Abstract
Previously it was established that infusion of glial cell line-derived neurotrophic factor (GDNF) protein into grafts of embryonic dopamine cells has a neurotrophic effect on the grafted cells. In this study we used a nonviral technique to transfer the gene encoding for GDNF to striatal cells. Plasmid DNA encoding for GDNF was compacted into DNA nanoparticles (DNPs) by 10 kDa polyethylene glycol (PEG)-substituted lysine 30-mers (CK30PEG10k) and then injected into the denervated striatum of rats with unilateral 6-hydroxy-dopamine lesions. Sham controls were injected with saline. One week later, experimental animals received either a ventral mesencephalic (VM) tissue chunk graft or a cell suspension VM graft implanted into the denervated striatum. Grafts were allowed to integrate for 4–6 weeks and during this period we monitored spontaneous and drug-induced motor activity. Using stereological cell counting we observed a 16-fold increase in the number of surviving TH+ cells within tissue chunk grafts placed into the striatum pretreated with pGDNF DNPs (14,923 ± 4,326) when compared to grafts placed into striatum pretreated with saline (955 ± 343). Similarly, we observed a sevenfold increase in the number of TH+ cells within cell suspension grafts placed into the striatum treated with pGDNF DNPs when compared to cell suspension grafts placed into the saline dosed striatum. Behaviorally, we observed significant improvement in rotational scores and in spontaneous forepaw usage of the affected forelimb in grafted animals receiving prior treatment with compacted pGDNF DNPs when compared to grafted animals receiving saline control pretreatment. Data analysis for protein, morphological, and behavioral measures suggests that compacted pGDNF DNPs injected into the striatum can result in transfected cells overexpressing GDNF protein at levels that provide neurotrophic support for grafted embryonic dopamine neurons.
Keywords: Glial cell line-derived neurotrophic factor (GDNF), DNA nanoparticles, Gene transfer, Parkinson’s disease
INTRODUCTION
Experimental studies using fetal implants to improve motor function in animals with experimental Parkinson’s disease have shown promise (2,4,8,53) while clinical trials using fetal implants have shown only limited therapeutic benefit to Parkinson’s patients (15,19,22,23, 26,32,35). The limited success of neural transplants may be attributed to the significant cell loss that occurs following transplantation into the brain. Experimental evidence suggests that a majority of grafted ventral mesencephalic cells succumb to necrotic or apoptotic cell death soon after the grafting procedure and as early as the first 4 days after grafting (11,31,42,43). The adult brain may not contain the appropriate amount of growth factors that are required for the maintenance and survival of grafted neurons, particularly following the influx of a large numbers of exogenous cells into a brain region that harbors a limited source of nutrients and growth factors. To overcome this obstacle, attempts have been made to supplement neural grafts with growth factors as a means to enhance the survival of the grafted dopamine neurons [for review see (9,52)]. In particular, glial cell line-derived neurotrophic factor (GDNF) has been used as a neurotrophic factor supplement to increase the survival of grafted dopamine neurons. It has been shown that pre-treatment of grafts with GDNF (3,44), direct infusion of GDNF protein into the graft (36,41,49), release of GDNF from cells encapsulated by polymers (1,38), or cografts of cells secreting GDNF (48) increase of the survival and function of grafted dopamine neurons. More recently, viral vector technology has been used to overexpress GDNF at the graft site as a means to enrich the neurotrophic environment for grafted cells (12,16). In the present study we used a nonviral gene therapy technique to compact plasmid DNA encoding for GDNF and then injected these nanoparticles into the denervated striatum in order to transfect striatal cells and overexpress GDNF prior to the grafting procedure.
Compacted DNA nanoparticles (DNPs) represent a promising nonviral technology that is safe and has been shown to be effective in the lung (54,55), nasal mucosa (19), eye (13), and brain (51). Single molecules of plasmid DNA can be compacted by polycations to form colloidally stable nanoparticles that have the minimum possible theoretical size based on the partial specific volumes of the constituent components (24). A preferred polycation is polyethylene glycol substituted lysine 30-mer peptides, which facilitate cell entry and nondegradative trafficking to the nuclei of postmitotic cells by associating with cell surface nucleolin protein (10,24,54); other laboratories have shown that nanoparticles <25 nm in size use nondegradative pathways to reach the perinuclear region (20). These particles are completely synthetic, can be designed to diminish an adverse immune response, are stable in saline and nuclease-rich environments, and can be formulated as particles with minimum diameters of 8–11 nm (14). In postmitotic cells, strands of naked DNA that ultimately survive cell entry and the cytoplasmic environment must then pass through the nuclear membrane pore, which presents another obstacle given the extended size of hydrated DNA. Nanoparticle technology can circumvent these problems by: 1) protecting DNA from degradation, and 2) facilitating transit of compacted DNA, if the nanoparticles are sufficiently small, across the 25-nm nuclear membrane pore of post-mitotic cells (24). When dosed in the lung, compacted DNA generates several hundred fold increased transgene activity compared to naked DNA in postmitotic lung epithelial cells (52). In the mouse retina, local delivery of compacted DNA transfects >90% of postmitotic photo-receptor cells (13).
In this study we evaluated compacted DNPs following direct injection into the rat brain adjacent to the graft site. The study was designed to determine whether this technique could be used to overexpress GDNF protein in the denervated striatum to levels that provided neurotrophic support for embryonic dopamine neurons grafted as tissue chunks or cell suspensions.
MATERIALS AND METHODS
Plasmid Construction
DNA vectors were constructed using standard molecular biology techniques, following by restriction analysis and sequencing of subcloned regions (37). pKCPIRlucBGH (5,352 bp) is described in Ziady et al. (54). pUL: The CpG-depleted luciferase gene was amplified from the plasmid pModLucSh (Invivogen) using 5′-ATACAC CATGGAGGATGCCAAGAATATTAAG and 5′-ATA CAACTAGTCTAGATTATTTGCCACCCTTCTTGG CCT primers. The stop codon (TAA) and two restriction sites (XbaI and SpeI) were added to the 3′ end during amplification. The obtained PCR fragment was digested with NcoI and SpeI and subcloned into the pCpG-mcs/NcoI/NheI vector (Invivogen). Then hEF1-α promoter and synthetic intron were deleted using SpeI and NcoI. The polyubiquitin C promoter, first exon and intron sequence were amplified from the pUbLux plasmid (17) using 5′-ACATATCTAGACTGCAGGCCTCC-GCGC CGGGTTTTG and 5′-GTCTTCCATGGTGGCTAGCT CGTCTAACA primers. Additional XbaI, PstI, and NcoI sites were added during amplification. The PCR fragment was digested with XbaI and NcoI and subcloned into the prepared promoterless SpeI/NcoI vector described above. The mCMV enhancer was deleted from the obtained vector using PstI. pGDNF (3,997 bp): The GDNF gene open reading frame was cut from pLenti-GDNF-IRES-GFP/BamHI (blunted with Klenow)/NheI (5,6) and subcloned into pUL/XbaI (blunted with Klenow)/NheI vector. Figure 1 shows the plasmid map for pGDNF.
