Abstract
Background
Sequelae of Perthes disease commonly manifests as complex hip pathomorphology including coxa magna, coxa brevis, and acetabular dysplasia. These abnormalities contribute to femoroacetabular impingement and early osteoarthritis. This report describes our experience with correction of the proximal femoral deformity associated with Perthes disease via surgical dislocation, osteochondroplasty (SDO), trochanteric advancement, and treatment of intra-articular chondrolabral injury.
Methods
Between January 2003 and January 2009, 14 patients with Perthes disease (4 female and 10 male patients) with an average age of 19.6 years (range 14 – 28) were treated with SDO and trochanteric advancement. One patient had a subsequent staged periacetabular osteotomy to improve acetabular coverage. Patient histories, physical exams, operative findings, and pre and postoperative radiographs were evaluated.
Results
Operative findings demonstrated 6 acetabular cartilage lesions, 6 labral lesions, and 4 femoral osteochondritis dissecans (OCD) lesions treated with autografts. Mean center trochanteric distance improved from −20 mm to −1 mm. 4/14 hips deteriorated 1 Tönnis grade and 1/14 hips 2 grades. The Harris hip scores improved from an average of 62 preoperatively (range 51–72) to 95 postoperatively (range 93–97) versus 71 (range 65–76) to 88.6 (range 63–100) in the hips without OCD lesions. There was no statistically significant difference in the age, pre or postoperative HHS between the OCD and non-OCD groups. Mean follow up was 45 months. There were no major perioperative complications and all patients in both groups have their native hip to date.
Conclusions
The typical adult sequelae of Perthes disease predispose the hip to the development of chondrolabral injury and poor clinical function. Treatment with SDO and trochanteric advancement reduces impingement, improves hip biomechanics and allows treatment of intra-articular pathology. The described approach is associated with clinical improvement without major perioperative complication. Additionally, we have found a high rate of OCD lesions of the femoral head in Perthes hips undergoing surgical dislocation. Osteochondral autograft transfer from the resected femoral head-neck junction been found in the 4 patients treated thus far to be safe and effective with comparable clinical and radiographic outcomes to those hips without OCD lesions.
INTRODUCTION
Patients with Legg-Calve-Perthes disease (Perthes) transition to adulthood having had acquired pathologic hip morphology which predisposes them to femoroacetabular impingement (FAI) and subsequent degenerative joint disease1. While some have demonstrated successful treatment of young patients with advanced cartilage degeneration using hip arthroplasty2, 3, the authors of this study have dealt with a patient population who are symptomatic but do not yet demonstrate severe radiographic degenerative changes.
Recently, two different approaches to joint salvage of the young adult with Perthes changes of the hip have been forwarded. Ganz et al. have demonstrated that their surgical approach to the hip with dislocation and osteochondroplasty of the head-neck junction can be applied to the Perthes hip4, 5. Likewise, Clohisy et al. concluded that periacetabular osteotomy combined with femoral valgus-producing osteotomies can be beneficial in addressing morphological abnormalities6.
Surgical dislocation and osteochondroplasty (SDO) with trochanteric advancement via trochanteric osteotomy and advancement or distalization addresses the pathologic femoral head morphology and improves the abductor leverage by increasing center-trochanteric distance (CTD)7. This can be performed alone, or can be combined or staged8 with periacetabular osteotomy to improve acetabular coverage if needed. This combined approach is useful in treating patients with considerable acetabular deficiency in combination with the typical femoral changes of Perthes disease. The intention of this study was to perform a review of clinical and intraoperative findings collected prospectively as well as and pre and postoperative radiographs of patients who underwent surgical dislocation with osteochondroplasty for the treatment of the symptomatic hip with Perthes disease.
PATIENTS AND METHODS
We performed a database review the patients with Perthes disease treated with hip salvage procedures at our tertiary referral center between January 2003 and January 2009. Eighteen consecutive patients with Perthes disease were identified: three patients had primary periacetabular osteotomies to improve femoral head coverage coupled with a simultaneous osteochondroplasty of the femoral head through an anterior arthrotomy. Fifteen subjects were treated with surgical dislocation, osteochondroplasty of the femoral head-neck junction, fourteen of patients also had a relative neck lengthening via trochanteric advancement. Two of these patients underwent a staged surgical dislocation and osteochondroplasty with trochanteric advancement followed by a periacetabular osteotomy to correct acetabular deficiency8. We elected to retrospectively analyze the presentation, surgical treatment and clinical and radiographic outcomes of the fourteen patients that underwent both SDO and trochanteric advancement. All fourteen hips met the inclusion criteria which also included availability of pre and postoperative radiographs, histories and physical exams, and postoperative followup of six months or greater.
Of the fourteen patients who underwent trochanteric advancements via surgical dislocation, there were four female and ten male patients, with an average age of 19.6 years old (range 14–28). Prospectively collected clinical data on all patients, including presenting medical history, pre and postoperative Harris Hip Scores, clinical, operative, and radiographic findings were reviewed retrospectively. Patients typically had signs and symptoms of impingement as well as discomfort with activity.
Radiographs demonstrated a shortened femoral neck (coxa breva) in varus (coxa vara) as well as a flat, mushroom shaped head (coxa magna), and a relative overgrowth of the trochanter with a decreased or negative center-trochanteric distance (CTD)7, 9. (Figure 1) In addition to dysmorphic proximal femurs, hips with Perthes disease sometimes also have shallow acetabuli, up-sloping sourcil (acetabular roof) with varying degrees of anterior and/or lateral deficiency. (Figure 2)
FIGURE 1.
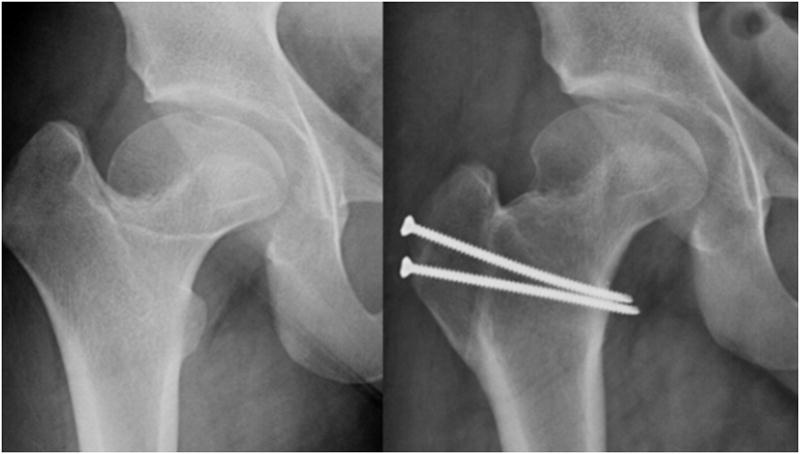
Anteroposterior radiographs pre and post surgical dislocation and relative femoral neck lengthening demonstrating improved head-neck offset and center trochanteric distance.
