Abstract
The immunomodulator drug Gilenya (FTY720), marketed as the first oral sphingosine 1-phosphate receptor (S1P-R) modulator for treatment of Multiple Sclerosis (MS) also inhibits lysosomal acid sphingomyelinase (ASMase). Treatment of cultured cells for 24h with FTY720 (up to 10μM) inhibited ASMase by >80% and this could be reversed by pre-treatment with the cathepsin protease inhibitor leupeptin (5μM). In contrast, neutral sphingomyelinase activity was unaffected and sphingosine-1-phosphate treatment had no effect on ASMase. RT-PCR revealed no inhibition of ASMase mRNA and there was no direct (in vitro) inhibition of ASMase by either FTY720 or FTY720-phosphate. This suggests that its mechanism of inhibition is similar to that of tricyclic anti-depressants such as desipramine, which are also amphiphilic cationic drugs. Both Desipramine and FTY720 treatment reduced ASMase without significant inhibition of other lysosomal hydrolases but most hydrolases showed increased secretion (up to a 50% increase) providing more evidence of lysosomal disruption by these drugs.
Keywords: Galinya, myriocin analog, acid sphingomyelinase, lysosomal hydrolases, amphiphilic cation, tricyclic anti-depressant
1. Introduction
The C19H33NO2 immunomodulator drug FTY720 (CH3 (CH2)7-phenyl-(CH2)2-C (NH2) (CH2OH)–CH2OH) (Gilenya), is an analog of naturally occurring myriocin, which inhibits de novo sphingolipid synthesis (1). In cultured non-neural cells it has been shown to inhibit ceramide synthase-2 (2,3), leading to a reduction in ceramide levels in one study (2). It also appears to inhibit the de novo biosynthesis of sphingosine-1-phosphate (S1P) (2), a sphingolipid with roles in the regulation of cell growth, death, senescence, adhesion, migration, angiogenesis and intracellular trafficking (4–6). FTY-720’s therapeutic action in Multiple Sclerosis is to inhibit the exit of autoreactive memory T-cells from secondary lymphoid organs to produce a peripheral lymphopenia (7). However, it also localizes to CNS white matter along the myelin sheath and gets converted to FTY720-P (presumably as the result of sphingosine kinase-2 action) after crossing the blood-brain barrier (2,8). FTY720-P is believed to act as a functional S1P antagonist which induces complete internalization and desensitization of lymphocyte G-protein-coupled receptors, since it is not as easily degraded as S1P (7). Because of its reported role in promoting remyelination (9), its clinical importance and observed side-effects it is important to understand the action of FTY720 in full. We now report that it is also a potent inhibitor of lysosomal acid sphingomyelinase (ASMase), most likely by a mechanism similar to desipramine and other tricyclic antidepressants (10–13).
Tricyclic antidepressants such as desipramine are believed to induce intracellular degradation of acid sphingomyelinase (10–12) and acid ceramidase (13) by interfering with the binding of the enzyme to the inner membranes of late endosomes and lysosomes. The effect is seen with cationic amphiphilic drugs at 5μM but not by neutral or anionic ones (10–13). Inhibition is typically not more than 90% so there is only modest accumulation of sphingomyelin and depletion of ceramide, unlike that observed in cells totally deficient in ASMase (Niemann-Pick disease) (14). ASMase has an isoelectric point of 6.8 so is positively charged at lysosomal pH 4.5 and is believed to bind the lysosome/endosome-specific phospholipid BMP (LBPA) (11). Displacement of ASMase from the membrane by cationic lipophilic drugs is hypothesized to expose ASMase to proteases(14) and the desipramine-induced degradation of ASMase (and ACeramidase) can be blocked by both leupeptin and CA074Me (a cathepsin B/L inhibitor) but not by pepstatin.
2. Materials and Methods
2.1 Chemical and Cell culture
2-Amino-2-[2–4(0ctylphenyl) ethyl] 1,3propanediol (FTY720) and 2-amino-2[2-(4-octylphenyl) ethyl]-1,3-propanediol, mono dihydrogen phosphate ester (FTY720-P) was obtained from Cayman Chem. Co. (Ann Arbor, MI); Sphingosine-1-phosphate from Avanti Polar Lipids, Inc (Alabaster, Al) and Leupeptin from Roche (Indianapolis, IN).