Figure 1.
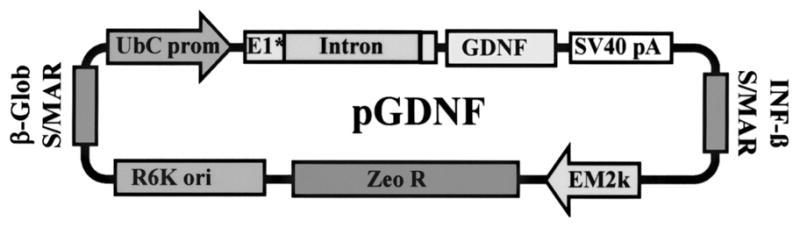
Plasmid map for pGDNF.
Preparation of Condensing Peptide
L-Cysteinyl-poly-L-lysine (UCB Bioproducts, Inc.) was conjugated with 10-kDa polyethylene glycol (PEG) (Nektar Therapeutics) as described in Liu et al. (24) except that trifluoroacetate counterion was replaced with acetate by size-exclusion chromatography on Sephadex G-25 before lyophilization of the PEGylated peptide.
Formulation of Compacted DNA Nanoparticles
Compacted DNA was manufactured by adding 20 ml of DNA solution (0.1 mg/ml in water) to 2.0 ml of PEGylated condensing peptide (3.2 mg/ml in water) at a rate of 4 ml/min by a syringe pump and through sterile tubing ended with a blunt cannula. During this addition, the tube with peptide was vortexed at a controlled rate so that the two materials mixed instantaneously. Peptide and DNA were formulated at a final amine-to-phosphate ratio of 2:1. The compacted DNA was then filtered through a vacuum-driven sterile filter with a 0.2-μm polyethersulfone membrane. The filtered compacted DNA was then concentrated 20–30-fold using VIVASPIN centrifugal concentrators (MWCO 100k). The concentrated DNA was then diluted 20–30-fold with sterile 0.9% NaCl for injection (Baxter) and concentrated again 20–30-fold to remove excess peptide and exchange water with physiologic saline. The final concentration of compacted DNA was 3.5 mg/ml. After formulation, the compacted DNA underwent several quality control tests, including sedimentation, turbidity, gel electrophoresis, transmission electron microscopy, and fluorescamine assays, as described in Ziady et al. (54) and Liu et al. (24). Also, endotoxin levels were checked using an ENDOSAFE® PTS (Portable Test System) manufactured by Charles River Laboratories and were <5 EU/mg of DNA. Estimated number of pGDNF nanoparticles are 8.0 × 1011 particles/μl.
Animals
Male Sprague-Dawley rats were obtained from Harlan Farms and used in this study. Animals were housed in environmentally regulated rooms and had free access to food and water for the duration of the study. All animal procedures were conducted in strict compliance with approved institutional protocols, and in accordance with the provisions for animal care and use described in the Guide for the Care and Use of Laboratory Animals (28).
6-Hydroxydopamine Lesion
To establish the Parkinson’s disease model, all rats were given unilateral 6-hydroxydopamine (6-OHDA) lesions of the left nigrostriatal pathway; 6-OHDA (Sigma) was dissolved in 0.9% saline (containing 0.2% ascorbic acid) at a concentration of 3.0 μg/μl and stereotaxically injected into the nigrostriatal pathway of anesthetized rats at a rate of 1.0 μl/min for 2 min. Each rat received two injections of 6-OHDA: one injection in the medial forebrain bundle (AP −4.4, ML 1.2, DV −7.5) area and the other in the rostral pars compacta of the substantia nigra (AP −5.3, ML 2.0, DV −7.5); all coordinates reported in this study represent millimeter adjustments from bregma (AP, ML) and below the dural surface (DV) with the top of the skull in a flat position. This technique routinely produces complete lesions of dopamine neurons in the A9 and A10 midbrain regions, and near complete denervation of dopaminergic fibers innervating the ipsilateral striatum.
Compacted pGDNF DNPs Injections
Five weeks following 6-OHDA lesion, animals were divided into two groups: compacted pGDNF injection or saline (sham). Animals were anesthetized and placed in a stereotaxic apparatus. A 30-gauge needle was used to administer a left intrastriatal injection of 28.0 μg of compacted pGDNF DNPs (3.5 μg/μl) or saline at a rate of 0.5 μl/min in four sites (AP +0.5, ML +2.5, DV1 −6.5, DV2 −5.0; AP +0.5, ML +4.0, DV1 −6.5, DV2 −5.0). Needle was left in place for 2 min following each injection and withdrawn from brain at a rate of 1 mm/min. Prior studies indicate effective brain delivery of the nanoparticles with minimal backflow (51).
Tissue Dissection for ELISA Assays
For striatal dissections, brains were removed from euthanized animals and then placed upside down (ventral surface facing upwards) into a stainless steel brain matrix (RMB-4000C, ASI Instruments). For the following descriptions, the approximate stereotaxic coordinates are listed in parentheses. The initial coronal cut was targeted at a level just caudal to the optic chiasm (~AP −0.5) and a second cut was made 3.0 mm anterior to the initial cut. The 3.0-mm-thick coronal slab formed by these two cuts was removed from the brain matrix and then divided into two pieces along the midline. Both the left and right striatum were dissected from the two pieces. A rectangular piece of tissue was dissected by making the following cuts: 1) a cut parallel to the midline was made just lateral to the lateral ventricle, 2) a cut parallel to the midline was made just medial to the lateral aspect of striatum at the striatum/corpus callosum border, 3) a cut perpendicular to the midline was made just ventral to the dorsal aspect of the striatum at the striatum/corpus callosum border, and 4) a cut perpendicular to the mid-line was made just dorsal to the anterior commissure.
Quantification of GDNF by Enzyme-Linked Immunosorbent Assay (ELISA)
Protein levels of GDNF were measured in tissue samples. Each tissue sample was weighed and immediately frozen on dry ice. Subsequently, each tissue sample was homogenized in 300 μl volume of homogenate buffer [400 mM NaCl, 0.1% Triton X, 2.0 mM EDTA, 0.1 mM benzethonium chloride, 2.0 mM benzamidine, 0.1 mM PMSF, aprotinin (9.7 TIU/ml), 0.5% BSA, 0.1 M phosphate buffer, pH 7.4]. The homogenate was centrifuged for 10 min at 10,000 × g at 4°C. Tissue homogenates were assayed using a GDNF Emax™ ImmunoAssay System (Promega; Madison, WI).
Ventral Mesencephalic Tissue Chunk Grafts
One week after the compacted pGDNF nanoparticle injections, animals were anesthetized and placed in a stereotaxic apparatus. At the same time, the ventral mesencephalon was dissected from E14 fetuses obtained from timed pregnant Sprague-Dawley rats (Harlan Farms) and stored individually in a cold, sterile, calcium/magnesium-free buffer (CMF; 0.15 M NaCl, 8.0 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KHPO4, 26.0 mM NaHCO3, 0.1% glucose, 100 mg/ml streptomycin, 2.5 mg/ml fungizone, pH 7.2). The ventral mesencephalon from a single fetus was drawn into the blunt end of a 22-gauge spinal needle and stereotaxically placed into the denervated striatum of the recipient animal at the following coordinates: AP +0.5, ML +3.3, DV −5.8.
