Abstract
PURPOSE
To examine associations between recreational physical activity and quality of life (QOL) in a multi-ethnic cohort of breast cancer survivors, specifically testing whether associations are consistent across racial/ethnic groups after accounting for relevant medical and demographic factors that might explain disparities in QOL outcomes.
METHODS
Data were collected from a population-based cohort of non-Hispanic White (n=448), Black (n=197), and Hispanic (n=84) breast cancer survivors (Stage 0-IIIa) in the Health, Eating, Activity, and Lifestyle (HEAL) Study. Physical activity was assessed approximately 2.5 years breast cancer diagnosis, with QOL assessed on average 6–12 months later. We used structural equation modeling to examine relationships between meeting recommended levels of physical activity and QOL, stratifying by race/ethnicity and adjusting for other demographic, comorbidity, and treatment effects.
RESULTS
Structural equation modeling indicated that meeting recommended levels of physical activity had significant positive associations with QOL for Black and for non-Hispanic White women, (p<0.05). Fewer Black women reported meeting recommended physical activity levels (p<0.001); but meeting recommendations was associated with better QOL. Post-hoc tests showed that meeting physical activity recommendations was specifically associated with better vitality, social functioning, emotional roles, and global QOL (all p’s< 0.05).
CONCLUSIONS
These results suggest that meeting recommended levels of physical activity is associated with better QOL in non-Hispanic White and Black breast cancer survivors. Findings may help support future interventions among breast cancer survivors and promote supportive care that includes physical activity; although more research is needed to determine these relationships among Hispanic and other ethnic minority women.
Keywords: Physical Activity, Quality of Life, Ethnicity, Breast Cancer
Introduction
More than 2.3 million women are living with breast cancer in the United States [1]. While breast cancer patients are living longer, they may not be symptom-free. Survivors report psychosocial distress and physical symptoms such as fatigue, hormonal symptoms and decrements in physical functioning, both in the short term and over time [2, 3]. Importantly, evidence suggests that lifestyle behaviors, such as physical activity (PA), may improve breast cancer prognosis [4, 5] can have beneficial effects on QOL [6], and therefore may be an important part of supportive care. The relationship between PA and breast cancer survivors’ QOL has been examined in a number of cross-sectional, prospective, and intervention studies [7, 8], however most studies are based on small, homogenous groups, with little evidence available on ethnically or economically diverse populations. Research suggests that Black and Hispanic breast cancer survivors report poorer QOL than their White counterparts [9, 10], but no information is available on the relationship between PA and QOL among ethnic minority women. It is also not known whether the level of PA in current public health recommendations [11, 12] is associated with better QOL among cancer survivors. To determine whether PA recommendations should be included as part of supportive care for breast cancer survivors, it is important to understand these relationships across various ethnic groups.
The current project examines associations between self-reported recreational PA and QOL in an ethnically diverse, population-based, prospective observational cohort study of breast cancer survivors. We first describe the prevalence of meeting PA recommendations of 30 minutes of moderate to vigorous activity on most days of the week [11, 12] approximately two and a half years after a breast cancer diagnosis. We then test the hypothesis that women who meet current public health PA recommendations report better QOL than women who do not meet these recommendations. We specifically examine whether this association is the same or varies among Black, Hispanic, and non-Hispanic White breast cancer survivors.
Materials and Methods
Study Design
Participants in this study are women enrolled in the Health, Eating, Activity, and Lifestyle (HEAL) Study, a multicenter, multiethnic, prospective study of women diagnosed with in situ or Stages I-IIIA breast cancer. In this observational cohort study, women were assessed at multiple time points and informed consent was obtained from participants at each assessment. No intervention was administered. Study protocols were approved by the Institutional Review Board at each participating center, in accordance with an assurance filed with and approved by the US Department of Health and Human Services.
Eligibility and Recruitment
Details of the study aims, design and recruitment have been published previously [13, 14]. Briefly, breast cancer patients were recruited from the Surveillance Epidemiology and End Results (SEER) registries in three geographic regions of the US: New Mexico, Western Washington, and Los Angeles County. Eligible participants were diagnosed with Stage 0-IIIa breast cancer during a defined time frame, were able to participate in an interview within a 12-month period after diagnosis, and had no prior breast cancer diagnosis. A total of 1,183 women completed the baseline interview; 944 (80%) participated in a second interview, approximately 24 months after the baseline survey; and 858 (73%) participated in a QOL assessment approximately six months after the 24 month assessment. For the current analysis, we excluded 53 women who were diagnosed with a recurrence or a new primary breast cancer by the date of the QOL assessment, 24 women who reported their race as “other” and 52 women with incomplete data. The final sample size for analyses was 729: Black (n=197), non-Hispanic White (n=448), and Hispanic (n=84) survivors.