FIGURE 2.
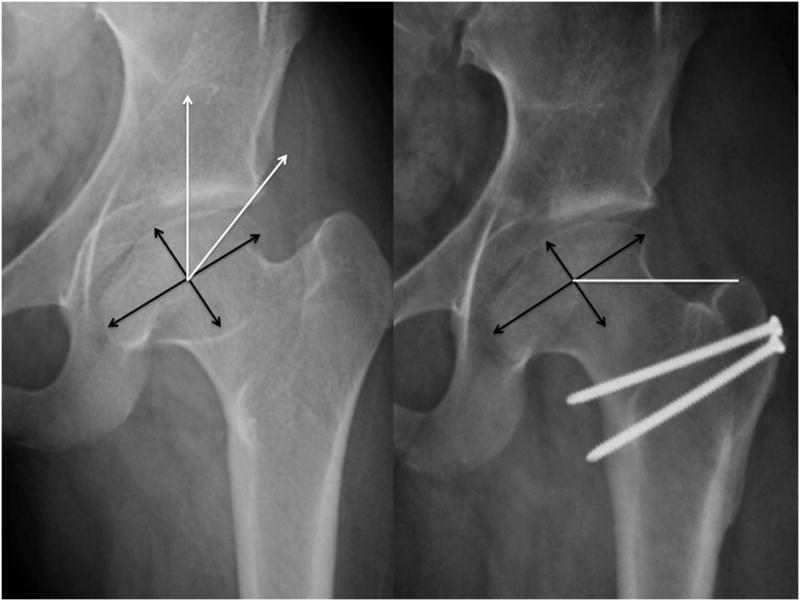
Anteroposterior radiographs pre (left) and post surgical dislocation and relative femoral neck lengthening (center) andpost staged periacetabular osteotomy (right). Post surgical dislocation radiograph demonstrates improved head-neck offset andcenter head distance. Post periacetabular osteotomy demonstrates improved lateral acetabular coverage.
Preoperative radiographs were evaluated for standard measurements including acetabular index, anterior and lateral center edge angles, and acetabular angle of Sharp. The center of the femoral head used for measuring radiographic measurements such as center-edge angles and CTD was determined by the intersecting of the longest and shortest diameters of the femoral head “egg.” (Figure 3) The modified classification system of Stulberg et al was used: Stulberg class-II femoral heads fit within 2 mm of a circle on both anteroposterior and frog-leg lateral radiographs; class-III were aspherical by more than 2 mm on either view; class-IV femoral head as one with at least 1 cm of flattening of the weight-bearing articular surface10. Radiographs were evaluated for crossover sign11–13, signifying acetabular retroversion, and posterior wall sign, signifying posterior acetabular deficiency11, 12.
FIGURE 3.
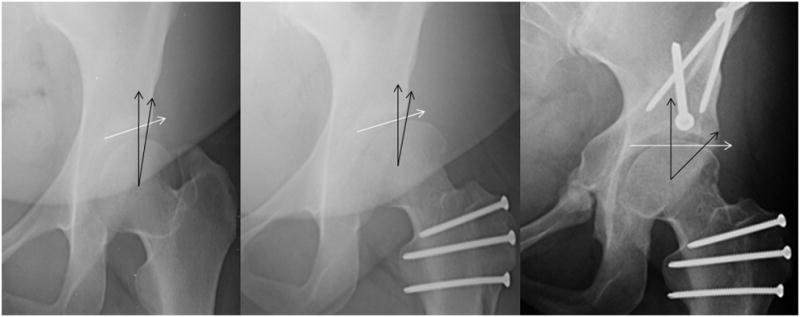
Anteroposterior hip radiographs. Preoperative radiograph on the left demonstrates a method for determining acetabular coverage when measuring center edge angles in hips with aspherical femoral heads associated with Perthes disease. Postoperative radiograph on the right utilizes the same technique when determining the center trochanteric distance, thedistance in mm that the trochanter is above (negative) or below (positive) the center of the femoral head.
Additionally, we measured pre and postoperative CTD,7 which is recorded in millimeters, indicating if the tip of the trochanter is above or below the level of the center of the femoral head, rather than the articulo-trochanteric distance (ATD)14, 15 which merely notes whether the tip of the trochanter is positive or negative, above or below the femoral head’s center. (Figure 3) Omeroğlu et al. described pathological CTD as distances greater than +7 millimeters and less than −17 millimeters. We were not aware of this classification or recommended range for normal prior to the surgeries reported in this study7.
Surgical Dislocation and Osteochondroplasty Technique
We utilize a lateral skin incision that was carried down through the subcutaneous fat and through the fascia lata using sharp dissection. The vastus lateralis was sharply elevated off the anterior proximal femur. The interval posteriorly between the medius and the posterior edge of the trochanter was developed. A trochanteric osteotomy was performed using a inch oscillating saw and completed with a straight osteotome. The gluteus minimus and medius were left attached to the superior aspect of the trochanteric fragment and the vastus lateralis remained attached to the inferolateral aspect as described by Ganz et al5. A Wagner retractor was placed over the anterior rim of the acetabulum to facilitate exposure. The interval between the piriformis and the short external rotators was left intact. With external rotation of the hip, the entire anterior hip capsule extending from the piriformis posteriorly to the anterior-inferior portion of the hip capsule was exposed.
A Z-shaped capsulotomy was then performed and sutures are placed in the flap corners. At this point, range of motion assessed including flexion as well as internal and external rotation which helps identify areas of impingement16. After transecting the ligamentum teres, the femoral head was dislocated without difficulty.
Special attention was given to identifying femoral head-neck morphology including offset, as well as severity and location of damage to the femoral and acetabular articular cartilage and the acetabular labrum. Osteochondritis dissecans (OCD) lesions of the femoral head were diagnosed on preoperative radiographs and magnetic resonance arthrography when present and confirmed intraoperatively. The lesions were treated with osteochondral autograft from the anterolateral aspect of asphericity of the femoral head that is removed with osteotomes to improve femoral head morphology. After the OCD lesion was debrided and burred to bleeding bone, the autograft was shaped with rongeurs and osteotomes to size and press fit into the lesion, attempting to make the cartilage surface flush with the adjacent articular surface. An absorbable pin (Arthrex Trim-It Drill Pin™, 2mm × 100mm) was utilized in one hip due to concern regarding autograft stability.