Ventral Mesencephalic Tissue Cell Suspension Grafts
One week after the compacted pGDNF nanoparticle injections, the ventral mesencephalon was dissected from E14 fetuses obtained from timed pregnant Sprague-Dawley rats (Harlan Farms) and stored in a cold, sterile, oxygenated calcium/magnesium-free buffer (CMF; 0.15 M NaCl, 8.0 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KHPO4, 26.0 mM NaHCO3, 0.1% glucose, 100 mg/ml streptomycin, 2.5 mg/ml fungizone, pH 7.2). Embryonic tissue was pooled and rinsed with CMF; a cell suspension was prepared using a 0.125% trypsin solution (Invitrogen, Carlsbad, CA) followed by inactivation with 1.0 ml newborn calf serum (Invitrogen) and 500 μl of DNase (0.5 mg/ml) in 3.5 ml Hank’s balanced salt solution (HBSS) (Invitrogen). Cells were triturated with a 22-gauge needle 15 times, placed drop-wise on top of sterile fetal bovine serum (Invitrogen), and spun at 1000 rpm for 10 min. Fetal bovine serum was aspirated and 1 ml of HBSS was added to cell pellet and triturated to create a single cell suspension. Cells were counted using the trypan blue exclusion technique and a suspension was made at a concentration of 200,000 cells/μl. Cells were drawn up in a 30-gauge needle and 2 μl of cell suspension was injected into the denervated striatum at a rate of 0.5 μl/min at the following coordinates: AP +0.5, ML +3.3, DV −5.8.
Spontaneous Motor Test (Cylinder Test)
Spontaneous exploratory limb usage was analyzed using a modified forelimb asymmetry technique (39). Rats were placed into a clear, Plexiglas cylinder (25 cm tall, 16 cm ID) and spontaneous activity was videotaped for 5 min. Videotapes were played back in order to quantify the number of times the rat placed the left, right, or both forepaws on the wall of the chamber; the experimenter was unaware of the treatment group. The total number of forepaw touches was counted for each animal during the 5-min session and the percent usage of the left or right forepaw was calculated by dividing the counts for individual left or right forepaw touches by the total number of forepaw touches. Animals with severe 6-OHDA lesions typically use the ipsilateral fore-paw 90% of the time (e.g., a rat with a lesion of the left nigrostriatal pathway will use the left forepaw 90% of time during exploratory behavior). On the average, naive rats show equal usage of right and left forepaws during exploratory behavior.
Amphetamine-Induced Rotational Behavior
Animals with 6-OHDA lesions were given a systemic injection of d-amphetamine (5 mg/kg) in saline and placed inside opaque 16-in.-diameter cylindrical chambers that were positioned directly beneath a video camera. The video camera was connected to a Videomex V image motion computer system (Columbus Instruments, Columbus, OH). The total number of 360° clockwise or counterclockwise rotations was measured during each 90-min test session. Scores were taken at 4 and 5 weeks postlesion and 4 and 5 weeks postgraft.
Immunohistochemistry
All rats were anesthetized with Fatal-Plus™ (0.88 ml/kg, IP; Vortech Pharmaceuticals, Dearborn, MI) and transcardially perfused with cold 0.9% saline followed by 4% buffered paraformaldehyde (pH 7.4 in 0.1 M phosphate buffer). Brains were postfixed overnight in 4% paraformaldehyde and transferred to 30% sucrose. Sections (40 μm) were cut using a sliding microtome and placed into a cryoprotectant solution at −20°C (47). For immunohistochemical detection of markers, free-floating sections were first rinsed in 0.1 M phosphate buffer (22 mM NaH2PO4 and 80 mM K2HPO4, pH 7.2) followed by 3% H2O2 treatment to inhibit endogenous peroxidase activity. Sections were then rinsed in 0.1 M PO4 and 0.1 M PO4-Triton followed by an overnight incubation in primary antisera containing a monoclonal antibody against tyrosine hydroxylase (TH; 1:4000; Chemicon). The sections were then incubated in an affinity-purified biotinylated goat anti-mouse IgG secondary antibody (1:400, Chemicon) and then incubated in an avidin-biotin-peroxidase complex (Vector Laboratories). Staining was completed by placing sections in a 0.003% H2O2 solution containing diaminobenzidine chromagen to visualize the reaction.
Stereology
An Olympus BH-2 microscope was used to visualize tissue sections. We used Bioquant Life Science version 8.12.20 for stereological cell quantification using the optical fractionator method (40). The graft was delineated at 4× and TH+ cells were subsequently counted at 40× with a 100 × 100-μm2 grid cell area and a 100 × 100 μm2 counting grid. We examined every third section (40 μm thick) through the entire rostral–caudal length of the graft. Optical dissector height was 30 μm with a guard volume set at 5 μm. Graft volume estimates were made using the Cavalieri method (27).
Statistical Analyses
The α level of significance was set at p < 0.05. Analysis of variance (ANOVA) or t-tests were used for statistical analyses; choice of test was dependent on the experimental design. For rotational behavior and cylinder test scores, data were analyzed using repeated measures ANOVA. Where appropriate, all statistically significant interactions were followed up with the post hoc Tukey Test for mean comparisons. For descriptive statistics, means are reported with their corresponding SEM.
RESULTS
Experimental Design
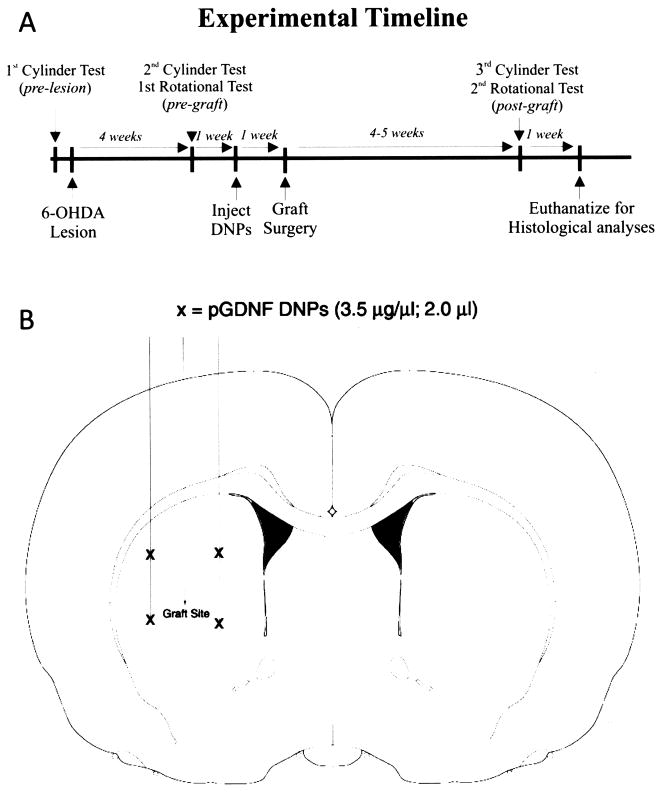
Figure 2A illustrates the experimental timeline of these studies. Rats received unilateral 6-OHDA lesions of the left nigrostriatal pathway. Lesion severity was determined 4 weeks later using amphetamine-induced rotational behavior and the cylinder test. One week later, animals received four deposits of pGDNF DNPs or sham injections of saline into the denervated (left) striatum as shown in Figure 2B. One week after the intrastriatal injections of pGDNF DNPs or saline, either a cell suspension or tissue chunk graft of fetal VM tissue was placed between the injection sites of DNPs, as shown in Figure 2B. Grafts were allowed to integrate for a 6-week postgraft period.