Measures
Background variables
Data are derived from the three assessments, medical record abstraction and SEER registry records. A baseline in-person interview or self-administered questionnaire, conducted on average 6.1 months after diagnosis, provided data on age, education, and race/ethnicity. Marital status, menopausal status, smoking and weight were collected at a second in-person interview or self-administered questionnaire, conducted, on average, 24.4 months after the baseline interview. Height was measured in the clinic at two centers (Washington and New Mexico) and was self-reported in Los Angeles. Body mass index (BMI) was calculated based on weight (kg) divided by measured or self-reported height (m2). At the 24-month interview, participants reported whether they had been diagnosed by a physician with any of 18 chronic medical conditions (e.g. angina, arthritis, osteoporosis, chronic lung disease, diabetes, other cancers), and if yes, whether such condition limited their current activities of daily living. Medical comorbidity was calculated as the number of conditions that participants’ reported as limiting their current activities of daily living. Participants also reported the name, dose and frequency of any medications used at time of the 24-month interview.
Diagnosis, Stage of breast cancer, and treatment
Diagnosis date and stage of disease were assessed using SEER data. Treatment data (surgeries, radiation therapy, and chemotherapy) were abstracted from medical records and SEER registry records. Tamoxifen use was collected by medical abstraction and was self-reported during the 24-month interview.
Physical Activity
Recreational PA was measured at the 24-month assessment using a modified version of the Modifiable Activity Questionnaire [15], and assessed the type, duration and frequency of 20 activities (e.g. walking, jogging, aerobics, tennis) during the past year. The intensity of each activity was classified as light-, moderate-, or vigorous based on its rating in the Compendium of Physical Activities [16]. Hours per week of moderate to vigorous sports and recreational PA were combined into a total score and were then categorized based on that score according to criteria set by the American College of Sports Medicine, the American Heart Association, and the American Cancer Society’s PA recommendations of 30 minutes of moderate to vigorous activity on most days of the week [11, 12]. Occupational and household activities were not covered in this score. Participants were categorized as Sedentary (0 hours/week), Low Active (<2.5 hours/week) or Met Recommendations (≥2.5 hours/week).
Quality of Life
QOL was assessed at the third assessment (on average 34.5 months following the baseline interview). HRQOL was assessed using the Medical Outcomes Study short form 36 (SF-36) [17, 18]. Raw scores from the four psychosocial subscales, Vitality, Social Functioning, Role-Emotional and Mental Health, were used in the present study (a companion paper assesses the impact of PA on the physical subscales [19]). We measured the psychosocial impact of cancer using the Social/Emotional subscale of the Brief Cancer Impact Assessment (BCIA [20]). This subscale (Cronbach’s α=0.75) assesses the impact of cancer on family plans, love life, emotional or psychological needs, social life, and living arrangements. Global QOL was assessed using the widely used [21] Ladder of Life [22], on a 10-point scale from 1 (the worst possible life) to 10 (the best possible life).
Data Analysis
Data were analyzed using Mplus, version 4.21 [23] and SPSS for Windows, version 12. We used a Structural Equation Model (SEM) framework to examine relationships between PA and QOL by race/ethnicity, accounting for relevant demographic and clinical covariates. Analyzing the data with SEM allowed us to reduce the number of comparisons we were modeling, as QOL was conceptualized as one “latent” variable made up of six measured QOL scales. Latent variables are not directly observed, but are inferred from measured variables or scores, (e.g. socioeconomic status is inferred from education and income). The benefits of SEM, over standard multiple regression modeling, are that it can handle complex models estimating effects of multiple independent variables on multiple dependent variables; it tests multiple regressions among continuous latent variables while accounting for correlations among predictors and outcomes; and it can account for continuous or categorical variables. Estimated associations among variables account for measurement error; the error is estimated and removed, leaving only common variance. An SEM approach maximizes power to detect associations; in this case it maximized power to determine whether there were significant relationships between PA and QOL after accounting for multiple covariates.
Using SEM, we ran a Multiple Group Multiple-Indicator/Multiple-Cause (MIMIC) model to examine whether the relationship between PA and QOL varied based on race/ethnic group (Black, non-Hispanic White, or Hispanic). Standard SEM models that include a race variable can only determine if QOL is different by racial group and if race is related to PA, but do not allow us to examine the possibility that PA is differentially associated with QOL, depending on racial group. Multiple Group MIMIC models allow us to determine if PA (or any other covariate in the model) has a differential influence on QOL based on race/ethnicity. With this technique, we were able to: 1) evaluate if PA has an influence on QOL, and 2) evaluate if that relationship varied based on racial/ethnic group.
To test these we employed a step-by-step nested model design. We compared a fully constrained model (i.e., one that assumes that the structural relationship among PA, its covariates, and the QOL variable are equivalent with respect to racial/ethnic group) to a model that allows the relationships between PA (and covariates) and QOL to vary by race/ethnicity. Since these are nested models, we used the chi-square statistic to evaluate the change in model fit based on freeing up the parameters of interest.
Covariates in the SEM included age, education, marital status, smoking status, time since diagnosis, menopausal status, treatment, comorbidity status, and antidepressant use. Stage of disease and treatment history were both considered, but stage was excluded due to significant collinearity with treatment. A similar decision was made to exclude study site, as it was closely associated with race. To develop a more parsimonious model, variables that were not associated with QOL and did not add significantly to the SEM (BMI and tamoxifen use) were excluded from the final model. Marital status was coded dichotomously (married/partnered relationship vs. single/widowed/divorced).