When Outerbridge III and IV acetabular cartilage damage or labral lesions were encountered the labrum was sharply transected from the affected rim with a long handled knife, leaving the anterior and posterior labrum attached like a “bucket-handle” as described by Espinosa et al17. The labrum was then debrided back to non-degenerated labrum and the cartilage lesions were treated with resection of the acetabular rim back to normal cartilage. A burr was used to create a bleeding cancellous bed along the extra-articular acetabular rim allowing for reattachment of the labrum via suture anchors. The femoral head-neck junction was debrided with the use of osteotomes and a high-speed burr to improve offset. The femoral head was returned to the acetabulum and correction of impingement was confirmed.
TROCHANTERIC ADVANCEMENT
Using towel clips for traction, the trochanteric fragment was advanced distally and inspected for position using fluoroscopy. When a satisfactory increase in the CTD was accomplished, a high-speed bur was used to create a smooth bleeding bone bed along the lateral surface of the proximal femur for stable positioning of the distalized greater trochanter. Attention should be made to limit the thickness of the trochanteric fragment and observe the degree trochanteric prominence after distalization. Too much offset has been shown to increase the risk for trochanteric bursitis and therefore debulking the trochanteric fragment in some cases may be necessary18.
K-wires were used for preliminary trochanteric fixation. After fluoroscopic confirmation of satisfactory distalization, two fully threaded, large-fragment cortical lag screws were placed. After distalization of the trochanteric fragment, there was usually a remaining spike or bulge of the previous medial aspect of the stable greater trochanter still attached to the superior base of the femoral neck. A combination of osteotomes, rongeurs, and a high-speed bur were used to contour the newly formed anterior and superior femoral neck-trochanter transition. A Freer elevator was used to elevate the retinaculum off the femoral neck during contouring to protect the blood supply. The capsule was then repaired without tension using interrupted absorbable suture, before closing the fascia, subcutaneous, and skin layers.
Occasionally, soft-tissue tension of the posterior retinaculum and soft tissues was noted when there was scarring from previous surgeries or need for greater distalization. In this situation, pedicalization of the posterior trochanteric fragment may be advisable19. This was accomplished by dissecting the vascular pedicle posteriorly before performing the initial trochanteric osteotomy. A small osteotomy of the posterior greater trochanter may be performed to allow the retinaculum to fall posteriorly and distally without tension on the retinacular vessels. The osteotomized posterior trochanteric fragment can then be debrided with a rongeur.
Statistical Methods
We compared the age, and preoperative and postoperative HHS of subgroups using Student’s t test. All of the collected data were analyzed using a commercially available software package (FileMaker® Pro 7.0, FileMaker, Inc, Santa Clara, CA; and Microsoft® Excel®, Microsoft Corp, Redmond, WA).
RESULTS
The Harris hip scores20 improved significantly from an average of 66 preoperatively (range 45–76) to 87 postoperatively (range 65–100) with a mean follow up of 45 months (p<0.0001). There were no major perioperative complications and all patients have their native hips to date. (Table 1) Eleven of the patients ambulated with a limp or aid prior to surgery compared to two patients after surgery.
Table 1.
Pre and Postoperative Clinical and Radiographic Findings
| Center Trochanteric Distance | Operative Findings | Tönnis Grade | Harris Hip Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Age | Stulberg Classification | Preop | Postop | Change | Acetabular | Femoral | Preop | Postop | Preop | Postop |
| M18 | III | −14 | −6 | 8 | Delamination | OCD (2×2cm) | 1 | 2 | 71 | 97 |
| M23 | III | −28.6 | −14 | 14.6 | OB3-4 | 0 | 1 | 71 | 97 | |
| M20 | III | −20 | −3 | 17 | 1 | 1 | 71 | 100 | ||
| M22 | IV | −33 | −16.8 | 16.2 | Labral Tear | 2 | 2 | 71 | 65 | |
| F15 | IV | −40 | −13 | 27 | OB 2 | 1 | 1 | 76 | 93 | |
| M21 | IV | −25.3 | −4 | 21.3 | OCD (1×1cm) | 1 | 1 | 71 | 97 | |
| F28 | IV | −25 | 4.5 | 29.5 | 0 | 2 | 65 | 67 | ||
| F18 | IV | −6.6 | 18 | 24.6 | 2 | 2 | 75 | 91 | ||
| M16 | IV | −15 | −1 | 14 | Delamination/Labral Tear | 1 | 1 | 73 | 91 | |
| F14 | IV | −21 | −3 | 18 | OB1/Labral Tear | 1 | 1 | 65 | 65 | |
| M19 | III | −11 | 0 | 11 | Labral Tear | OCD (1.5×2.5cm) | 1 | 1 | 51 | 90 |
| M19 | III | −15 | 0 | 15 | Delamination | 1 | 2 | 53 | 85 | |
| M21 | IV | −5 | +15 | 20 | Delamination/Labral Tear | 2 | 2 | 45 | 85 | |
| M20 | IV | −20 | +5 | 25 | OB2/Labral tear | OB2/OCD (1.5×3cm) | 2 | 3 | 62 | 93 |
| Avg | −20 | −1 | 18.7 | 66 | 86.9 | |||||
| Stddev | 9.9 | 9.6 | 6.2 | 9.6 | 12.3 | |||||
| p <0.0001 | ||||||||||
OCD: Osteochondral Defect
OB: Outerbridge
Operative findings at time of surgical dislocation and trochanteric advancement demonstrated the following: Six of fourteen hips had acetabular hyaline cartilage lesions, four of which were full thickness delamination lesions with a free edge adjacent to the labrum. Two hips with delamination were Class III and two were Class IV based upon the modified Stulberg radiographic classification system10. These lesions were addressed by sharply freeing the labrum from the acetabular rim in the area of the lesion, and debridement unstable cartilage. After resecting the damaged acetabular rim back to stable cartilage, the labrum was advanced and refixed with suture anchors to the ‘new’ acetabular rim. Six hips had discrete labral tears or labral detachments not associated with acetabular cartilage lesions. These lesions were treated by labral takedown, debridement of degenerated labrum, and refixation of the labrum by suture anchors to an acetabular rim debrided of soft tissue. Four hips had osteochondral defects of the femoral head that were treated with an autografts from the osteochondroplasty of the femoral head neck junction. (Table 1) (Figure 4A–F)
FIGURE 4.