Figure 2.
(A) Experimental protocol timeline of 6-OHDA, DNP (or saline), and graft injections, including time points for functional and immunohistochemical analyses. (B) Diagram showing injection sites for DNPs, saline, or grafts. Each “X” indicates a site where 2.0 μl of saline (sham) or pGDNF DNPs (3.5 μg/μl) were injected. Saline or DNPs were injected into the striatal sites 1 week prior to graft surgery. Cell suspension or chunk grafts were placed in between the four deposit sites.
GDNF Protein Levels in the Striatum Transfected With pGDNF DNPs
Three naive animals received left intrastriatal injections of pGDNF DNPs in the same sites shown in Figure 2B but did not receive grafts. One week later animals were euthanatized and the left and right striata were dissected for protein analysis. For GDNF protein levels, there was a significant effect of side [t(4) = 9.57, p < 0.001] with GDNF levels of 0.55 ± 0.04 ng/g tissue on the injected side when compared to 0.07 ± 0.02 ng/g tissue of GDNF on the noninjected side. Previously we reported a short term (1–2 week) upregulation of GDNF protein in the denervated striatum following a 6-OHDA lesion (50); however, subsequent unpublished data from our laboratory has shown that at 4 weeks postlesion, GDNF protein levels in the denervated striatum are equivalent to GDNF protein levels in the intact striatum so we do not anticipate there was any compensatory upregulation of GDNF within the denervated striatum that was due to the lesion itself at the time the samples were taken.
Effect of Brain Transfection/Graft on Behavior (Rotational Behavior and Cylinder Test)
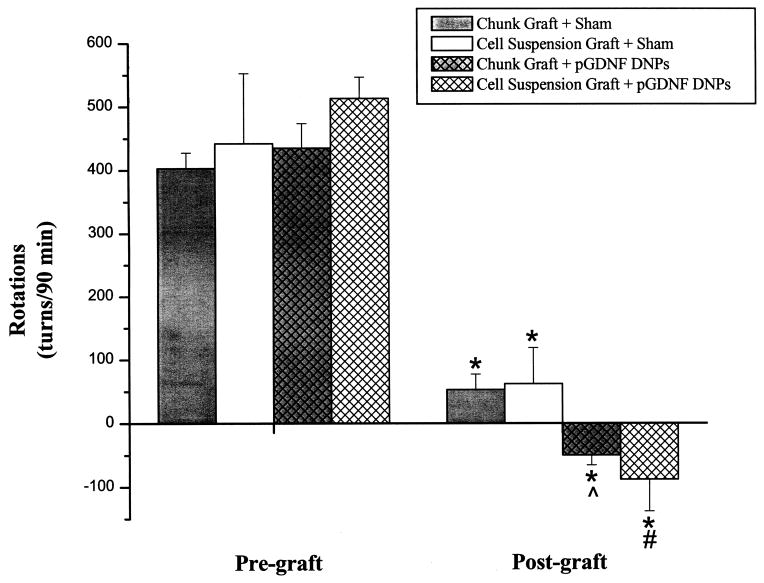
Rotational behavior was initially tested 4 weeks following a unilateral 6-OHDA lesion of the left nigrostriatal pathway. Pregraft scores, shown in Figure 3, indicate that animals in each treatment group had similar lesions. Rotational scores were tested again 4–5 weeks postgraft. Figure 3 shows grafts in all treatment groups successfully attenuated rotational behavior. Statistical analysis of rotational scores revealed a significant Treatment × Time interaction for the chunk graft treatments [F(1, 6) = 4.40, p = 0.04] and cell suspension graft treatments [F(1, 14) = 4.31, p = 0.04]. Furthermore, post hoc analysis of mean rotational scores indicate postgraft rotational scores for the pGDNF DNP treatment groups were significantly lower than scores for saline treatment groups for both graft types [p = 0.02 pGDNF DNPs + chunk graft vs. saline + chunk graft (postgraft); p = 0.03 pGDNF DNPs + cell suspension graft vs. saline + cell suspension graft (postgraft)].
Figure 3.
Rotational behavior. Amphetamine was administered (5.0 mg/kg, IP) 4 weeks after the 6-OHDA lesion (Pre-graft) and 4–5 weeks after the graft procedure. Bars represent the mean rotational scores (+SEM) for animals receiving pGDNF DNPs + cell suspension graft (n = 9), saline + cell suspension graft (n = 7), pGDNF DNPs + chunk graft (n = 5), or saline + chunk graft (n = 5). *p < 0.05 Post-graft versus Pre-graft for all treatment groups; ^p < 0.05 pGDNF DNPs + chunk (Post-graft) versus saline + chunk graft (Post-graft), #p < 0.05 pGDNF DNPs cell suspension (Post-graft) versus saline + cell suspension graft (Post-graft).
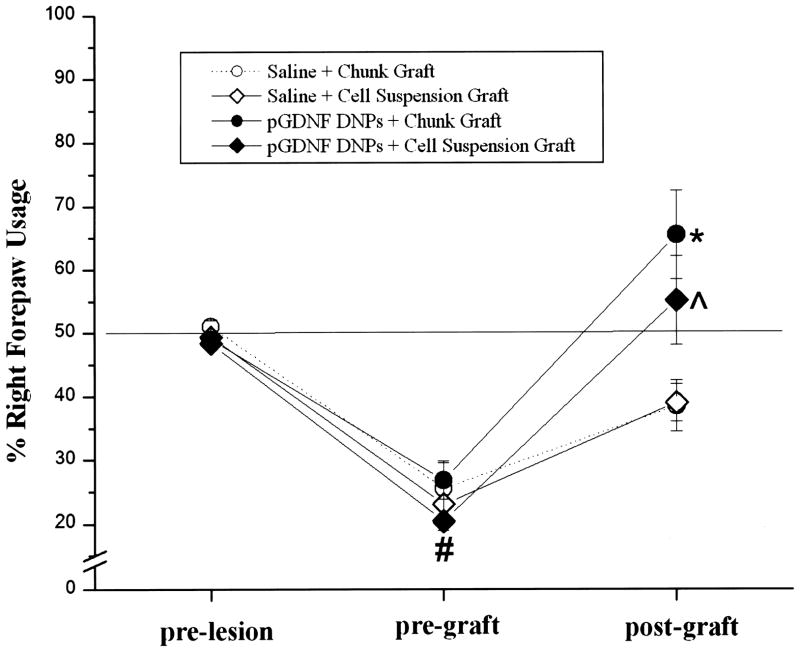
Spontaneous forelimb activity was monitored in each animal using the cylinder test. Cylinder tests scores were obtained 1 week prior to the 6-OHDA lesion (prelesion), 4 weeks postlesion and 1 week before sham or DNP treatment (pregraft), and 4–5 weeks postgraft. Figure 4 summarizes the results of these tests. Statistical analysis revealed a significant Treatment × Time for these experiments: for tissue chunk grafts [F(1, 6) = 10.12, p < 0.01] and for cell suspension treatments [F(1, 8) = 6.40, p < 0.01]. All groups showed a significant reduction in contralateral forepaw usage following the lesion (pre-graft). Animals receiving tissue chunk grafts or cell suspensions grafts that were also pretreated with pGDNF DNPs showed a statistically higher percentage of right forepaw usage at the postgraft time point when compared to grafted animals receiving saline pretreatment.