Overall model fit was evaluated using the comparative fit index (CFI), the Tucker-Lewis Index (TLI), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR), with recommended criteria for good fit being CFI>0.90, TLI>0.90, RMSEA<0.08, SRMR<0.08 [24–26]. Each goodness of fit index has strengths and weaknesses depending on the data characteristics. The group of indices as a whole was used to determine model fit. After identifying meaningful associations within the larger SEM, we followed up with targeted (post-hoc) tests to better describe the effects of PA on individual QOL outcomes. We examined least squares means of each QOL outcome stratified by race/ethnicity. Pairwise comparisons were examined for each outcome adjusting for the same covariates as used in the SEM modeling.
Results
Sample Characteristics
These analyses included 736 women, aged 31–88 years (mean=57 yrs). Overall, 34% of this sample reported an amount of PA that met public health recommendations, 47% were classified as “low active” and 19% were sedentary. Black women were less likely to meet recommended PA levels than all other women, and Hispanic women were more likely to meet recommended levels than non-Hispanic White women. Table 1 presents demographic and health related indices by race/ethnicity. Compared to Hispanic and non-Hispanic White participants, Black women tended to be younger, heavier, less likely to be married, less likely to have taken tamoxifen, and diagnosed with more advanced disease; and all were recruited from Los Angeles. Hispanic women were less educated, more likely to meet PA recommendations than NHW women, and were recruited primarily from New Mexico. Non-Hispanic White women reported more antidepressant medication use and reported fewer comorbidities that limited their activities.
Table 1.
Demographics and health indices 2.5 years after breast cancer diagnosis by race/ethnicity
| Black n (%) | Non-Hispanic White n (%) | Hispanic n (%) | p-value* | |
|---|---|---|---|---|
| Total | 197 (27.0) | 448 (61.5) | 85 (11.5) | |
| Age (y), Mean ± SD | 53.7 ± 7.9 | 58.9 ± 10.4 | 58.0 ± 11.4 | <0.001 |
| Physical Activity Level | 0.004 | |||
| Sedentary | 52 (26.4) | 77 (17.2) | 12 (14.3) | |
| Low Active | 97 (49.2) | 204 (45.5) | 38 (45.2) | |
| Met Recommendations | 48 (24.4) | 167 (37.3) | 34 (40.5) | |
| Site | <0.001 | |||
| Western Washington | 1 (0.5) | 132 (29.5) | 2 (2.4) | |
| New Mexico | 0 (0.0) | 316 (70.5) | 82 (97.6) | |
| Los Angeles | 196 (99.5) | 0 (0.0) | 0 (0.0) | |
| Education | <0.001 | |||
| High School or less | 71 (36.0) | 76 (17.0) | 36 (42.9) | |
| Some College | 86 (43.7) | 159 (35.5) | 30 (35.7) | |
| College Grad | 23 (11.7) | 107 (23.9) | 9 (10.7) | |
| Grad school | 17 (8.6) | 106 (23.7) | 9 (10.7) | |
| Marital Status | <0.001 | |||
| Married or Partnered | 82 (41.6) | 292 (65.2) | 48 (57.1) | |
| Single | 115 (58.4) | 156 (34.8) | 36 (42.9) | |
| Stage | 0.001 | |||
| In situ | 41 (20.9) | 102 (22.5) | 17 (20.0) | |
| Local | 92 (46.9) | 272 (59.9) | 53 (62.4) | |
| Regional | 63 (32.1) | 80 (17.6) | 15 (17.6) | |
| Months Since Diagnosis | ||||
| Mean ± SD | 33.4 ± 4.7 | 29.7 ± 2.6 | 28.9 ± 2.4 | <0.001 |
| Treatment | <0.001 | |||
| Surgery-only | 71 (36.0) | 133 (29.7) | 29 (34.5) | |
| Radiation | 47 (23.9) | 191 (42.6) | 30 (35.7) | |
| Chemotherapy | 34 (17.3) | 31 (6.9) | 5 (6.0) | |
| Radiation + Chemotherapy | 45 (22.8) | 93 (20.8) | 20 (23.8) | |
| Tamoxifen | 0.40 | |||
| No | 116 (58.9) | 238 (53.1) | 46 (54.8) | |
| Yes | 81 (41.1) | 210 (46.9) | 38 (45.2) | |
| Body Mass Index, Mean ± SD | 31.0 ± 7.3 | 26.9 ± 5.6 | 27.6 ± 5.0 | <0.001 |
| Smoking Status | 0.15 | |||
| Never | 95 (48.2) | 213 (47.5) | 47 (56.0) | |
| Former | 72 (36.5) | 189 (42.2) | 26 (31.0) | |
| Current | 30 (15.2) | 46 (10.3) | 11 (13.1) | |
| Menopausal Status | 0.42 | |||
| Premenopausal | 36 (18.3) | 80 (17.9) | 18 (21.4) | |
| Postmenopausal | 146 (74.1) | 349 (77.9) | 61 (72.6) | |
| Unknown | 15 (7.6) | 19 (4.2) | 5 (6.0) | |
| Current antidepressant use | <0.001 | |||
| No | 186 (94.4) | 364 (81.3) | 73 (86.9) | |
| Yes | 11 (5.6) | 84 (18.7) | 11 (13.1) | |
| Comorbidity limitations | 0.18 | |||
| None | 140 (71.1) | 341 (76.1) | 61 (72.6) | |
| 1 condition | 34 (17.3) | 80 (17.9) | 15 (17.9) | |
| 2 or more | 23 (11.7) | 27 (6.0) | 8 (9.5) |
p-value derived from either analysis of variance comparing mean values or chi-square tests comparing distributions of characteristics by race/ethnicity.