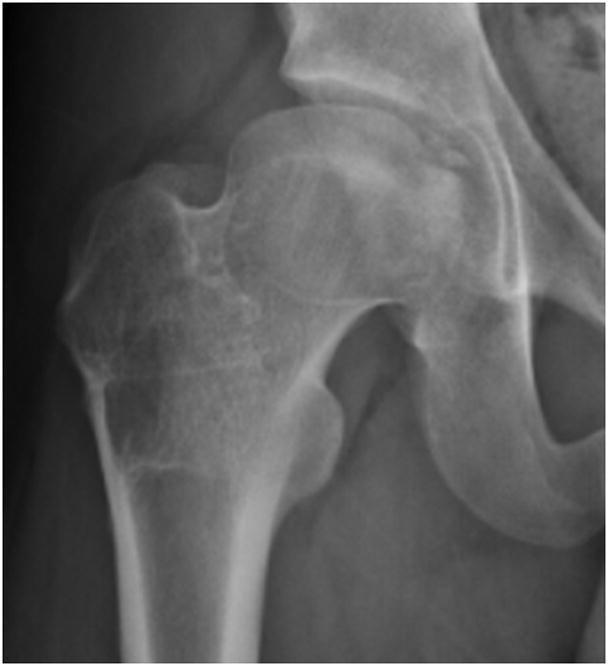
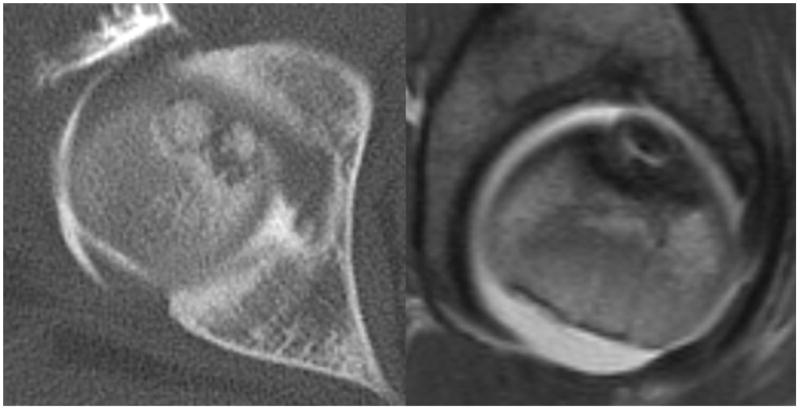
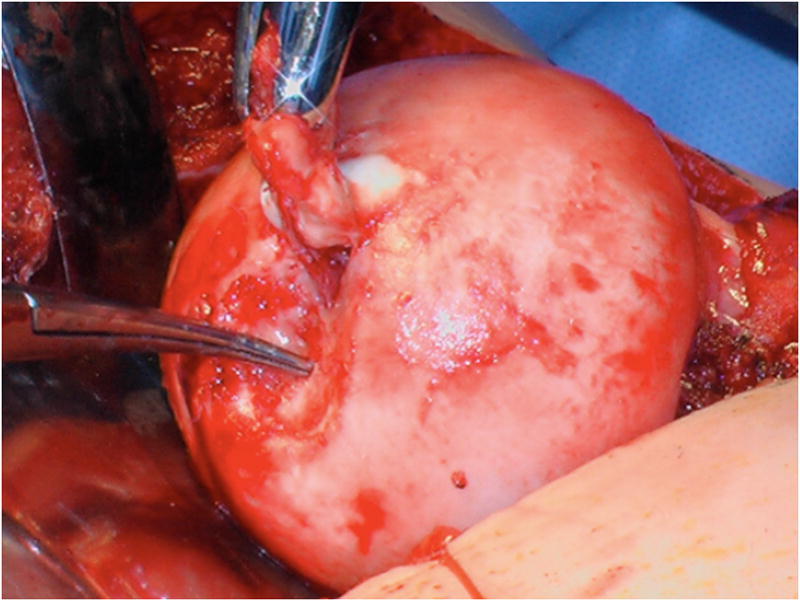
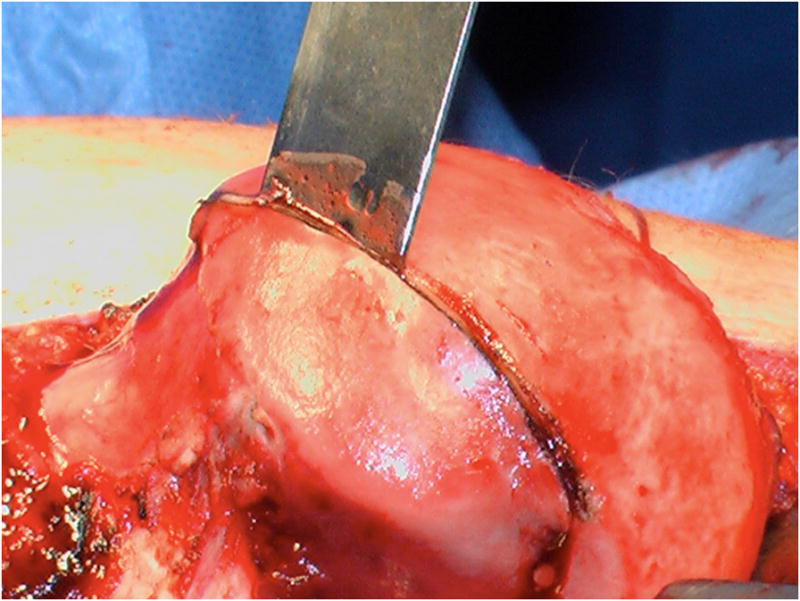
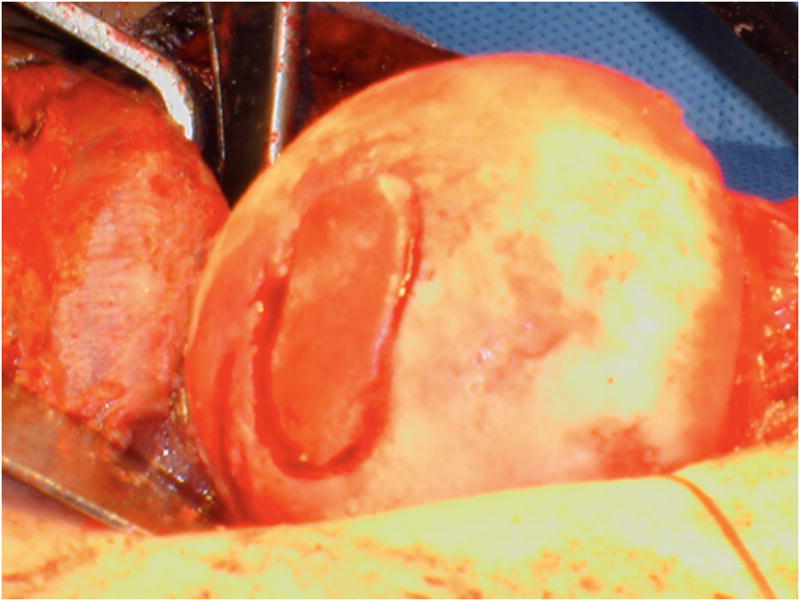
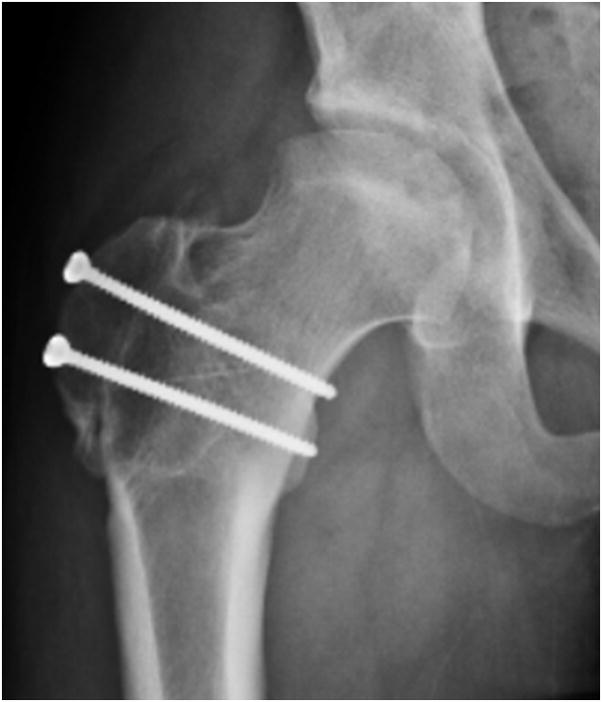
A–F, Preoperative MR arthrographic and radiographic images of a femoral OCD lesion and intraoperative pictures of the subsequent treatment. A, Preoperative anteroposterior radiographic demonstrating reduced CTD, a mushroom shaped femoral headand femoralOCD lesion. B, Preoperative sagittal (left) and axial (right) MR arthrogram images demonstrating a femoral OCD lesion. C, Intraoperative photographs of a femoral head OCD lesion undergoing debridement. D, Intraoperative photographs of anosteochondroplasty of the femoral head-neck juntion being performed which will be used as the osteochondral autograft for the femoral head OCD lesion. E, Intraoperative photographs and postoperative radiographs of an osteochondral autograft of a femoral head OCD lesion. F, Postoperative anteroposterior radiographic demonstrating improved CTD and head-neck offset, and healing off emoral OCD lesion. CTD indicates center trochanteric distance; OCD, osteochondral defect; MR, magnetic resonance.
Operative time averaged 130 minutes and estimated blood loss averaged between 525 milliliters (range 200–1500 ml; standard deviation 374 ml). Our postoperative protocol included fifty percent weight-bearing with crutches for six weeks after surgery after which physical therapy was utilized to address abductor weakness and range of motion restriction.
Average preoperative anterior center edge angle was 19 degrees and the average lateral center edge angle was 17 degrees. Acetabular index averaged 11 degrees and angle of Sharp averaged 42 degrees. Seven of the 14 hips had a posterior wall sign and six had a crossover sign, one of which was postoperatively corrected to due to rim resection for an acetabular hyaline cartilage delamination lesion. Mean CTD7(height of trochanter relative to center of femoral head) improved an average of 18.7 millimeters from −20 to −1.3 millimeters. Six of fourteen hips did not have a “pathologic” CTD (> +7 or < −17 millimeters) prior to surgery. Additionally, two hips which were within Omeroglu’s recommended CTD range prior to surgery had their trochanters distalized beyond − 17 millimeters. 0/14 hips in our study had CTD’s less than −17 millimeters after surgery. There was no significant difference between those hips with CTD’s within Omeroglu’s recommended range (12/14 hips) and those outside (2/14 hips) though our study numbers were small (p=0.89). Five hips were Class III and nine hips Class IV based upon the modified Stulberg radiographic classification10.
Four of 14 hips deteriorated one Tönnis grade and one hip deteriorated two grades on most recent follow up radiographs21. (Table 1)