Figure 4.
Cylinder test. Animals were tested 1 week before receiving a unilateral 6-OHDA lesion (pre-lesion), 4 weeks after the lesion but before the transfection procedure (pre-graft), and then 4–5 weeks after receiving a fetal VM graft (post-graft). Symbols represent the average percent right forepaw usage (±SEM) for animals receiving pGDNF DNPs + cell suspension graft (n = 9), saline + cell suspension graft (n = 7), pGDNF DNPs + chunk graft (n = 5), or saline + chunk graft (n = 5). *p < 0.05 pGDNF DNPs chunk graft (post-graft) versus saline + chunk graft (post-graft) or pGDNF DNPs + chunk graft (pre-graft); ^p < 0.05 pGDNF DNPs + cell suspension graft (postgraft) versus saline + cell suspension graft (post-graft) or pGDNF DNPs + cell suspension graft (pre-graft); #p < 0.05 prelesion versus pre-graft for all treatment groups.
Histological Analysis of Grafts Implanted Into the Striatum Transfected With pGDNF DNPs
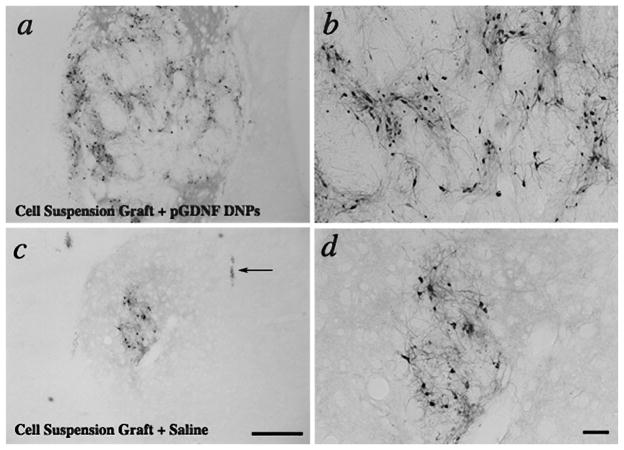
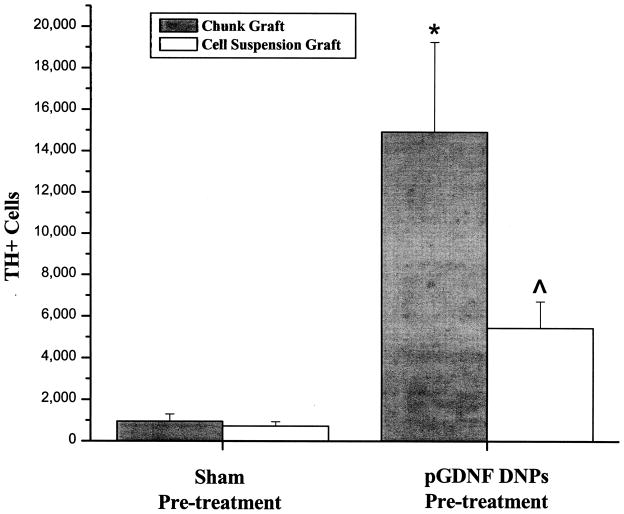
Figures 5 and 6 show typical examples of TH immunohistochemistry for cell suspension or chunk grafts 6 weeks postgrafting. In grafts containing 400,000 cells prior to transplantation, we normally see a low yield of TH+ cells 1 month following transplantation, as shown in the left photomontage in Figure 7 for a suspension graft. On the other hand, grafts placed into the striatum treated with pGDNF DNPS show larger grafts and more extensive fiber outgrowth from the graft. Moreover, using a stereological cell counting technique to quantify the number of TH+ cells within each graft (Fig. 8), we determined there was a statistically significant effect of treatment, with 16-fold more TH+ cells within chunk grafts placed into the striatum pretreated with pGDNF DNPs (14,923 ± 4,326) than in chunk grafts placed into the sham pretreated striatum (955 ± 343) [t(6) = 3.49, p < 0.05]. Similarly, for cell suspension grafts we determined there was a statistically significant effect of treatment, with sevenfold more TH+ cells within cell suspension grafts placed into the striatum pretreated with pGDNF DNPs (5,445 ± 1,272) than in suspension grafts placed into the sham pretreated striatum (727 ± 208) [t(6) = 2.73, p < 0.05].
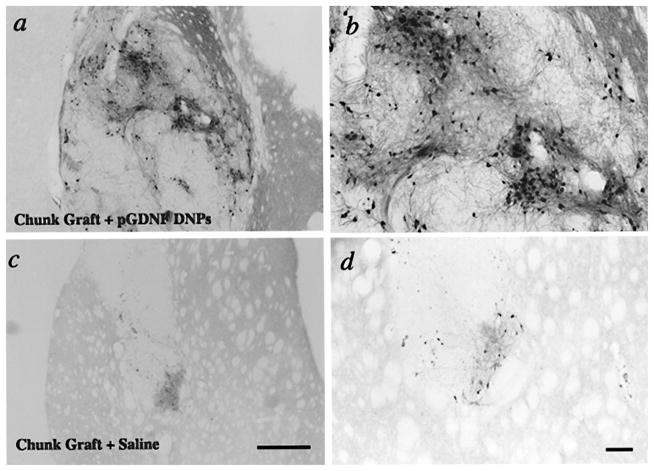
Figure 5.
Low power (a, c) and higher power (b, d) photomicrographs of cell suspension grafts immunostained for tyrosine hydroxylase (TH) 6 weeks postgrafting. Grafts were pretreated with compacted pGDNF DNPS (a, b) or saline (c, d) 1 week prior to graft surgery. Arrow in (c) points to cannula track for sham injection. Scale bars: (c) 500 μm, (d) 100 μm.
Figure 6.
Low power (a, c) and higher power (b, d) photomicrographs of chunk grafts immunostained for tyrosine hydroxylase (TH) 6 weeks postgrafting. Grafts were pretreated with compacted pGDNF DNPS (a, b) or saline (c, d) 1 week prior to graft surgery. Scale bars: (c) 500 μm, (d) 100 μm.