PA and QOL by Race/Ethnicity
The SEM multiple group MIMIC model depicting the relationship between PA and QOL is illustrated in Figure 1. Because four of the observed subscales come from one instrument (SF-36), the associated errors were correlated in the SEM but not presented in Figure 1. Overall model fit was good across the different indices: CFI (0.91), TLI (0.89), RMSEA (0.06), and SRMR (0.05).
Figure 1.
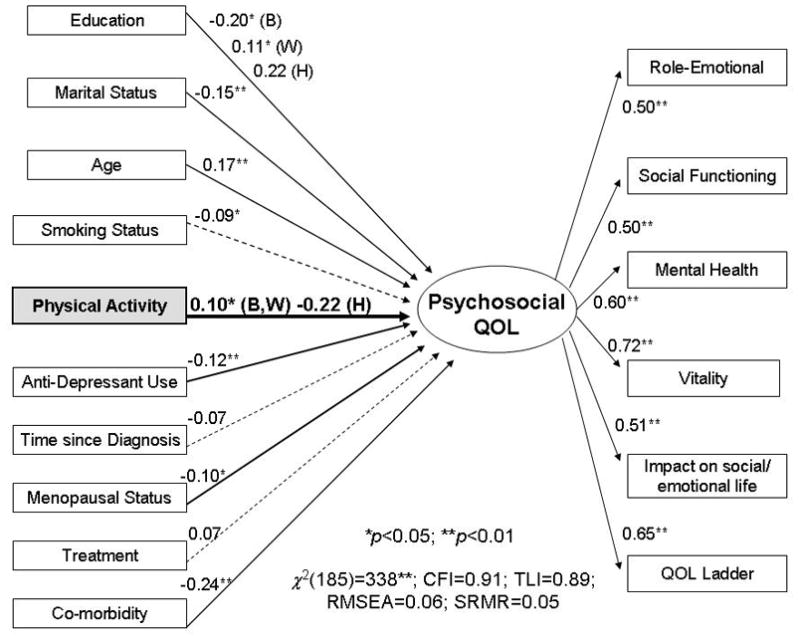
A multi-group multiple-indicator/multiple-cause structural equation model testing the association between physical activity and quality of life. Chi-square (χ2) =388.36; df =185; p<.001; comparative fit index =0.91; Tucker-Lewis index =0.89; root mean square error of approximation=0.06; standardized root mean square residual=0.05; Solid lines represent significant paths, B=Black, W=non-Hispanic White, H=Hispanic, **p<.01; *p<.05.M
Paths (γ coefficients) between observed measures (in rectangles) on the left side and the latent outcome variable (in the oval) show the influence of PA and the covariates on QOL. The paths (λ coefficients) from the latent variable to the observed measures on the right side indicate that QOL is measured by six scales or subscales. When the relationship between PA (or a covariate) and QOL varied by race/ethnicity, path coefficients are provided for each racial/ethnic group.
The association between PA and QOL was similar for Black and non-Hispanic White women but statistically different for Hispanic women (χ2(1)=3.99, p<0.05) in hierarchically-nested models that accounted for the effects of covariates. Higher PA levels for both Black and non-Hispanic White women were positively associated with QOL (γ=0.10; p=0.03). Among Hispanic women, a negative, though not statistically significant, relationship between PA and QOL was observed (γ=−0.22; p=0.08).