The four hips with OCD lesions had improvement in the Harris hip scores from an average of 64 preoperatively (range 51–72) to 94 postoperatively (range 90–97) versus 67 (range 45–76) to 84 (range 65–100) in the hips without OCD lesions (p=0.65 and p=0.16 respectively). Average age was 19.6 years old (range 14–28 years) in the non-OCD group and 19.5 (range 18–21 years) in the OCD group (p>0.05). Two hips with OCD lesions were Stulberg Class III and two were Class IV. Mean follow up was 57 months compared to 42 months in the group without OCD lesions. There were no major perioperative complications and all patients in both groups with and without OCD lesions have their native hip to date. Of the four hips with OCD lesions of the femoral head, two deteriorated at least one grade using the Tönnis radiographic arthritis scale versus 3/11 of the hips without OCD lesions. (Table 1)
Two patients at latest follow-up did not have improvement in HHS compared to before surgery. The first patient was 22 years old at time of surgery with a preoperative HHS of 75 and a history of a prior proximal femoral varus derotation osteotomy as a youth. He had a small labral tear but no significant cartilage lesion at time of surgery. His HHS was above 90 until 4 years after surgery when it declined to 65 after he began working as a heavy laborer though on radiographs his Tönnis grade remained stable at grade 2. The second patient was a 14-year-old female with severe proximal femoral deformity and a preoperative HHS of 65. At surgery she had a small labral detachment and Outerbridge grade 1 softening of the anterosuperior acetabulum. Radiographs at latest followup demonstrate no deterioration. While she has had no complications and has her native hip to date, her postoperative HHS was 65 at 36 months of followup and she has been unsatisfied with her activity tolerance.
The third patient, a 28 year-old female, presented with significant acetabular deficiency as well as a mushroomed shaped head and reduced CTD. Preoperative planning included a staged surgical approach. A SDO and trochanteric advancement to address the femoral-sided deformity was performed first followed by a PAO after initial recovery to improve acetabular coverage of the femoral head. While she did not significantly improve clinically after the SDO (HHS improved from 65–67) her HHS improved to 94 after the PAO. All three of the failures were Class IV based upon the modified Stulberg classification, indicating severe asphericity and flattening of the femoral head. The postoperative HHS was not found to be statistically significant between the Class III and IV hips (average 94 versus 83; p=0.12) but was likely due to limited numbers.
DISCUSSION
Challenges of treatment of Perthes sequelae
Legg-Calve-Perthes disease (Perthes) is a disorder with no clear etiologic explanation though a number of factors have been implicated including genetic, post-traumatic, endocrinopathies, inflammatory, nutritional, as well as protein C and S disorders. Endochondral ossification of the femoral head temporarily ceases secondary to the vascular insult while the articular cartilage, which is nourished by synovial fluid, continues to grow. This leads to a smaller ossification center and thickening of the femoral head’s cartilage medially22.