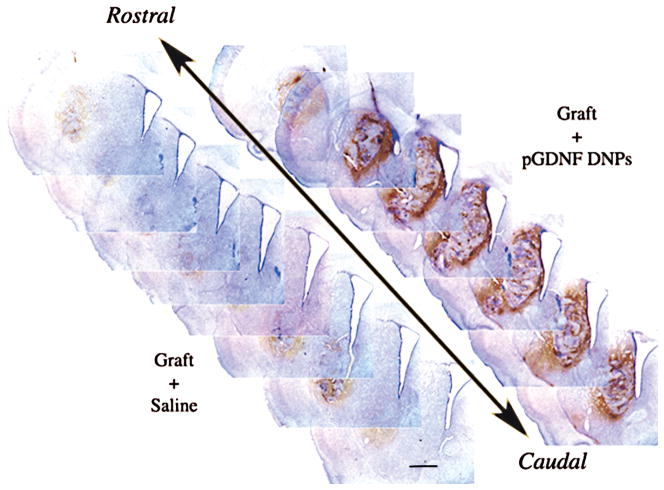
Figure 7.
Photomontage of serial brain sections immunostained for tyrosine hydroxylase (TH) and showing the denervated/grafted striatum in an animal that was pretreated with compacted pGDNF DNPS (right) or saline (left) 1 week prior to suspension graft surgery. Chronology of treatments: animals received a complete lesion of the nigrostriatal pathway, 6 weeks later either compacted pGDNF nanoparticles or saline was injected into the denervated striatum and then 1 week later a fetal VM cell suspension grafts was implanted into the denervated/transfected striatum. Grafts developed in the striatum for 6 weeks before animals were euthanatized and brains removed for histological examination. Brain sections are 40 μm thick and every third section through the graft site is shown for each animal; sections are arranged so that the rostral striatum is near the top and caudal striatum is at bottom. Graft cells were taken from the ventral mesencephalon of E13–E14 fetuses (200,000 cells/μl; 2.0 μl). Scale bar: 1 mm.
Figure 8.
pGDNF DNPs increases the number of TH+ cells in cell suspension or chunk grafts. Tissue was analyzed 6 weeks postgrafting. Bars represent mean number of TH+ cells in each graft (+SEM). *p < 0.05 pGDNF DNPs + chunk graft versus saline + chunk graft; ^p < 0.05 pGDNF DNPs + cell suspension graft versus saline + cell suspension graft.
Graft volumes were estimated using the Cavalieri method and are reported in Table 1. We observed an eightfold increase in graft volume for chunk grafts implanted into the striatum pretreated with pGDNF DNPs and a fourfold increase in graft volume size for cell suspension grafts implanted into striatum transfected with pGDNF DNPs.
Table 1.
Graft Volume (mm3)
| Treatment | Graft Type |
|
|---|---|---|
| Chunk | Cell Suspension | |
| pGDNF DNPs | 1.28 ± 0.31* | 0.85 ± 0.11* |
| Saline | 0.15 ± 0.02 | 0.19 ± 0.04 |
p < 0.05 versus saline.
DISCUSSION
Here we report that plasmid DNA encoding for the neurotrophic factor GDNF can be compacted into rodlike nanoparticles ranging in size from 8 to 11 nm in diameter, injected into the denervated striatum, and effectively transfect cells in the brain so that transgene expression of GDNF attained levels that are neurotrophic for grafted dopamine neurons. This nonviral nucleic acid delivery technology has largely overcome various physiologic barriers to introducing DNA into the nuclei of nondividing cells. This technology is based on condensation of single molecules of nucleic acids with polyethylene glycol (PEG)-substituted lysine peptides to formulate nanoparticles having the minimum possible theoretical size based on the partial specific volumes of lysine and DNA (24). These DNA nanoparticles are stable >3 years at 4°C, nonimmunogenic (repeat dosing in the lungs of mice has been demonstrated), noninflammatory (55), and very effective in the lung (18,54), eye (14), and more recently in the brain (51). In an intranasal cystic fibrosis clinical trial, no adverse events were attributed to the DNPs and most subjects demonstrated partial to complete electrophysiologic correction of the CFTR chloride channel defect (19).
This study was designed to examine the short term (4–6 weeks) effects of this nonviral gene therapy technique. A previous study that used these same DNPs demonstrated reporter gene expression in the striatum 8–11 weeks following the injection of DNPs (51). We also observed overexpression of GDNF in the striatum that lasted at least 3 weeks postinjection (longest time point measured). Therefore, injecting the DNPs 1 week prior to grafting would provide at least 2 weeks of GDNF overexpression during the postgraft period. Previously we showed that osmotic minipump infusion of GDNF protein into the graft site during the first 2 weeks postgrafting substantially increased the survival of TH+ cells in single chunk grafts (47). In terms of the survival of TH+ cells, the effect of transfecting the striatum with pGDNF DNPs and overexpressing GDNF protein in the transplant site exceeded what we observed following the direct infusion of GDNF protein into the transplant site, which was a three to fourfold increase in the number of surviving TH+ cells (49). While it is possible that GDNF overexpression may have continued beyond the 2-week postgrafting period in the present study and for this reason may have contributed to the greater survival of grafted TH+ cells, there may be other reasons why we observed a difference between the studies. As discussed earlier, grafted embryonic cells are most susceptible to necrotic and apoptotic cell death during the first week postgrafting. Neurotrophic factor intervention during this brief period may be all that is required to keep these cells viable in the short term. We must also consider that in the present study four transfection sites surrounding the graft were used for pGDNF DNP treatment while only a single injection site was used in the earlier GDNF protein infusion study, which may have resulted in smaller brain volumes having neurotrophic support. In summary, we speculate that GDNF protein levels as well as volume of brain distribution may account for the superior results seen in this DNP gene transfer study compared to the prior GDNF protein infusion protocol.
Volumetric analysis indicates graft size increased four to eightfold when the grafts were implanted in between the four sites injected with pGDNF DNPs. One of the grafts in the chunk graft/pGDNF DNP group was 14 times larger (2.1 mm3) than the mean volume measurement for grafts in the chunk graft/sham group (0.15 mm3). Not only did we observe an increase in the survival of grafted TH+ cells, we observed larger grafts in general suggesting a trophic effect to the nondopaminergic components of the grafts as well. We were unable to measure fiber outgrowth in this study because some of the pGDNF DNP-treated grafts were so large that they occupied most of the medial-to-lateral extent of the striatum (Fig. 5a). Future studies will require an adjustment of the DNP dosing in order to correct for oversized grafts.