Post-hoc tests of PA and QOL Scales
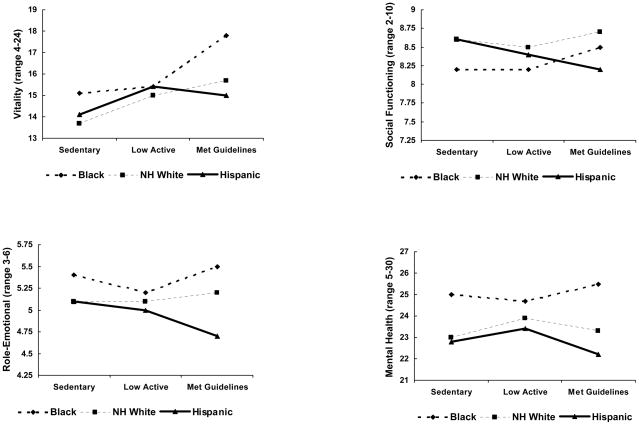
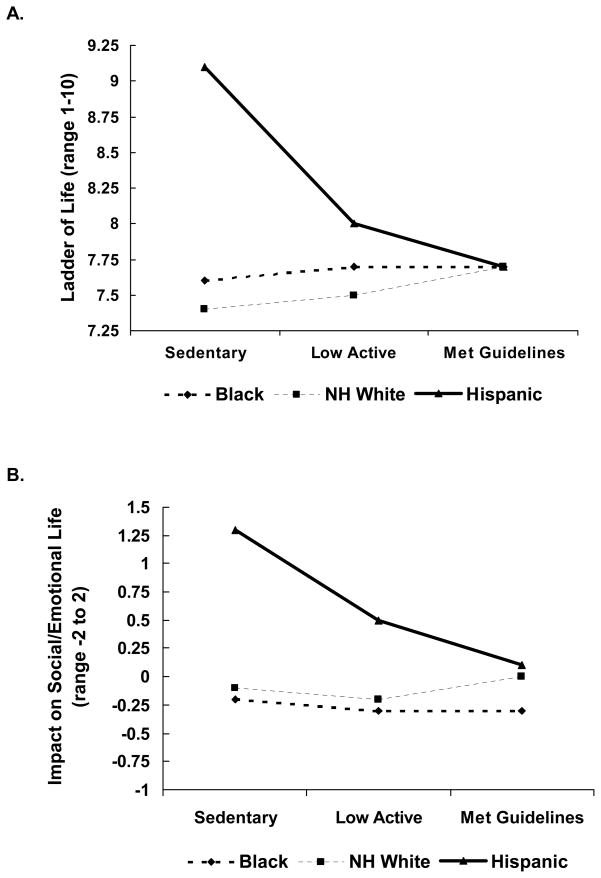
After performing the omnibus test of PA and the QOL latent factor in the SEM, post-hoc tests were used to investigate relationships between PA and each individual QOL outcome stratified by race/ethnicity to describe the effects of PA on specific aspects of QOL. Consistent with the SEM, associations between PA and QOL scales showed similar, positive patterns as PA levels increased among Black and non-Hispanic White women, while for Hispanic women relationships between PA and QOL subscales showed a distinctly different pattern, appearing flat or negative (see Figures 2,3). Pairwise comparisons of adjusted means are presented in Table 2. Both Black and non-Hispanic White women who met the recommended levels of PA had higher Vitality and Social Functioning scores, and higher overall QOL on the Ladder of Life. Higher scores on Emotional Roles were also found for non-Hispanic White women meeting the recommended levels.
Figure 2.
Least squares mean values of SF-36 subscales within physical activity and Race/Ethnicity categories
Figure 3.
Least squares mean values of A) Ladder of Life and B) Impact on social/emotional life scales within physical activity and race/ethnicity categories
Table 2.
Individual quality of life scores by sports/recreational physical activity and race/ethnic categories
| Met Recommendations (≥2.5 hours/week) n=250 | Low Active (<2.5 hours/week) n=344 | Sedentary (0 hours/week) n=142 | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | LSM1 | Mean (SD) | LSM1 | Mean (SD) | LSM1 | |
| SF-36 Scale | |||||||
| Vitality (Range 4 to 24) | |||||||
| Black | 197 | 18.0 (4.5) | 17.8 a | 15.5 (4.4) | 15.4 b | 14.7 (4.9) | 15.1 b |
| Non-Hispanic White | 448 | 16.0 (4.0) | 15.7 a | 14.7 (4.3) | 14.9 b | 13.5 (4.0) | 13.7 b |
| Hispanic | 84 | 14.1 (4.8) | 14.0 | 15.5 (3.6) | 15.4 | 13.3 (4.9) | 14.1 |
| Social Functioning (Range 2 to10) | |||||||
| Black | 197 | 8.7 (2.0) | 8.5 a | 8.3 (2.1) | 8.2 | 7.8 (2.4) | 8.2 b |
| Non-Hispanic White | 448 | 8.9 (1.5) | 8.7 a | 8.5 (1.8) | 8.5 | 8.4 (1.9) | 8.6 b |
| Hispanic | 84 | 8.3 (2.0) | 8.2 | 8.4 (1.8) | 8.4 | 8.3 (1.9) | 8.6 |
| Role-Emotional (Range 3 to 6) | |||||||
| Black | 197 | 5.5 (1.0) | 5.5 | 5.3 (1.2) | 5.2 | 5.3 (1.1) | 5.4 |
| Non-Hispanic White | 448 | 5.3 (0.9) | 5.2 | 5.0 (1.1) | 5.1 | 4.9 (1.1) | 5.1 |
| Hispanic | 84 | 4.7 (1.2) | 4.7 | 5.0 (1.2) | 5.0 | 4.9 (1.2) | 5.1 |
| Mental Health (Range 5 to 30) | |||||||
| Black | 197 | 25.6 (4.8) | 25.5 | 24.8 (4.2) | 24.7 | 24.7 (4.4) | 25.0 |
| Non-Hispanic White | 448 | 23.7 (4.0) | 23.3 | 23.7 (3.9) | 23.9 | 23.0 (4.6) | 23.0 |
| Hispanic | 84 | 22.4 (4.9) | 22.1 | 23.0 (4.2) | 23.4 | 23.2 (5.4) | 22.8 |
| QOL Ladder (Range 1 to 10) | |||||||
| Black | 197 | 7.8 (1.9) | 7.7 | 7.8 (1.9) | 7.7 | 7.8 (1.7) | 7.6 |
| Non-Hispanic White | 448 | 7.8 (1.5) | 7.7 a | 7.4 (1.8) | 7.5 | 7.3 (2.0) | 7.4 b |
| Hispanic | 84 | 7.8 (1.6) | 7.7 a | 7.9 (1.5) | 8.0 | 9.0 (1.2) | 9.1 b |
| Impact on Social/Emotional (Range −2 to 2) | |||||||
| Black | 197 | −0.3 (2.4) | −0.3 | −0.3 (2.4) | −0.3 | −0.4 (2.2) | −0.2 |
| Non-Hispanic White | 448 | 0.0 (3.2) | −0.2 | −0.2 (2.8) | −0.1 | −0.2 (2.8) | −0.1 |
| Hispanic | 84 | 0.1 (3.5) | 0.1 a | 0.6 (2.8) | 0.5 | 1.1 (2.7) | 1.3 b |
LSM – Least Squares Means are adjusted for age, education, marital status, smoking status, menopausal status, time since diagnosis, breast cancer treatment, comorbidity status, and antidepressant use.
