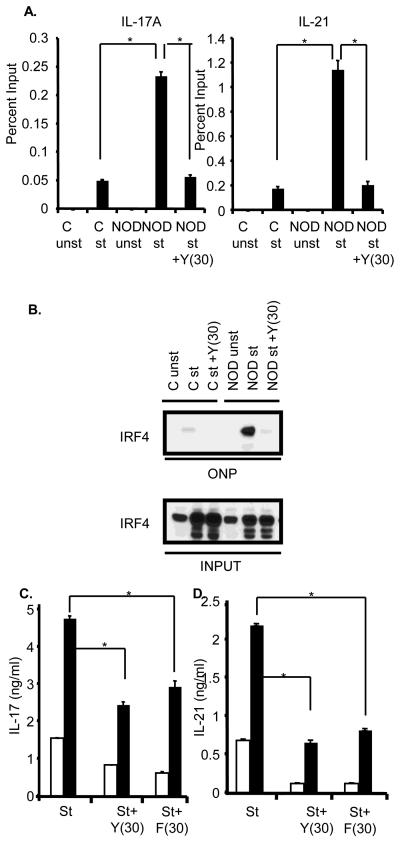
Phosphorylation of IRF4 by ROCK2 increases its ability to target the IL-17 and IL-21 promoters [16]. We thus employed chromatin immunoprecipitation (ChIP) assays to assess IRF4 binding to the IL-17 and IL-21 promoters. As compared to stimulated control CD4+ T cells, stimulated NOD CD4+ T cells exhibited enhanced binding of IRF4 to the IL-17 and IL-21 promoters (Figure 2A). Addition of Y27632 profoundly inhibited the increased IRF4 binding observed in stimulated NOD CD4+ T cells (Fig. 2A). Similar results were obtained by employing an oligonucleotide precipitation assay (ONPs) with an oligonucleotide containing the IRF4 binding site from the IL-21 promoter (Fig. 2B). These data thus indicate that the aberrant activation of ROCK2 detected in NOD CD4+ T cells leads to deregulated IRF4 function.
Figure 2.
ROCK inhibition interferes with IRF4 function and diminishes the production of IL-17 and IL-21 by NOD CD4+ T cells. A. CD4+ T cells from either Balb/c (C) or NOD mice were cultured as described in Fig. 1B. Chromatin immunoprecipitation assays were then carried out with either an IRF4 antibody or a control antibody. Quantification of IRF4 binding to the IL-17A and IL-21 promoters was performed using QPCR and is shown as percent input. The data are representative of two independent experiments. The error bars represent Mean ± S.D. *P≤0.0008. B. CD4+ T cells were purified from Balb/c (C) or NOD mice, were cultured as described in Fig. 1B, and then subjected to an oligonucleotide precipitation assay (ONP) with a biotin-labelled oligonucleotide corresponding to the IRF4 binding site within the IL-21 promoter. Precipitates were analyzed by Western blotting with an IRF4 antibody (top panel). The levels of IRF4 in the input samples are shown in the lower panel. Data are representative of three independent experiments. C. Purified CD4+ T cells from Balb/c (white bars) and NOD (black bars) mice were subjected to a primary stimulation and then restimulated with αCD3 and αCD28 for 48 hrs in presence or absence of 30 μM of Y27632 (Y(30)) or 30 μM of Fasudil (F(30)). Supernatants were assayed for IL-17 (left panel) and IL-21 (right panel) production by ELISA. The data are representative of three independent experiments. The error bars represent Mean ± S.D. *P≤0.004.
Given that IRF4 is a critical regulator of IL-17 and IL-21 production we next evaluated whether ROCK inhibitors could affect the ability of NOD CD4+ T cells to produce IL-17 and IL-21. When CD4+ T cells from NOD mice were isolated, cultured under neutral conditions and then restimulated, they produced increased levels of IL-17 and IL-21 as compared to control T cells (Fig. 2C). Addition of the ROCK inhibitor Y27632 diminished the enhanced production of IL-17 and IL-21 by NOD CD4+ T cells. A similar inhibitory effect was observed with a distinct type of ROCK inhibitor, Fasudil (Fig. 2C). These data thus support the idea that aberrant activation of ROCK2 in NOD CD4+ T cells leads to enhanced production of IL-17 and IL-21.