Neural-derived cells (LA-N-5 and HOG) and mouse skin fibroblasts were grown in monolayer culture in DMEM supplemented with 10% fetal calf serum as described previously (15). Drugs were dissolved in ethanol and added to the culture medium to produce final concentrations of 5–15μM.
2.2 Lysosomal hydrolase assays
Cells were harvested, the pellets resuspended and lysed in 25 mM Tris-HCl, 150 mM NaCl and 1% Triton X-100 pH 7.4 and aliquots (10–20 μg protein) and assayed with the appropriate 4MU or HMU substrate as described previously (16). For ASMase the incubation was carried out at pH 4.5 in 150 mM sodium acetate buffer containing 1mM EDTA to block any NSMase activity. The HMU released was followed fluorometrically in a 96-well FLX microplate reader. The enzyme activities were calculated from the slope of the graph of intrinsic fluorescence plotted against time and standardized by μg of protein
2.3 Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using a Qiagen total RNA extract kit (Valencia, CA), and RT-PCR was executed with an RT-PCR one step kit (Qiagen, Valencia, CA) using specific primer-pairs. Primers used were human ASMase, Forward 5′-CAGGGTTCCTGGCTGGGCAGCA-3′; Reverse 5′-GGTCCTGGACCATGAGACCTAC-3′; β-actin primer: Forward 5′-ATTGGCAATGAGCGGTTCC-3′; Reverse 5′-GGTAGTTTCGTGGATGCCACA-3′.
The reaction mixture was prepared in PCR tubes according to the kit menu and put into a Perkin Elmer GeneAMP PCR System 2400. The RT-PCR reaction was at 50°C for 30 min, 95°C for 15 min; then 94°C, 60°C, 72°C, 1 min each, 35 cycles; 72°C with 10min for extension. The RT-PCR amplified samples were visualized on 1.2% Agarose gels using ethidium bromide.
2.4 Western blot analysis
Electrophoresed proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA), and Western blotting carried out with the anti-ASMase polyclonal antibody (Santa Cruz, Santa Cruz, CA) according to the manufacturer’s instructions. Positive bands were detected with a chemiluminescence kit from Fisher Scientific (Pittsburgh, PA).
3. Results
3.1 FTY720 inhibited ASMase in a dose-dependent manner over the concentration range 0–10μM
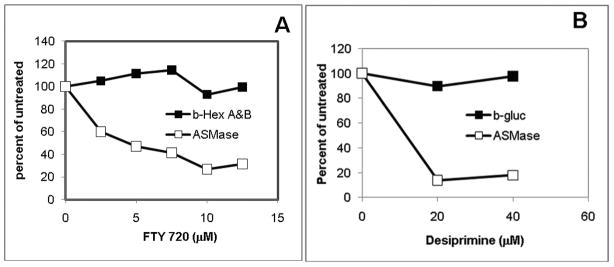
The inhibition of ASMase by FTY720 (Fig.1A) was similar to that previously reported for desipramine (Fig.1B); Representative other lysosomal hydrolases (β-D-hexosaminidase (Fig.1A) and β-D-glucosidase (Fig.1B) were not inhibited.
Fig.1. Dose-response of inhibition of ASMase by FTY-720.
Cells were incubated with FTY720 (Panel A) or desipramine (Panel B) at the concentration indicated for 24h, harvested, lysed and the specific activity of ASMase and other lysosomal hydrolase activities (β-D-hexosaminidase (Fig.1A) and β– D- glucuronidase (Fig.1B) were determined as described in the text.