Different superscripts across a row indicate activity groups with statistically significant differences in means; p<0.05.
Discussion
The aim of this investigation was to determine whether recreational PA two years after a breast cancer diagnosis is associated with better QOL in a diverse, population-based cohort. The secondary aim was to evaluate whether these relationships were consistent across racial/ethnic groups. Findings suggest that meeting general public health recommendations for PA is associated with better psychosocial outcomes among Black and non-Hispanic White women, particularly in terms of vitality, social functioning, and global QOL. In contrast, among Hispanic survivors, PA was not associated with better QOL, and even appeared to have negative relationships, although associations did not reach statistical significance. Although fewer Black women reported meeting PA recommendations than did non-Hispanic White or Hispanic women (24% compared to 37% and 41%, respectively), those who did meet recommendations reported better QOL than their less physically active counterparts. These findings have important implications, particularly as Black cancer survivors often report worse QOL than other women [9, 10]. Results from this study suggest that PA may be a relevant part of supportive care for Black women, and may be influential in encouraging Black women to be more active. To our knowledge, this is the first study to examine associations between PA and QOL in a large, multiethnic sample of breast cancer survivors in the US. Our study provides an important addition to the growing body of literature on PA and QOL in cancer survivors among understudied racial/ethnic groups.
Some clinicians are beginning to include PA as part of supportive care for cancer [27], and ultimately clinicians need ways to motivate their patients to be physically active. Accruing evidence that meeting public health PA recommendations also improves QOL may provide additional incentive for breast cancer survivors to increase their PA and may be a target in interventions. The majority of women in the HEAL study did not meet recommended levels of PA two years after their diagnosis [14] which is comparable to national survey data on PA in cancer survivors [28,29]. At this time, the currently accepted public health recommendations for PA are being evaluated. Comprehensive federal public health guidelines, “The Physical Activity Guidelines for Americans” will be issued in late 2008 by the Department of Health and Human Services (http://www.health.gov/PAguidelines/) and will include evidence on cancer. The current study adds to the evidence, suggesting that getting the equivalent of 30 minutes per day, 5 days per week of moderate to vigorous activity is related to better QOL among Black and White breast cancer survivors. While our data provides some evidence that meeting PA recommendations is associated with better QOL, further research is needed among Hispanic breast cancer survivors.
Interpreting the results for the Hispanic women in this study is difficult, particularly as there is no other published study of PA and QOL among Hispanic breast cancer survivors for comparison. The effect sizes for the PA and QOL associations (represented by the path coefficient in the SEM and the means of the individual QOL subscales) were larger in magnitude for Hispanics than the associations found for Black and non-Hispanic White women. The lack of statistical significance in our models may be due to sample size. However, this does not explain the direction of the relationships. Previous research among Hispanic prostate cancer survivors suggests that poorer QOL is related, in part, to lower levels of PA [30]. Further, national data indicate that Hispanic women are less likely to meet PA recommendations than non-Hispanic White women and that they are more comparable to Black women [31, 32]. However, in our sample, compared to non-Hispanic White women, Hispanic women were more likely to meet PA recommendations, while Black women reported less activity. The negative association among Hispanic women is also not explained by lower overall QOL. Previously published data from the HEAL study showed no significant differences in QOL reported by Hispanic compared to non-Hispanic White women after accounting for relevant covariates [33]. Additionally, PA was associated with better physical functioning in both Hispanic and non-Hispanic White women [19]. One explanation of the ethnic group differences may have to do with cultural expectations and linguistic differences in interpreting the meaning of psychosocial QOL items on self-report measures. Research has shown that acculturation, cultural beliefs, family and religion are significantly associated with QOL [32, 34, 35] and that there may be cultural bias in health surveys and in perceptions and definitions of QOL [36–38]. The standard QOL measures used in this study have not been tested for cultural or linguistic equivalence [39]. These methodological considerations represent important areas for future research.
