Abstract
OBJECTIVE
To evaluate the association of urinary symptoms with risk of falls in community-dwelling elderly men.
PATIENTS AND METHODS
We evaluated 5872 participants in MrOS, a prospective cohort study of risk factors for falls and osteoporotic fractures among community-dwelling men aged 65 years and older. We used Poisson regression models with a robust variance estimator to evaluate the association of urinary symptoms at study entry with falls occurring during 1 year of follow-up. We considered age, history of falls, history of dizziness, multiple physical performance measures, body size, and medication use as potential confounders.
RESULTS
At baseline, 3188 (54%) reported mild, 2301 (39%) moderate, and 383 (7%) severe symptoms. Compared to men with mild symptoms, the adjusted 1-year cumulative incidence of falls was significantly higher among men with moderate or severe symptoms. Risk of at least 1 fall was increased by 11% among those with moderate (relative risk [RR] 1.11, 95% confidence interval [95% CI] = 1.01 - 1.22; P = 0.02) and by 33% among those with severe symptoms (RR 1.33, 95% CI = 1.15 - 1.53; P < 0.001). Further, those with moderate had a 21% (RR 1.21, 95% CI = 1.05 - 1.40; P = 0.01) and those with severe symptoms a 63% (RR 1.63, 95% CI = 1.31 - 2.02; P < 0.001) increased risk of at least 2 falls. Symptoms most strongly associated with falls were urinary urgency, difficulty initiating urination, and nocturia.
CONCLUSION
Moderate and severe urinary symptoms independently increase the 1-year risk of falls—particularly recurrent falls—in community-dwelling older men.
Keywords: falls, benign prostatic hyperplasia, lower urinary tract symptoms, risk factor, overactive bladder, elderly, American Urological Association Symptom
Introduction
Male lower urinary tract symptoms represent a cluster of highly prevalent chronic urinary disorders that occurs among 15% to 60% of males over the age of 40 years.[1-5] The prevalence increases sharply with age.[3, 5, 6] The most common etiologies for chronic lower urinary tract symptoms are benign prostatic hyperplasia and overactive bladder, conditions that collectively afflict tens of millions of older men.[1, 7] Benign prostatic hyperplasia affects 70% of men aged 60 to 69 years and 80% of those 70 years or older.[1] Among men aged 75 years or older, 42% describe symptoms consistent with overactive bladder.[7]
Nearly 1 in 4 community-dwelling men over the age of 65 years experiences one or more falls each year.[8, 9] A substantial proportion of these individuals will suffer serious injury, pain, depression, and other morbidities. Multifaceted interventions directed toward known risk factors are an effective means of preventing falls in men living in the community.[10]
Lower urinary tract symptoms are a potential risk factor for falls in community-dwelling men. Published reports have suggested that nocturia and urinary incontinence are associated with an increased likelihood of falls in older men.[11-15] The mechanistic inference is that these symptoms may force abrupt, unexpected alterations upon daily physical routines and compel affected individuals to engage in potentially risky behaviors, such as arising several times a night to urinate. Thus, if this association is confirmed, urinary symptoms may be a potential target for preventive interventions to reduce fall risk in community-dwelling men.
Limitations in previous studies of the association of urinary symptoms with falls in men include use of cross-sectional study designs, use of nonvalidated instruments for urinary symptom measurement, study populations composed predominantly of women, and analyses that accounted for limited numbers of confounders. Moreover, previous studies focused primarily on nocturia or urinary incontinence without considering other common urinary symptoms that are the clinical hallmarks of benign prostatic hyperplasia and overactive bladder.[11-13, 15]
Therefore, in the current study, we prospectively investigated the independent association of lower urinary tract symptoms as measured by a validated instrument with incident falls in a large cohort of community-dwelling older men.