3.2 FTY720 and inhibition of ASMase is blocked by the co-addition of leupeptin
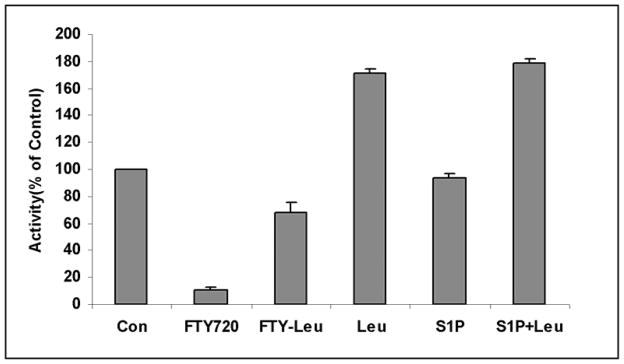
Treatment of LA-N-5 cells with FTY720 (10μM) induced a 90% inhibition of ASMase activity, reversible with co-addition of leupeptin (5μM) (FTY+Leu)(Fig.2). Leupeptin alone (5μM) stimulated ASMase but S1P (10μM) had no effect. Similarly, Myriocin, which is a zwitterionic, atypical amino acid (and therefore not an amphiphilic cation), did not inhibit ASMase activity under similar conditions.
Fig.2. FTY720 (but not S1P) inhibits ASMase in cultured cells, and leupeptin (Leu) can reverse the inhibition.
FTY720 and S1P were added at 10μM and leupeptin at 5μM as described in the text. Results were based on multiple assays run in triplicate and are expressed as percent of control activity.
Similar results were observed in other cell lines (eg. HOG and mouse fibroblasts, data not shown).
3.3 FTY720 had no effect on mRNA levels for ASMase but reduced ASMase enzyme protein
Treatment of cells with FTY720 (10μM) did not have any effect on mRNA levels (Fig.3A) but reduced ASMase protein levels in parallel with the reduction in enzyme activity (Fig 3B).
Fig.3. FTY720 does not affect the message levels for ASMase even when inhibiting activity >90%.
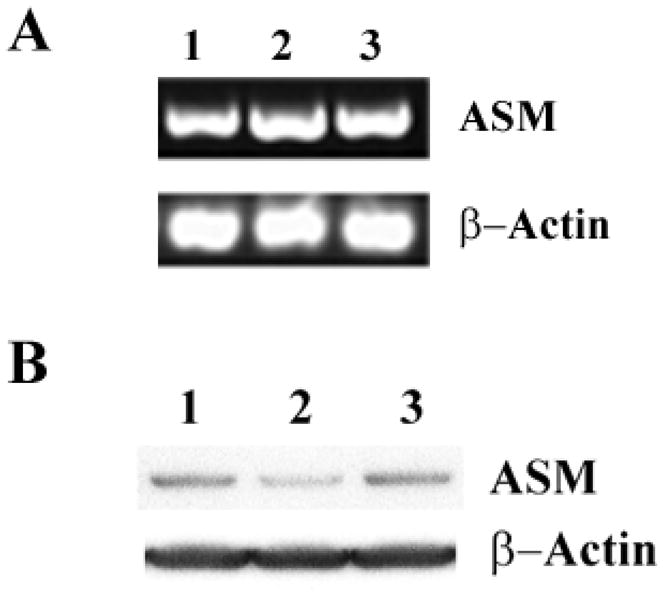
Panel A: RT-PCR of human LA-N-5 cells. Lane 1, control; lane 2, 10μM FTY720 treatment; Lane 3, 5μM Leupeptin pre-incubation for 1h followed by 10μM FTY720 treatment. β-Actin is the control for sample application. Panel B: Western blot of human LA-N-5 cells. Lane1, control; Lane2, 10μM FTY720 treatment; Lane 3, 5μM Leupeptin pre-incubation for 1h then 10μM FTY720 treatment for 24h. β-Actin is the control for sample application.
3.4. Neither FTY720 nor FTY720 phosphate inhibited ASMase in vitro
Since phosphates are potent non-competitive ASMase inhibitors in vitro (16) we compared the effect of FTY720 and FTY720-P (10μM) in intact cells (24h) with that in cell homogenates (up to 2h) and saw no in vitro inhibition at concentrations up to 50μM (Fig.4). Treatment with chloroquine or ammonium chloride reduced the levels of most lysosomal hydrolases and increased their secretion (data not shown) but there was no specificity for ASMase.
Fig.4. FTY720 and FTY720 phosphate have no inhibitory effect on ASMase in vitro.