Several resulting deformities include: coxa vara (decreased angle of the femoral shaft to center of the femoral head resulting in a shortened leg), coxa plana or magna (an enlarged, flattened, mushroom shaped head), and a relative overgrowth of the unaffected trochanter. The prominent trochanter paired with a shortened femoral neck and mushroom shaped femoral head can lead to femoroacetabular impingement and resultant chondrolabral damage1, 23.
The proximal femoral morphological abnormalities characteristic of Perthes disease as a group contribute to and are a well known cause of femoroacetabular impingement (FAI)24. The mushroom shaped head with decreased head/neck offset leads to abutment within the acetabulum or upon the acetabular rim that frequently leads to intra-articular damage25–27. Our study supports this concept as six of the fourteen hips in our series had labral lesions, six had significant acetabular hyaline cartilage damage, and four had osteochondral defects of the femoral head. (Table 1) It has shown that unless these acetabular lesions are addressed, osteoarthritis may progress rapidly16. We favor cartilage lesion debridement, labral takedown, rim resection, and labral advancement over microfracture or debridement alone16, 17. Preoperative advanced imaging including magnetic resonance arthrography has been helpful in our practice to evaluate the condition of the acetabular and femoral cartilage. Anecdotally, several patients who we were planning on performing hip preservation surgery for Perthes disease have been found to have severe supra-focal cartilage loss on preoperative magnetic resonance imaging despite maintained joint space on plain radiographs and thus were treated with total joint arthroplasty.
The short, thickened femoral neck associated with Perthes disease not only contributes to FAI but also plays a role in a reduction of the abductor lever arm. This leads to a deficiency of abductor strength, contributing to the limp and exercise induced fatigue and lateral pain associated with Perthes disease. In our series, eleven patients ambulated with a limp or aid prior to surgery. More recently, it has shown that high greater trochanter to femoral head center relationship caused by either overgrown trochanter or a varus femoral neck is associated with progression of osteoarthritis in hips with pistol grip deformity28. Interestingly, our only hip with Perthes disease to go on to total joint arthroplasty after surgical dislocation had only an osteochondroplasty without trochanteric advancement. In our series postoperative HHS and failures did not correlate with presence of acetabular cartilage lesion, likely indicating that the lesions were satisfactory addressed. However, failures were isolated to the Stulberg Class IV group, indicating that severity of asphericity and flattening of the head may be the biggest factor in postoperative clinical success.
Traditionally, surgical treatment of the adult hip with symptomatic Perthes disease was focused on one or more of the following: abductor weakness, acetabular dysplasia, FAI, and trochanteric impingement. The standard approach to treatment remains controversial. There are advocates of various methods from watch and wait29, 30 to those who argue for femoral sided osteotomies31–34, Salter35, 36 and Bernese6, 37, 38 peri-acetabular osteotomies, as well as combinations of these and others39, 40. To date, no gold standard for treatment of the characteristic adult Perthes deformities has been established.
Rational for SDO and trochanteric advancement to address Perthes Pathomorphology
When coupled with a trochanteric advancement, surgical dislocation is capable of addressing most of the pathology associated with Perthes disease, especially in those hips with only a mild acetabular deficiency. Trochanteric advancement improves the abductor leaver arm leading to more normal abductor muscle function. This is evidenced in our study by only two patients ambulating with a limp or aid after surgery compared to eleven prior. The lateral impingement secondary to the reduced CTD is improved and the neck is relatively lengthened by the advancement and removal of stable greater trochanteric bone. The surgical dislocation also permits osteochondroplasty of the head-neck junction and complete treatment of chondrolabral pathology resulting from FAI. Additionally, we have found a significant rate of osteochondritis dissecans lesions of the femoral head in Perthes hips undergoing surgical dislocation. Osteochondral autograft transfer from the resected femoral head-neck junction has in the four patients treated thus far been found to be safe and effective with postoperative clinical and radiographic outcomes comparable to those Perthes hips without OCD lesions and no complications. While we did not use a commercially available system to transfer the grafts, we believe this would be feasible option.
Other surgical options
Alternative treatments for adults with sequelae of Perthes disease are many. Isolated trochanteric distalization has been performed as a biomechanical correction to increase the abductor lever arm and therefore improve abductor function18, 41, 42. Its success is limited by its failure to address the morphological changes of the acetabulum and the femoral head.
Inter-trochanteric osteotomy attempts to address the coxa vara common to Perthes disease 33, 34, 40, 43–46. The valgus creating osteotomy increases the neck/shaft angle, which improves abductor function. The CTD is also increased which reduces trochanteric impingement. However, this surgical technique does not address the mushroom-shaped head or head/neck offset and can place the head in a plane that is no longer congruent with the dysplastic acetabulum.
Periacetabular osteotomy with anterior arthrotomy and debridement has been described by Clohisy6 among others47–50 as a successful method for the treatment of Perthes disease. In Clohisy’s series of 24 hips (20 patients) the periacetabular osteotomy was combined with proximal femoral valgus producing osteotomy in 13 hips, which can reduce the leg length inequality commonly seen in Perthes disease 40, 46, 51. Harris hip scores in Clohisy’s series improved from a preoperative average of 69 to a postoperative average of 91, which was comparable to our series (66 to 87) with similar follow-up (54 months versus 45 in our series). While this combination of procedures does allow many of the pathological features of Perthes disease to be addressed simultaneously, neither periacetabular nor proximal femoral osteotomies are technically simple procedures on their own, and when preformed simultaneously offer considerable technical challenges. Furthermore, the anterior arthrotomy utilized during periacetabular osteotomy does not allow the full visualization or treatment of acetabular cartilage lesions.
Limitations of SDO with trochanteric advancement
Our study has several limitations. This series has a relatively small number of patients and with limited clinical and radiographic followup. However, many patients with Perthes disease develop significant symptoms and physical limitations during early adulthood or even late childhood. Furthermore, there are limited surgical options for this young group of patients with joint preservation being preferable when possible over total joint arthroplasty. One limitation of the technique described is that it does not permit correction of leg length discrepancy. However, compared to other options such as the inter-trochanteric valgus producing osteotomy, the recovery is shorter and less morbid with less potential for complications while improvement in abductor function helps with the associated limp. A second limitation is that acetabular dysplasia is not addressed. However, many patients with Perthes have both morphology and symptoms that can be improved by isolated treatment of the femoral side of the hip. Developmentally, the acetabulum forms by guidance from the femoral head and even in the dysmorphic hips associated with Perthes disease the majority of acetabula are not grossly dysplastic.