Administration of Fasudil to NOD mice ameliorates the development of diabetes
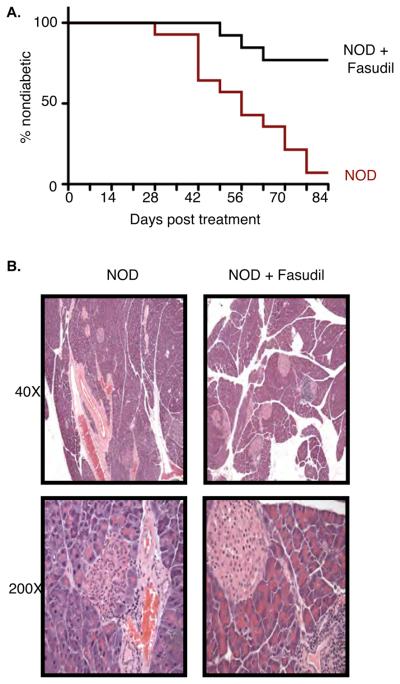
Deregulated production of IL-17 and, particularly of IL-21, plays a key role in the development of diabetes in NOD mice [4; 5; 6; 8; 10]. Since ROCK inhibitors decreased the production of IL-17 and IL-21 in vitro we explored the possibility that ROCK inhibition in vivo could ameliorate the development of diabetes in NOD mice. Fasudil (100mg/kg) was administered orally to NOD mice starting at 10 weeks of age when the majority of NOD mice have already developed insulitis. Treatment was continued for a period of 12 weeks. Consistent with previous reports and our own experience [16; 19], Fasudil treatment was well tolerated. Strikingly, administration of Fasudil led to a remarkable decrease in the incidence of diabetes in NOD mice (Fig. 3A). Only 20% of the Fasudil treated NOD mice were diabetic by 22 wks of age compared to 95% of the untreated NOD mice. Consistent with these findings, histological analysis revealed that, while the pancreata from the untreated NOD mice contained fewer and smaller islets infiltrated by lymphocytes and occasional plasma cells with many displaying degenerative features, the pancreata from Fasudil treated mice still had more islets of variable sizes with inflammatory infiltrates but also a few islets of nearly normal size devoid of inflammation (Figure 3B and Supplementary Table I). The composition of inflammatory cells and the density or pattern of inflammation in the affected islets was not significantly different in the two groups. Administration of Fasudil can thus ameliorate the development of diabetes.
Figure 3.
ROCK inhibition protects from the development of diabetes in NOD mice. A. Diabetes incidence in NOD mice (red line) (n=13) and NOD mice treated with Fasudil (black line) (n=12) for 12 weeks starting at 10 wks of age. Onset was determined after two consecutive readings of blood glucose levels >250mg/dl. *P≤0.0003 by Log-rank test. B. Evaluation of insulitis by hematoxylin/eosin staining of pancreata of NOD mice and NOD mice treated with Fasudil for 12 weeks starting at 10 wks of age. Representative photomicrographs showing differences in islet size and number are shown (magnification 40X and 200X, as indicated).
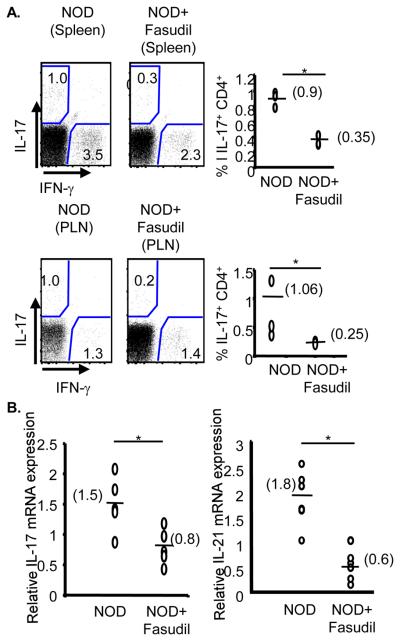
Administration of Fasudil to NOD mice did not affect the frequency of CD4+ T cells or their activation status as gauged by the expression of CD44, CD62L, or ICOS (Supplementary Fig. 1A). Furthermore, no effects on regulatory T cells or B cells were observed in NOD mice (Supplementary Figs. 1B and 1C). However, consistent with the in vitro effects of Fasudil on the production of IL-17 and IL-21 by NOD CD4+ T cells, administration of Fasudil decreased the frequency of IL-17-producing CD4+ T cells in both the spleen and pancreatic lymph nodes (Fig. 4A). Additionally, a marked decrease in the expression of both IL-17 and IL-21 was observed in the spleen of Fasudil treated NOD mice (Fig. 4B). Taken all together these studies suggest that ROCK inhibition slows the development of diabetes by interfering with the production of IL-17 and IL-21.
Figure 4.