Patients and Methods
Study population
The Osteoporotic Fractures in Men (MrOS) Study is a cohort study of older men designed to identify risk factors for falls and fractures. MrOS enrolled 5995 participants from March 2000 through April 2002 as previously described.[16, 17] Briefly, recruitment occurred at 6 US academic medical centers in Birmingham, AL, Minneapolis, MN, Palo Alto, CA, Pittsburgh, PA, Portland, OR, and San Diego, CA. Recruitment was accomplished primarily through targeted mailings based on motor vehicle registration, voter registration, and Veteran’s Administration databases. Eligible participants were at least 65 years of age, able to walk without assistance from another person, and had not had bilateral hip replacement surgery. Each study site enrolled approximately 1,000 men. All participants gave written informed consent.
Lower urinary tract symptoms
At the baseline study visit, participants completed questionnaires and interviews regarding demographic characteristics, lifestyle factors, and medical conditions including lower urinary tract symptoms. Lower urinary tract symptoms were assessed with the American Urological Association Symptom Index (AUA-SI)[18] on a self-reported questionnaire. The AUA-SI is a validated and reliable quantitative instrument for measuring urinary symptoms in men.[19, 20] It contains 7 items on the amount of time in the past month the respondent has experienced incomplete bladder emptying, frequent urination, intermittence, urgency, weak urinary stream, hesitancy, and nocturia. Responses range from 0 (“not at all” or “0 times”) to 5 (“almost always” or “5 or more times”) and are summed across all items to yield a total symptom score. Overall urinary symptom severity is categorized as mild (0 to 7 points), moderate (8–19 points), or severe (20 to 35 points).[18]
Demographics, lifestyle, and medical history
Demographic characteristics were age, race/ethnicity (white, black/African American, Asian, Hispanic, other), education level, marital status, and self-reported health (excellent, good, fair, poor, or very poor). Lifestyle factors were cigarette smoking (current, past, or never), current alcohol consumption (nondrinker, 1-<7 drinks/week, and ≥7 drinks/week) and physical activity. Responses to the physical activity scale for the elderly (PASE), a validated instrument designed specifically for use among older adults, provided an overall physical activity score based on household, leisure, and occupational activities.[21] Medical history included self-report of falls in the 12 months before baseline, trouble with dizziness, or any of several medical conditions diagnosed by a doctor including arthritis, diabetes, hypertension, angina, myocardial infarction, stroke, prostate cancer, prostatitis, or benign prostatic hyperplasia. Men were classified as using herbal supplements for urinary symptoms if they reported currently using saw palmetto, South African star grass, stinging nettle, rye grass pollen, pumpkin seed, or African plum. Prescription medications used in the past 30 days were recorded from the information on packages that men brought to their baseline visit and matched to their ingredients based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA). Men were classified as taking prescription urological medications if they were currently using 5-alpha reductase inhibitors, alpha-blockers, or urinary-specific antispasmodics. Men were classified as users of central nervous system prescription medications if they were currently taking selective serotonin reuptake inhibitors (SSRIs), benzodiazapine, or a non-benzodiazapine anticonvulsant.[22]
Body size and physical performance
Several variables were measured by study staff at the baseline visit. Body size included height measured using a Harpenden stadiometer, weight measured on balance beam or digital scales with the participant wearing indoor clothing except shoes, and body mass index (BMI) calculated as weight divided by the square of height in meters (kg/m2). Physical performance and function included grip strength, gait speed, narrow walk, use of walking aids, and self-report of mobility limitations. Grip strength was assessed as maximum force in kilograms using a hand dynamometer (Jamar, Bolingbrook, IL). Gait speed (m/s) was calculated from the time to walk a 6-meter course at a usual walking pace. The narrow walk time was recorded as the number of seconds to walk the same 6-meter course while keeping each foot inside a 20-cm wide lane. A narrow walk trial was considered successful if there were no more than 2 deviations from the lane. Participants had up to 3 trials to complete 2 successfully. The number of successful trials was categorized as 0, 1, or 2. Men who used a cane, walker, or other assistive device were classified as walking-aid users. Mobility limitation was classified as self-report of difficulty walking 2 to 3 blocks, climbing up 10 steps, or both.[23]
Ascertainment of falls
Follow-up for falls was conducted via questionnaires mailed to each participant triannually in March, July, and November beginning after his enrollment. For example, the first questionnaire was mailed on July 1, 2000 to men enrolled in the MrOS cohort by that time. Participants were asked if they had fallen in the past 4 months and the months of recall were listed (eg, the July 1 questionnaire lists March, April, May, and June). Those who reported falling were asked the number of times they fell (1, 2, 3, 4, or ≥ 5). Telephone interviews were used to obtain information from participants who did not return the questionnaire. Response to each follow-up questionnaire was 99%.