While several intervention studies have shown positive effects of exercise on QOL, most have shown better physical functioning, with fewer studies investigating psychosocial effects [7, 40,41]. There is some evidence that meeting PA recommendations is associated with better QOL in the general population [42] and in breast cancer and other cancer survivors [43–45]; however, PA recommendations have not previously been examined with respect to QOL in an ethnically diverse group of breast cancer survivors. Compared to sedentary individuals, meeting recommendations was associated with better QOL on four out of the six dimensions measured in non-Hispanic White participants and on three of six dimensions in Black participants. Activity levels below the recommended amount did not appear to be related to QOL. These results suggest that existing public health PA recommendations are important and meaningful predictors of psychosocial outcomes in Black and White breast cancer survivors. However, as Hispanic women did not report better QOL related to their PA levels, it is possible that messages and interventions will need to be structured differently for Hispanic women.
The current investigation had several strengths and limitations. A major strength was the diverse sample and the use of latent variable modeling. Using an SEM approach, we were able to maximize statistical power without increasing the Type I error associated with multiple comparisons generated from multiple regression model runs. Further, our model accounted for several covariates, showing that PA is related to QOL over and above the effects of sociodemographic, treatment and behavioral variables as well as the effects of other medical conditions. With few exceptions [e.g., 46], research on PA and QOL in cancer survivors has not accounted for measures of health-related comorbidities. Of the variables examined in the present study, comorbidity had the strongest association with QOL, suggesting that it should be included in any investigation of PA and QOL.
A limitation of this study is the uneven distribution of race/ethnicity across the study sites; all participants from the Los Angeles site were Black and the majority of Hispanic participants were recruited from New Mexico. Another limitation is that the observational cohort design did not allow us to conclusively determine the direction of the PA and QOL relationships. Because we do not have longitudinal assessments of PA and QOL from diagnosis onward, it is possible that those with better QOL were more likely to engage in PA. However, for most women in the sample, PA was assessed prior to the QOL assessment, suggesting that PA 2–3 years after breast cancer diagnosis is associated with better subsequent QOL.
In conclusion, our results highlight the importance of examining racial/ethnic effects on PA and QOL separately and in combination in future studies. While the number of exercise trials being conducted among cancer survivors is increasing [40], most are focused on White women and they may not generalize to all women. When providing supportive care to cancer patients, it is important to recognize that not all participants may positively respond to PA and tailored interventions may be warranted. Further investigation is needed to elucidate possible QOL differences among different Hispanic populations, and to understand the role of cultural expectations and linguistic interpretation of outcome measures. Further study is also needed of Asian and other ethnic minority groups where research on PA and QOL is also lacking. Ultimately it is important for clinicians and policy makers to know if public health PA recommendations are useful for interventions to improve QOL for all cancer survivors. Future randomized trials are needed to examine these effects prospectively and to better understand the influence of race/ethnicity and culture on these relationships. The knowledge gained from such studies will help in designing and evaluating culturally sensitive interventions to improve QOL among all breast cancer survivors.
Acknowledgments
This research was supported by contracts from the National Cancer Institute (N01-CN-75036-20, N01-CN-05228, N01-PC-67010).
References
- 1.Ries LAG, Eisner MP, Kosary CL, et al., editors. SEER Cancer Statistics Review, 1975–2001. National Cancer Institute; Bethesda, MD: 2004. [Google Scholar]
- 2.Hegel MT, Moore CP, Collins ED, et al. Distress, psychiatric syndromes and functional impairment in women with newly diagnosed breast cancer. Cancer. 2006;107(12):2924–2931. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 4.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson PE, Gammon MD, Lund MJ, et al. Recreational physical activity and survival among young women with breast cancer. Cancer. 2006;107(8):1777–1785. doi: 10.1002/cncr.22201. [DOI] [PubMed] [Google Scholar]
- 5.Irwin ML, Smith AW, McTiernan A, et al. Association of pre- and post-diagnosis physical activity with mortality in breast cancer survivors: The Health Eating Activity and Lifestyle (HEAL) Study. J Clin Oncol. doi: 10.1200/JCO.2007.15.9822. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 8.Pinto BM, Maruyama NC. Exercise in the rehabilitation of breast cancer survivors. Psychooncology. 1999;8(3):191–206. doi: 10.1002/(SICI)1099-1611(199905/06)8:3<191::AID-PON355>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Powe BD, Hamilton J, Hancock N, Johnson N, Finnie R, Ko J, Brooks P, Boggan M., Jr Quality of life of African American cancer survivors. A review of the literature. Cancer. 2007;109(2 Suppl):435–445. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 10.Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: Different types of antecedents predict different classes of outcomes. Psychooncology. 2006;15(9):749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- 11.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 12.Kushi LH, Byers T, Doyle C, et al. The American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee, American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer With Healthy Food Choices and Physical Activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21(10):1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 15.Kriska A. Modifiable activity questionnaire. Med Sci Sports Exerc. 1997;29(6):S73–S78. [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE., Jr . The SF-36 health survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2. Philadelphia, PA: Lippincott-Raven; 1996. pp. 337–345. [Google Scholar]