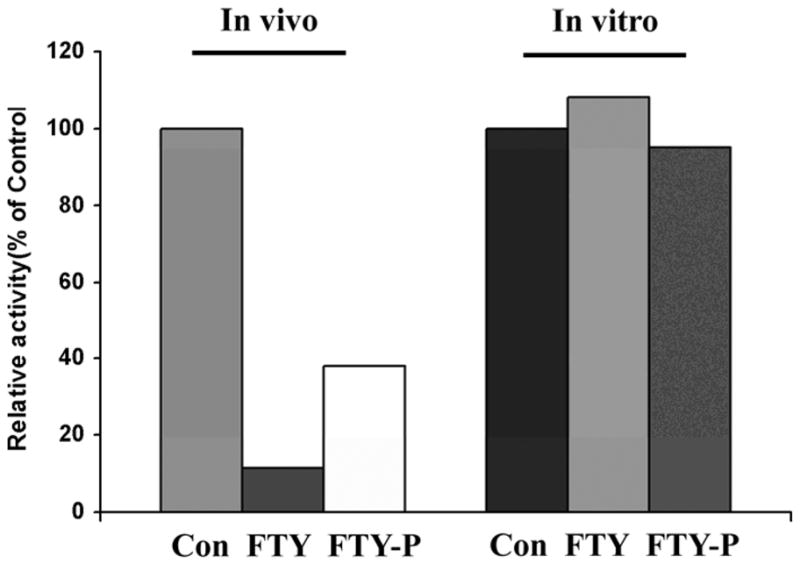
Both FTY720 and FTY720-P (10μM) for 24h inhibited ASMase in vivo. Treatment of homogenates with either drug at concentrations up to 50μM and times up to 2h did not result in any inhibition of ASMase activity, measured as described in the text.
4. Discussion
Our results suggested that FTY720 inhibits ASMase activity by a mechanism which involves the proteolytic degradation of the enzyme complex. In this regard it appears to be similar to the mechanism proposed for desipramine and amytryptaline action on ASMase and ACerase (11–14). Although FTY720 acts predominantly on the immune system and is believed to exert other important actions on S1P receptors after being converted to FTY720-P, this conversion is very small (<1% (2). FTY720 has been shown to affect the metabolism of a wide range of sphingolipids, possibly because it is structurally related to the inhibitor of de novo sphingolipid synthesis, myriocin (2, 3). However, neither myriocin nor S1P (which are zwitterionic) were inhibitory to ASMase, supporting the mechanism of amphiphilic cation action to release ASMase for degradation (11–14).
Unlike ceramide synthase-2 (2, 3) we observed no direct in vitro inhibition of ASMase by FTY720 but 70–90% inhibition in whole cells. Analyses of FTY720-treated cells showed a modest 10% increase in sphingomyelin and confirmed that over the lower concentration range used (0–10μM) there was very little decrease (10%) in ceramide levels (2). The reported 2-fold decrease in both sphingosine and S1P (2) may result from the reported inhibition of ceramide synthase (2, 3) and the likely inhibition of acid ceramidase which is sensitive to desipramine (12). At high concentrations (250–500μM), drugs such as Cocaine (a membrane-permeate weak base), are concentrated by acid tropic sequestration and rapidly inactivate acid sphingomyelinase, (a 10-fold decrease in Vmax with identical Km) as well as phospholipase A1 (17). This was blocked by the proton ionophore, monensin, the vacuolar ATPase inhibitor, bafilomycin as well as cathepsin protease inhibitors such as leupeptin and E64a. High concentrations of cocaine also induced selective sphingomyelinase proteolysis and extensive lipid storage in lysosomes (electron-dense lamellar structures after 3 days) which was rapidly reversed by cocaine removal, suggesting fast ASMase turnover. Thus the effects of FTY720 could be cumulative.
5. Conclusion
Our novel finding is that FTY720 inhibits ASMase in a manner very similar to that of other amphpiphilic cationic drugs known as functional inhibitors of acid sphingomyelinase (18). Both FTY720 and FTY720-phosphate had no direct effect on ASMase but were able to expose the lysosomal ASMase to proteases, since ASMase degradation and loss of activity was blocked by pre-exposure to cathepsin inhibitors. Leupeptin alone also increased basal ASMase activity but had no effect on other lysosomal hydrolase activities, suggesting a uniquely high turnover rate for ASMase. A 10% increase in sphingomyelin and a 10% depletion of ceramide was observed which shows a functional effect of the inhibition might cumulatively increase when FTY720 is used over many years.