Functionally, grafts placed into the denervated striatum that was previously transfected with pGDNF DNPs provided better compensatory effects in terms of improved scores for forelimb activity. While both cell suspension and chunk grafts treated with saline showed a trend for improved cylinder test scores, their postgraft scores were not statistically greater than their pregraft scores (p = 0.20 for chunk grafts; p = 0.61 for cell suspension grafts). Rotational behavior indicates all grafts in all treatment groups were functional in terms of reducing amphetamine-induced rotational behavior, and prior transfection of the striatum with pGDNF DNPs resulted in an even higher reversal of rotational behavior than grafts alone. It is interesting that we observed over a 2.5-fold increase in the number of TH+ cells in the chunk graft/pGDNF DNP group when compared to the cell suspension graft/pGDNF DNP group and similar rotational and cylinder scores for both groups. In some cases it is possible that the large size of grafts in the chunk graft/pGDNF DNP group may have displaced a large amount of striatal tissue and actually interfered with the ability of the graft to successfully reinnervate the host striatum and, as a consequence, limited the degree of functional recovery.
The size of the DNPs used in this study may be an important factor for the successful transfection of brain. Several studies using DNPs with diameters in the range of 80–100 nm reported only short-term transgene expression and limited success. For instance, Leone et al. (21) used a nonviral, lipid entrapped, polycation condensed delivery system (LPD) to induce transgene expression in the brains of rodents, primates, and humans; these nanoparticles had an average particle size of 80–100 nm in diameter. However, this nonviral system demonstrated only limited success. Polyethylenimine/DNA (PEI/DNA) complexes have been used as a nonviral gene technique to delivery DNA to mammalian cells (29). Oh et al. injected PEI/DNA nanoparticles (~100 nm) into dog brain with minimal toxicity (30); however, in this same study it was also reported that intracerebral injection of plasmids encoding for reporter genes as naked plasmids or PEI/DNA complexes elicited nearly equivalent levels of transgene activity at 3 days, suggesting no real benefit for the PEI/DNA complexes. Efficiency problems in initial nanoparticle gene delivery studies were most likely due to the fact that these complexes were still too large to diffuse efficiently in the extracellular brain matrix (46) or cross the 25-nm nuclear membrane pore, or may have been degraded in the cell cytoplasm. More recently, we and others have demonstrated successful transfection of brain cells using smaller nanoparticles. For instance, Bharali et al. reported successful transgene expression of reporter genes and neurotrophic factor in brain following direct intracerebral injection of encapsulated DNA plasmids within ~30 nm silica nanoparticles (7). We reported successful long-term transgene expression in brain using PEG-substituted lysine peptide DNA rod-like nanoparticles (~8–12 nm in diameter) as a vehicle for gene delivery (51). Of note, polymeric small nanoparticles (<25 nm) but not larger nanoparticles (>42 nm) appear to enter cells and traffic through the cytoplasm of cells using a nondegradative pathway, which underscores the importance of using small size particles to deliver plasmid DNA to the nucleus (20).
Compacted DNA is an attractive alternative to viral vectors because of their noninflammatory and nonimmunogenic properties (13,55); immunogenicity is an inherent problem with many viral vectors (45), especially if viral vectors need to be redosed (25). In fact, compacted DNA can be repetitively dosed to murine airways without any decrement in transgene expression (Copernicus, unpublished data), suggesting that chronic administration is feasible. We observed only a minimal amount of immune activity within the injection site based upon macrophage (ED-1) and cytotoxic T cell (OX-8) immunohistochemical stains; these minor changes were found in both DNP and saline groups and therefore appear to be due to the injection procedure itself and not the DNPs (51). Because a major component of the nanoparticles is lysine polymers, the issue of poly-lysine toxicity needs to be addressed. One of the toxic effects of synthetic DNA complexes, such as poly-lysine or polyethyleneimine (PEI), is the activation of complement; the complement system, or the nonspecific defense system, protects vertebrate species from invasion by foreign organisms. Plank et al. demonstrated the activation of complement by poly-lysine is a function of the chain length of polylysine (33). In this study it was shown unbound long-chain (>50 mer) poly-lysine activated complement but that activation was substantially decreased for unbound short-chain (<50 mer) poly-lysine; this is one reason why we chose to use a small-chain 30-mer lysine polymer in our nanoparticle formulation. In a side-by-side comparison between poly-lysine and PEI, it was shown that unbound PEI was a much stronger activator of complement than unbound long-chain poly-lysine. Secondly, it was shown that the toxicity of poly-lysine is reduced when it is bound to other moieties, including DNA: poly-lysine/DNA complexes with neutral or slightly negative charge did not activate complement at all (33,34). This is significant because our compacted DNA nanoparticles are charge neutral or slightly negative. Moreover, conjugation of PEG and/or transferrin to unbound poly-lysine or poly-lysine/DNA complexes had a further protective effect against the activation of complement (33). These data suggest there is a minimal immunogenic response to the injection of poly-lysine/DNA nanoparticles.
In summary, we demonstrated a nonviral gene therapy technique can be used in combination with neural grafts to enhance both the survival of grafted embryonic dopamine neurons and functional recovery in an animal model of PD. DNA plasmids encoding for GDNF and compacted into nanoparticles were used to transfect the denervated striatum prior to graft implantation. Subsequently, grafts implanted into the denervated/transfected striatum were generally larger and contained substantially more TH+ cells than similar grafts implanted into the nontransfected denervated striatum. Functional recovery was also improved using this combined nonviral gene therapy/grafting technique. This study demonstrates the utility of using a nonviral, nanoparticle-based technology for transferring genes to brain cells as a possible therapeutic approach for treating neurodegenerative diseases.
Acknowledgments
This research was supported in part by NS50311 (D.M.Y.), Michael J. Fox Foundation for Parkinson’s Research (D.M.Y., M.J.C.), and the Jelm Foundation (D.M.Y.). M.J.C. is Senior VP Science and Medical Affairs at Copernicus, and holds stock and stock option equity interest in Copernicus.