Our approach in Perthes hips with major acetabular deficiency has been to proceed with periacetabular osteotomy with simultaneous arthrotomy and debridement of the head-neck junction. Hips with minimal to moderate acetabular deficiencies (center edge angles between 15 and 24 degrees) we have treated with surgical dislocation, osteochondroplasty of the head-neck junction to address the head deformity, and simultaneous trochanteric advancing osteotomy or trochanteric advancement to improve the CTD. We have found that patients with minimal acetabular deficiency tend to do well with this surgery. However, those with more moderate acetabular deficiency or acetabular cartilage lesions treated by acetabular rim resection and production of iatrogenic acetabular dysplasia may continue to have symptoms and may subsequently be treated with staged periacetabular osteotomy depending upon coverage and severity of symptoms8. (Figure 2)
Additionally, in our series clinical failure was associated with hips with severe asphericity and flattening of the femoral head (failure in 3/9 hips with Stulberg Class IV deformities). While these patients frequently have significant clinical symptoms and limited alternative options, preoperative counseling should encourage realistic expectations.
In conclusion, trochanteric distalization coupled with surgical dislocation and osteochondroplasty of the hip allows for safe inspection and treatment of intraarticular cartilage and labral pathology while providing an improvement in hip biomechanics.
Acknowledgments
NIH Grant# R01A053344
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Wenger DR, Kishan S, Pring ME. Impingement and childhood hip disease. J Pediatr Orthop B. 2006;15:233–243. doi: 10.1097/01202412-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz HC, Antoniades JT, Le Duff MJ. Results of metal-on-metal hybrid hip resurfacing for Crowe type-I and II developmental dysplasia. J Bone Joint Surg Am. 2007;89:339–346. doi: 10.2106/JBJS.F.00576. [DOI] [PubMed] [Google Scholar]
- 3.Boyd HS, Ulrich SD, Seyler TM, Marulanda GA, Mont MA. Resurfacing for Perthes disease: an alternative to standard hip arthroplasty. Clinical orthopaedics and related research. 2007;465:80–85. doi: 10.1097/BLO.0b013e318156bf76. [DOI] [PubMed] [Google Scholar]
- 4.Clohisy JC, Keeney JA, Schoenecker PL. Preliminary assessment and treatment guidelines for hip disorders in young adults. Clinical orthopaedics and related research. 2005;441:168–179. doi: 10.1097/01.blo.0000193511.91643.2a. [DOI] [PubMed] [Google Scholar]
- 5.Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. The Journal of bone and joint surgery. 2001;83:1119–1124. doi: 10.1302/0301-620x.83b8.11964. [DOI] [PubMed] [Google Scholar]
- 6.Clohisy JC, Nunley RM, Curry MC, Schoenecker PL. Periacetabular osteotomy for the treatment of acetabular dysplasia associated with major aspherical femoral head deformities. J Bone Joint Surg Am. 2007;89:1417–1423. doi: 10.2106/JBJS.F.00493. [DOI] [PubMed] [Google Scholar]
- 7.Omeroglu H, Ucar DH, Tumer Y. A new measurement method for the radiographic assessment of the proximal femur: the center-trochanter distance. Acta Orthop Traumatol Turc. 2004;38:261–264. [PubMed] [Google Scholar]
- 8.Anderson LA, Crofoot CD, Erickson JA, Peters CL. Staged surgical dislocation and redirectional periacetabular osteotomy: a report of five cases. J Bone Joint Surg Am. 2009;91:2469–2476. doi: 10.2106/JBJS.H.00066. [DOI] [PubMed] [Google Scholar]
- 9.Omeroglu H, Kaya A, Guclu B. Evidence-based current concepts in the radiological diagnosis and follow-up of developmental dysplasia of the hip. Acta Orthop Traumatol Turc. 2007;41(Suppl 1):14–18. [PubMed] [Google Scholar]
- 10.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86-A:2103–2120. [PubMed] [Google Scholar]
- 11.Jamali AA, Mladenov K, Meyer DC, Martinez A, Beck M, Ganz R, et al. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross-over-sign”. J Orthop Res. 2007;25:758–765. doi: 10.1002/jor.20380. [DOI] [PubMed] [Google Scholar]
- 12.Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A:278–286. doi: 10.2106/00004623-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. The Journal of bone and joint surgery. 1999;81:281–288. doi: 10.1302/0301-620x.81b2.8291. [DOI] [PubMed] [Google Scholar]
- 14.Kelikian AS, Tachdjian MO, Askew MJ, Jasty M. Greater trochanteric advancement of the proximal femur: a clinical and biomechanical study. Hip. 1983:77–105. [PubMed] [Google Scholar]
- 15.Tachdjian MO. Congenital Deformities, Pediatric Orthopedics. W.B. Saunders; Philadelphia, PA: 1990. [Google Scholar]
- 16.Peters CL, Erickson JA. Treatment of femoro-acetabular impingement with surgical dislocation and debridement in young adults. J Bone Joint Surg Am. 2006;88:1735–1741. doi: 10.2106/JBJS.E.00514. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa N, Beck M, Rothenfluh DA, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. Surgical technique. J Bone Joint Surg Am. 2007;89(Pt 1 Suppl 2):36–53. doi: 10.2106/JBJS.F.01123. [DOI] [PubMed] [Google Scholar]
- 18.Macnicol MF, Makris D. Distal transfer of the greater trochanter. The Journal of bone and joint surgery. 1991;73:838–841. doi: 10.1302/0301-620X.73B5.1894678. [DOI] [PubMed] [Google Scholar]
- 19.Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. Instr Course Lect. 2009;58:241–255. [PubMed] [Google Scholar]
- 20.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop. 1986:20–33. [PubMed] [Google Scholar]
- 21.Tonnis D, Heinecke A, Nienhaus R, Thiele J. Predetermination of arthrosis, pain and limitation of movement in congenital hip dysplasia (author’s transl) Z Orthop Ihre Grenzgeb. 1979;117:808–815. [PubMed] [Google Scholar]
- 22.Kaniklides C. Diagnostic radiology in Legg-Calve-Perthes disease. Acta Radiol Suppl. 1996;406:1–28. [PubMed] [Google Scholar]