Acknowledgments
We would like to acknowledge the excellent technical assistance of Sylvia Dawson and the partial support of USPHS Grant NS36866.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kappos L. Oral Fingolimod (FTY720) for relapsing multiple sclerosis. New Engl J Med. 2006;255:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 2.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an Acyl-CoA chain length-dependent manner. J Biol Chem. 2009;284:16084–16090. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan L, Kordula T, Spiegel S, Milstein S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008;1781:459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho RP, Saini HS, Sato-Bigbee C. Sphingosine-1-phosphate and oligodendrocytes: From cell development to the treatment of multiple sclerosis. Prostaglandins & other Mediators. 2009;91:139–144. doi: 10.1016/j.prostaglandins.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Takabe T, Paugh SW, Milstein S, Spiegel S. Inside-out signaling of sphingosine-1-phosphate therapeutic targets. Pharmacological Reviews. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. persistent signaling induced by FTY720-phosphate is mediated by internalized S1P receptors. Nature Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 8.Foster CA, Howard LM, Schweitzer A, Persohn E, Heistand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental Encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharm Exp Ther. 323:469–476. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 9.Miron VE, Ludwin SK, Darlington PJ, Jarjour AE, Soliven B, Kennedy TE, Antel J. Fingolimod enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176:2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz R, Ferlinz K, Sandhoff K. Biol. Chem Hoppe Seyler. Acid sphingomyelinase. 1994;375:447–450. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- 11.Kolzer M, Werth N, Sandhoff K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic anti-depressant desipramine. FEBS Lett. 2004;559:96–98. doi: 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- 12.Elojeimy S, Holman DH, Liu X, El-Zawahry A, Bielwaska A, Hannun Y, Norris JS. New insigts on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett. 2006;580:4751–4756. doi: 10.1016/j.febslet.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 13.Kornhuber J, Reichel M, Tripal P, Groemer TW, Henkel AW, Muhle C, Gulbins E. The role of ceramide in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2009;259(suppl 2):S199–S204. doi: 10.1007/s00406-009-0061-x. [DOI] [PubMed] [Google Scholar]
- 14.Buccinnà B, Piccinini M, Prinetti A, Scandroglio F, Prioni S, Valsecchi M, Votta B, Grifoni S, Lupino E, Ramondetti C, Schuchman EH, Giordana MT, Sonnino S, Rinaudo MT. Alterations of myelin-specific proteins and sphingolipids characterize the brains of acid sphingomyelinase-deficient mice, an animal model of Niemann-Pick disease type A. J Neurochem. 2009;109:105–15. doi: 10.1111/j.1471-4159.2009.05947.x. [DOI] [PubMed] [Google Scholar]
- 15.Kilkus J, Goswami R, Dawson SA, Testai FD, Berdyshev E, Han X, Dawson G. Differential regulation of sphingomyelin synthesis and catabolism in oligodendrocytes and neurons. J Neurochem. 2008;106:1745–1757. doi: 10.1111/j.1471-4159.2008.05490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testai FD, Landek MA, Goswami R, Ahmed M, Dawson G. Acid sphingomyelinase and inhibition by phosphate ion: role of PtdIns (3, 4, 5) P3 in oligodendrocyte signaling. J Neurochem. 2004;89:636–644. doi: 10.1046/j.1471-4159.2004.02374.x. [DOI] [PubMed] [Google Scholar]
- 17.Nassogne MC, Lizarraga C, N’Kuli F, Van Bambek F, Van Binst R, Wallemacq P, Tulkens PM, Mingeot-Leclercq MP, Levade T, Courtoy PJ. Cocaine induces a mixed lysosomal lipidosis in cultured fibroblasts, by inactivation of acid sphingomyelinase and inhibition of phospholipase A1. Toxicol Appl Pharmacol. 2004;194:1010–1018. doi: 10.1016/j.taap.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A Novel Pharmacological Group of Drugs with Broad Clinical Applications. Cell Physiol Biochem. 2010;26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]