References
- 1.Ahn YH, Bensadoun JC, Aebischer P, Zurn AD, Seiger A, Bjorklund A, Lindvall O, Wahlberg L, Brundin P, Kaminski Schierle GS. Increased fiber outgrowth from xeno-transplanted human embryonic dopaminergic neurons with co-implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor. Brain Res Bull. 2005;66(2):135–142. doi: 10.1016/j.brainresbull.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Annett LE. Functional studies of neural grafts in parkinsonian primates. In: Dunnett SB, Bjorklund A, editors. Functional neural transplantation. New York: Raven Press; 1994. pp. 71–102. [Google Scholar]
- 3.Apostolides C, Sanford E, Hong M, Mendez I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience. 1998;83(2):363–372. doi: 10.1016/s0306-4522(97)00369-2. [DOI] [PubMed] [Google Scholar]
- 4.Bankiewicz K, Mandel RJ, Sofroniew MV. Trophism, transplantation, and animal models of Parkinson’s disease. Exp Neurol. 1993;124(1):140–149. doi: 10.1006/exnr.1993.1185. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner BJ, Shine HD. Permanent rescue of lesioned neonatal motoneurons and enhanced axonal regeneration by adenovirus-mediated expression of glial cell-line-derived neurotrophic factor. J Neurosci Res. 1998;54(6):766–777. doi: 10.1002/(SICI)1097-4547(19981215)54:6<766::AID-JNR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner BJ, Shine HD. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci. 1997;17(17):6504–6511. doi: 10.1523/JNEUROSCI.17-17-06504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brundin P, Duan WM, Sauer H. Functional effects of mesencephalic dopamine neurons and adrenal chromaffin cells grafted to the rat striatum. In: Dunnett SB, Bjorklund A, editors. Functional neural transplantation. New York: Raven Press; 1994. pp. 9–46. [Google Scholar]
- 9.Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: A review over current approaches. Cell Transplant. 2000;9(2):179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16(2):333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- 11.Duan W-M, Widner H, Brundin P. Temporal pattern of host responses against intrastriatal grafts of syngeneic, allogeneic or xenogeneic embryonic neuronal tissue in rats. Exp Brain Res. 1995;104:227–242. doi: 10.1007/BF00242009. [DOI] [PubMed] [Google Scholar]
- 12.Elsworth JD, Redmond DE, Jr, Leranth C, Bjugstad KB, Sladek JR, Jr, Collier TJ, Foti SB, Samulski RJ, Vives KP, Roth RH. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211(1):252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, Moen RC, Cooper MJ. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13(13):1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- 15.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 16.Georgievska B, Carlsson T, Lacar B, Winkler C, Kirik D. Dissociation between short-term increased graft survival and long-term functional improvements in Parkinsonian rats overexpressing glial cell line-derived neurotrophic factor. Eur J Neurosci. 2004;20(11):3121–3130. doi: 10.1111/j.1460-9568.2004.03770.x. [DOI] [PubMed] [Google Scholar]
- 17.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, Hyde SC. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8(20):1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- 18.Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15(12):1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- 19.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 20.Lai SK, Hida K, Man ST, Chen C, Machamer C, Schroer TA, Hanes J. Privileged delivery of polymer nanoparticles to the perinuclear region of live cells via a non-clathrin, non-degradative pathway. Biomaterials. 2007;28(18):2876–2884. doi: 10.1016/j.biomaterials.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Leone P, Janson CG, Bilaniuk L, Wang Z, Sorgi F, Huang L, Matalon R, Kaul R, Zeng Z, Freese A, McPhee SW, Mee E, During MJ. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol. 2000;48(1):27–38. doi: 10.1002/1531-8249(200007)48:1<27::aid-ana6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 23.Lindvall O, Hagell P. Cell therapy and transplantation in Parkinson’s disease. Clin Chem Lab Med. 2001;39(4):356–361. doi: 10.1515/CCLM.2001.056. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, Payne JM, Miller TJ, Brunovskis P, Fink TL, Muhammad O, Moen RC, Hanson RW, Cooper MJ. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278(35):32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 25.Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: The role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7(5):347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14(5):507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouton PR. Principles and practices of unbiased stereology. Baltimore, MD: The Johns Hopkins University Press; 2002. [Google Scholar]
- 28.National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1997. [Google Scholar]
- 29.Nimesh S, Goyal A, Pawar V, Jayaraman S, Kumar P, Chandra R, Singh Y, Gupta KC. Polyethylenimine nanoparticles as efficient transfecting agents for mammalian cells. J Control Release. 2006;110(2):457–468. doi: 10.1016/j.jconrel.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, Pluhar GE, McNeil EA, Kroeger KM, Liu C, Castro MG, Lowenstein PR, Freese A, Ohlfest JR. Efficacy of nonviral gene transfer in the canine brain. J Neurosurg. 2007;107(1):136–144. doi: 10.3171/JNS-07/07/0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 1996;19(3):102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 32.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 33.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7(12):1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 34.Plank C, Mechtler K, Wagner E, Szoka FC. Complement activation by polylysine-DNA complexes. In: Grigoriadis G, McCormack B, editors. Targeting of drugs: Strategies for oligonucleotide and gene delivery in therapy. New York: Plenum Publishing Corp; 1996. pp. 125–130. [Google Scholar]
- 35.Redmond DE., Jr Cellular replacement therapy for Parkinson’s disease—where we are today? Neuroscientist. 2002;8(5):457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- 36.Rosenblad C, Martinez-Serrano A, Bjorklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75(4):979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sautter J, Tseng JL, Braguglia D, Aebischer P, Spenger C, Seiler RW, Widmer HR, Zurn AD. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp Neurol. 1998;149(1):230–236. doi: 10.1006/exnr.1997.6718. [DOI] [PubMed] [Google Scholar]
- 39.Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich D, Dean RL 3rd, Sanberg PR, editors. Innovative models of CNS disease: From molecule to therapy. Clifton, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- 40.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20(1):93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 41.Sinclair SR, Svendsen CN, Torres EM, Martin D, Fawcett JW, Dunnett SB. GDNF enhances dopaminergic cell survival and fibre outgrowth in embryonic nigral grafts. Neuroreport. 1996;7(15–17):2547–2552. doi: 10.1097/00001756-199611040-00029. [DOI] [PubMed] [Google Scholar]
- 42.Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169(1):23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- 43.Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: Implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165(2):268–277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan AM, Pohl J, Blunt SB. Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson’s disease. Eur J Neurosci. 1998;10(12):3681–3688. doi: 10.1046/j.1460-9568.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 45.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 46.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci USA. 2006;103(14):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 48.Wilby MJ, Sinclair SR, Muir EM, Zietlow R, Adcock KH, Horellou P, Rogers JH, Dunnett SB, Fawcett JW. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J Neurosci. 1999;19(6):2301–2312. doi: 10.1523/JNEUROSCI.19-06-02301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153(2):195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]
- 50.Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891(1–2):228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- 51.Yurek DM, Fletcher AM, Smith GM, Seroogy KB, Ziady AG, Molter J, Kowalczyk TH, Padegimas L, Cooper MJ. Long-term transgene expression in the central nervous system using DNA nanoparticles. Mol Ther. 2009;17(4):641–650. doi: 10.1038/mt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurek DM, Seroogy KB. Neurotrophic factor protection of dopaminergic neurons. In: Mocchetti I, editor. Neurobiology of the neurotrophins. New York: Sulburger & Graham Publishing; 2001. pp. 355–397. [Google Scholar]
- 53.Yurek DM, Sladek JR., Jr Dopamine cell replacement: Parkinson’s disease. Ann Rev Neurosci. 1990;13:415–440. doi: 10.1146/annurev.ne.13.030190.002215. [DOI] [PubMed] [Google Scholar]
- 54.Ziady AG, Gedeon CR, Miller T, Quan W, Payne JM, Hyatt SL, Fink TL, Muhammad O, Oette S, Kowalczyk T, Pasumarthy MK, Moen RC, Cooper MJ, Davis PB. Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol Ther. 2003;8(6):936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Ziady AG, Gedeon CR, Muhammad O, Stillwell V, Oette SM, Fink TL, Quan W, Kowalczyk TH, Hyatt SL, Payne J, Peischl A, Seng JE, Moen RC, Cooper MJ, Davis PB. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8(6):948–956. doi: 10.1016/j.ymthe.2003.09.002. [DOI] [PubMed] [Google Scholar]