- 19.Alfano CM, Smith AW, Irwin M, et al. Physical activity, long-term symptoms, and health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Survivorship Res Pract. doi: 10.1007/s11764-007-0014-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfano CM, McGregor BA, Kuniyuki A, et al. Psychometric evaluation of the Brief Cancer Impact Assessment among breast cancer survivors. Oncology. 2006;70(3):190–202. doi: 10.1159/000094320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. 2. New York: Oxford University Press; 1996. [Google Scholar]
- 22.Cantril H. The patterns of human concerns. New Brunswick, NJ: Rutgers University Press; 1965. [Google Scholar]
- 23.Muthen LK, Muthen BO. Mplus user’s guide. Los Angeles, CA: Muthen & Muthen; 1998. [Google Scholar]
- 24.Bentler PM. On the fit of models to covariances and methodology to the Bulletin. Psychol Bull. 1992;112(3):400–404. doi: 10.1037/0033-2909.112.3.400. [DOI] [PubMed] [Google Scholar]
- 25.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park: Sage; 1993. pp. 136–162. [Google Scholar]
- 26.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 27.Segal R, Evans W, Johnson D, Smith J, Colletta SP, Corsini L, Reid R. Oncology Rehabilitation Program at the Ottawa Regional Cancer Centre: program description. CMAJ. 1999;161:282–285. [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard CM, Cokkinides V, Courneya KS, Nehl EJ, Stein K, Baker F. A comparison of physical activity of posttreatment breast cancer survivors and noncancer controls. Behav Med. 2003;28(4):140–149. doi: 10.1080/08964280309596052. [DOI] [PubMed] [Google Scholar]
- 29.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 30.Penedo FJ, Dahn JR, Shen BJ, Schneiderman N, Antoni MH. Ethnicity and determinants of quality of life after prostate cancer treatment. Urology. 2006;67(5):1022–1027. doi: 10.1016/j.urology.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Jones DA, Ainsworth BE, Croft JB, Macera CA, Lloyd EE, Yusuf HR. Moderate leisure-time physical activity: who is meeting the public health recommendations? A national cross-sectional study. Arch Fam Med. 1998;7(3):285–289. doi: 10.1001/archfami.7.3.285. [DOI] [PubMed] [Google Scholar]
- 32.Crespo CJ, Smit E, Carter-Pokras O, Andersen R. Acculturation and leisure-time physical inactivity in Mexican American adults: results from NHANES III, 1988–1994. Am J Public Health. 2001;91(8):1254–1257. doi: 10.2105/ajph.91.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juarez G, Ferrell B, Borneman T. Perceptions of quality of life in Hispanic patients with cancer. Cancer Pract. 1998;6(6):318–324. doi: 10.1046/j.1523-5394.1998.006006318.x. [DOI] [PubMed] [Google Scholar]
- 35.Juarez G, Ferrell B, Borneman T. Influence of culture on cancer pain management in Hispanic patients. Cancer Pract. 1998;6(5):262–269. doi: 10.1046/j.1523-5394.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- 36.Pasick RJ, Stewart SL, Bird JA, D’Onofrio CN. Quality of data in multiethnic health surveys. Public Health Rep. 2001;116(Suppl 1):223–243. doi: 10.1093/phr/116.S1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res. 1993;2(6):451–459. doi: 10.1007/BF00422219. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt S, Bullinger M. Current issues in cross-cultural quality of life instrument development. Arch Phys Med Rehabil. 2003;84(4 Suppl 2):S29–S34. doi: 10.1053/apmr.2003.50244. [DOI] [PubMed] [Google Scholar]
- 39.Hahn EA, Bode RK, Du H, Cella D. Evaluating linguistic equivalence of patient-reported outcomes in a cancer clinical trial. Clin Trials. 2006;3(3):280–290. doi: 10.1191/1740774506cn148oa. [DOI] [PubMed] [Google Scholar]
- 40.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 41.Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients? Eur J Cancer. 2004;40(7):951–962. doi: 10.1016/j.ejca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Brown DW, Balluz LS, Heath GW, Moriarty DG, Ford ES, Giles WH, Mokdad AH. Associations between recommended levels of physical activity and health-related quality of life. Findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev Med. 2003;37(5):520–528. doi: 10.1016/s0091-7435(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 43.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Can Med Assoc J. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karvinen KH, Courneya KS, North S, Venner P. Associations between exercise and quality of life in bladder cancer survivors: a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16(5):984–90. doi: 10.1158/1055-9965.EPI-06-0680. [DOI] [PubMed] [Google Scholar]
- 45.Vallance JK, Courneya KS, Jones LW, Reiman T. Differences in quality of life between non-Hodgkin’s lymphoma survivors meeting and not meeting public health exercise guidelines. Psychooncology. 2005;14(11):979–991. doi: 10.1002/pon.910. [DOI] [PubMed] [Google Scholar]
- 46.Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity on quality of life in long-term breast cancer survivors. Qual Life Res. 2005;14(2):361–371. doi: 10.1007/s11136-004-1468-5. [DOI] [PubMed] [Google Scholar]