- 23.Johnston TL, Schenker ML, Briggs KK, Philippon MJ. Relationship between offset angle alpha and hip chondral injury in femoroacetabular impingement. Arthroscopy. 2008;24:669–675. doi: 10.1016/j.arthro.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop. 2003:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 25.Zebala LP, Schoenecker PL, Clohisy JC. Anterior femoroacetabular impingement: a diverse disease with evolving treatment options. Iowa Orthopaedic Journal. 2007;27:71–81. [PMC free article] [PubMed] [Google Scholar]
- 26.Tanzer M, Noiseux N. Osseous abnormalities and early osteoarthritis: the role of hip impingement. Clinical orthopaedics and related research. 2004:170–177. [PubMed] [Google Scholar]
- 27.Snow SW, Keret D, Scarangella S, Bowen JR. Anterior impingement of the femoral head: a late phenomenon of Legg-Calve-Perthes’ disease. Journal of Pediatric Orthopedics. 1993;13:286–289. doi: 10.1097/01241398-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Bardakos NV, Villar RN. Predictors of progression of osteoarthritis in femoroacetabular impingement: a radiological study with a minimum of ten years follow-up. The Journal of bone and joint surgery. 2009;91:162–169. doi: 10.1302/0301-620X.91B2.21137. [DOI] [PubMed] [Google Scholar]
- 29.Zarzycka M, Zarzycki D, Kacki W, Jasiewicz B, Ridan T. Long-term results of conservative treatment in Perthes’ disease. Ortop Traumatol Rehabil. 2004;6:595–603. [PubMed] [Google Scholar]
- 30.Nowacki W, Szymkowiak E, Futyma J, Stencel P. A comparative analysis of conservative and surgical treatment of Perthes’ disease. Ortop Traumatol Rehabil. 2004;6:748–750. [PubMed] [Google Scholar]
- 31.Sugioka Y. Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: a new osteotomy operation. Clinical orthopaedics and related research. 1978:191–201. [PubMed] [Google Scholar]
- 32.Sugioka Y. Transtrochanteric rotational osteotomy in the treatment of idiopathic and steroid-induced femoral head necrosis, Perthes’ disease, slipped capital femoral epiphysis, and osteoarthritis of the hip. Indications and results. Clinical orthopaedics and related research. 1984:12–23. [PubMed] [Google Scholar]
- 33.Haverkamp D, Eijer H, Patt TW, Marti RK. Multi directional intertrochanteric osteotomy for primary and secondary osteoarthritis--results after 15 to 29 years. Int Orthop. 2006;30:15–20. doi: 10.1007/s00264-005-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santore RF. Intertrochanteric osteotomy for osteonecrosis. Semin Arthroplasty. 1991;2:208–213. [PubMed] [Google Scholar]
- 35.Kacki W, Zarzycka M, Zarzycki D, Kalicinski M, Sienkiel W, Jasiewicz B, et al. Comparison of radiological results of conservative and operative treatment by Salter osteotomy in severe cases of Perthes’ disease. Ortop Traumatol Rehabil. 2004;6:740–747. [PubMed] [Google Scholar]
- 36.Jasiewicz B, Zarzycka M, Zarzycki D, Kalicinski M, Tesiorowski M, Sienkiel W, et al. Salter osteotomy in Perthes’ disease. Late radiological results. Ortop Traumatol Rehabil. 2004;6:733–739. [PubMed] [Google Scholar]
- 37.Leunig M, Siebenrock KA, Ganz R. Rationale of periacetabular osteotomy and background work. Instructional course lectures. 2001;50:229–238. [PubMed] [Google Scholar]
- 38.Leunig M, Ganz R. The Bernese method of periacetabular osteotomy. Orthopade. 1998;27:743–750. doi: 10.1007/pl00003460. [DOI] [PubMed] [Google Scholar]
- 39.Napiontek M, Pietrzak S. Double osteotomy in the surgical treatment of Perthes’ disease: Dega’s transiliac osteotomy and subtrochanteric osteotomy. Ortop Traumatol Rehabil. 2004;6:728–732. [PubMed] [Google Scholar]
- 40.Turgeon TR, Phillips W, Kantor SR, Santore RF. The role of acetabular and femoral osteotomies in reconstructive surgery of the hip: 2005 and beyond. Clinical orthopaedics and related research. 2005;441:188–199. doi: 10.1097/01.blo.0000193541.72443.73. [DOI] [PubMed] [Google Scholar]
- 41.Givon U, Schindler A, Ganel A, Levy O. Distal transfer of the greater trochanter revisited: long-term follow-up of nine hips. J Pediatr Orthop. 1995;15:346–348. doi: 10.1097/01241398-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Givon U, Ganel A. Distal transfer of the greater trochanter. The Journal of bone and joint surgery. 1995;77:333–334. [PubMed] [Google Scholar]
- 43.Hersche O, Casillas M, Ganz R. Indications for intertrochanteric osteotomy after periacetabular osteotomy for adult hip dysplasia. Clin Orthop. 1998:19–26. [PubMed] [Google Scholar]
- 44.Notzli HP, Chou LB, Ganz R. Open-reduction and intertrochanteric osteotomy for osteonecrosis and extrusion of the femoral head in adolescents. J Pediatr Orthop. 1995;15:16–20. doi: 10.1097/01241398-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Trousdale RT, Ekkernkamp A, Ganz R, Wallrichs SL. Periacetabular and intertrochanteric osteotomy for the treatment of osteoarthrosis in dysplastic hips. J Bone Joint Surg Am. 1995;77:73–85. doi: 10.2106/00004623-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Santore RF, Turgeon TR, Phillips WF, 3rd, Kantor SR. Pelvic and femoral osteotomy in the treatment of hip disease in the young adult. Instructional course lectures. 2006;55:131–144. [PubMed] [Google Scholar]
- 47.Peters CL, Erickson JA, Hines JL. Early results of the Bernese periacetabular osteotomy: the learning curve at an academic medical center. J Bone Joint Surg Am. 2006;88:1920–1926. doi: 10.2106/JBJS.E.00515. [DOI] [PubMed] [Google Scholar]
- 48.Hickman JM, Peters CL. Hip pain in the young adult: diagnosis and treatment of disorders of the acetabular labrum and acetabular dysplasia. Am J Orthop. 2001;30:459–467. [PubMed] [Google Scholar]
- 49.Trumble SJ, Mayo KA, Mast JW. The periacetabular osteotomy. Minimum 2 year followup in more than 100 hips. Clin Orthop. 1999:54–63. [PubMed] [Google Scholar]
- 50.Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop. 1999:93–99. [PubMed] [Google Scholar]
- 51.Kim HT, Wenger DR. Surgical correction of “functional retroversion” and “functional coxa vara” in late Legg-Calve-Perthes disease and epiphyseal dysplasia: correction of deformity defined by new imaging modalities. J Pediatr Orthop. 1997;17:247–254. doi: 10.1097/00004694-199703000-00020. [DOI] [PubMed] [Google Scholar]