ROCK inhibition decreases the production of IL-17 and IL-21 in vivo. A. The frequency of CD4+ IL-17+ T cells from spleens and pancreatic lymph nodes (PLN) of NOD mice (n=3) or of NOD mice treated with Fasudil (n=3) was evaluated by intracellular FACS. *P≤0.001. B. Spleens from NOD mice (n=5) and NOD mice treated with Fasudil (n=5) were collected and IL-17 and IL-21 gene expression was analyzed by real-time RT-PCR. *P≤0.02 for IL-17 and *P≤0.0002 for IL-21.
DISCUSSION
Aberrant CD4+ T cell function is known to play a crucial role in the pathogenesis of type I diabetes. The deregulated molecular mechanisms responsible for the abnormal activation of these cells in diabetes have not been fully delineated. Here we demonstrate that CD4+ T cells from the NOD mouse, a well-established mouse model of Type I diabetes, aberrantly activate the serine-threonine kinase ROCK2. Indeed while activation of ROCK2 in TH cells normally occurs only when these cells are exposed to TH-17 skewing conditions [16], NOD CD4+ T cells activate this kinase when cultured under neutral conditions. Furthermore, we demonstrate that the aberrant activation of ROCK2 in NOD CD4+ T cells leads to deregulated IRF4 function and enhanced IL-17 and IL-21 production in vitro. Importantly, in line with the recently recognized role of IL-17 and IL-21 in the pathogenesis of diabetes [3; 4; 5; 6; 8; 9; 10], interfering with ROCK activation diminished IL-17 and IL-21 production in vivo and markedly ameliorated the development of diabetes. Given that we have previously observed aberrant activation of ROCK2 in CD4+ T cells from other spontaneous mouse models of autoimmunity, deregulated activation of this kinase in CD4+ T cells may thus represent a common pathogenic mechanism underlying the development of autoimmunity.
Our findings support the idea that targeting Rho kinases may be beneficial in T1D. Interestingly, studies in streptozocin-induced models and in insulin-resistant models of diabetes have implicated abnormal activation of the RhoA/ROCK pathway in the pathogenesis of diabetic nephropathy due to the ability of this family of kinases to promote extracellular matrix deposition, ROS production and cell migration [20; 21; 22; 23]. A key role of the RhoA/ROCK pathway in diabetes-induced microvascular damage has also been demonstrated [24]. It is thus intriguing to postulate that abnormal activation of RhoA/ROCK pathway in T1D may play a multifaceted role in the pathogenesis of T1D via its ability to contribute not only to the autoimmune aspects of T1D but also to some of the complications that are known to accompany this disease. It is also important to note that the beneficial effects of Fasudil in the development of diabetes in NOD mice may reflect inhibition not only of ROCK2 but also of ROCK1 since Fasudil can interfere with the function of both isoforms. The use of genetic approaches and/or the development of selective ROCK2 inhibitors will provide important insights into this issue and further delineate the relative importance of these two kinases in the pathogenesis of T1D.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Alliance for Lupus Research and by the NIH (HL62215 to AP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Tisch R, Wang B. Dysregulation of T cell peripheral tolerance in type 1 diabetes. Adv Immunol. 2008;100:125–49. doi: 10.1016/S0065-2776(08)00805-5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 3.Datta S, Sarvetnick NE. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS One. 2008;3:e3118. doi: 10.1371/journal.pone.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 5.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A. 2008;105:14028–33. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland AP, Van Belle T, Wurster AL, Suto A, Michaud M, Zhang D, Grusby MJ, von Herrath M. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes. 2009;58:1144–55. doi: 10.2337/db08-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridgway WM, Peterson LB, Todd JA, Rainbow DB, Healy B, Burren OS, Wicker LS. Gene-gene interactions in the NOD mouse model of type 1 diabetes. Adv Immunol. 2008;100:151–75. doi: 10.1016/S0065-2776(08)00806-7. [DOI] [PubMed] [Google Scholar]
- 8.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–18. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–24. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Otonkoski T, Vaarala O. IL-17 immunity in human Type I Diabetes. J. Immunol. 2010 doi: 10.4049/jimmunol.1000788. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4 Binding Protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;299:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105:20846–51. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas P, Bhagat G, Pernis AB. IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunological Reviews. 2010;233:79–96. doi: 10.1111/j.0105-2896.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010 doi: 10.1172/JCI42856. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol. 2008;439:181–9. doi: 10.1016/S0076-6879(07)00414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–9. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 20.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;568:242–7. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi Y, Yamada M, Imakiire T, Kushiyama T, Higashi K, Hyodo N, Yamamoto K, Oda T, Suzuki S, Miura S. A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol. 2007;192:595–603. doi: 10.1677/JOE-06-0045. [DOI] [PubMed] [Google Scholar]
- 22.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–23. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 23.Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57:1683–92. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]
- 24.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–26. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.