Analytic cohort and fall outcomes
From the MrOS cohort of 5995, we excluded men from the analysis because of missing information on lower urinary tract symptoms (n = 4), a history of Parkinson’s disease (n = 52), death or termination from the study before their first triannual questionnaire (n = 9), or incomplete fall information during the first follow-up year (n = 58). The remaining 5872 made up the analytic cohort.
For this analysis, we used the first 3 triannual questionnaires for each participant, representing follow-up of 1 year after a participant’s baseline visit. Fall status was considered complete if a participant completed all 3 questionnaires, or if he had completed all questionnaires up to the time he died or requested termination from the study. Men who reported at least 1 fall on any questionnaire in the year of follow-up were classified as having fallen. To evaluate recurrent falling, men were also classified as falling at least 2 times (compared with 0–1 time) based on the sum of the numbers of falls reported on the questionnaires during the 1-year follow-up period.
Statistical analysis
Baseline characteristics were described according to category of lower urinary tract symptoms severity. Differences in means of continuous variables were compared with the F test from a one-way analysis of variance (ANOVA), and differences in proportions were compared with chi-square tests from contingency tables. For each lower urinary tract symptoms category, the 1-year cumulative incidence (risk) of ≥ 1 and of ≥ 2 falls was each computed as the number of men with the specific outcome divided by the total number of men in the symptom category.
Risk ratios (RR) were used as the measure of association between lower urinary tract symptoms severity and fall risk and were computed as the 1-year cumulative incidence in the moderate or the severe lower urinary tract symptoms category divided by the 1-year cumulative incidence in the referent (mild) category. RR and 95% confidence intervals (CI) were estimated from multivariable Poisson regression models with a robust variance estimator.[24] During variable selection, all of the previously noted demographic, lifestyle, medical history, prostate disease, urological medication, CNS medication, body size, and physical performance measures were evaluated as potential confounding factors. A variable was considered a confounder if it changed the RR estimates by at least 10%.[25] If a variable was a confounder, it was retained in the model. When several variables from the same group (eg, mobility limitation and narrow walk) were identified as confounders, we ranked them according to the strength of the confounding and their contribution to model fit using the change in the −2 log likelihood value. We then retained the first-ranked variable in the model and added the remaining variables sequentially by rank, retaining the added variable if it remained a confounder in the presence of the other variables already in the model. We repeated this procedure until all variables had been evaluated. We also evaluated which aspect of the same variable (eg, number of narrow-walk trials or time to complete the narrow walk) was the strongest confounder. Based on this selection procedure, age, history of falls, history of dizziness, mobility limitation, and number of narrow-walk trials completed were identified as confounding factors of the association of urinary symptoms with fall risk and were thus included in the final, multivariable model.
Results
Participants and urinary symptoms at baseline
The mean (SD) age of the participants was 74 (6) years; 90% were white, many had coexisting morbidities, and approximately 20% used medications for urological symptoms (Table 1). As reported previously in this cohort, nearly half of the men experienced moderate or severe urinary symptoms.[4] Mean (SD) AUA-SI for the cohort was 8.3 (6.3); for participants with mild, moderate, and severe urinary symptoms the mean scores were 3.7 (2.1), 12.1 (3.3), and 23.3 (3.4), respectively.
Table 1.
Baseline characteristics according to urinary symptom severity* among men ≥ 65 years: the MrOS study.
| All n = 5872 |
Mild urinary symptoms n = 3188 (54%) |
Moderate urinary symptoms n = 2301 (39%) |
Severe urinary symptoms n = 383 (7%) |
P value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 73.6 (5.8) | 73.1 (5.8) | 74.1 (5.9) | 74.6 (6.2) | < .0001 |
| Range | 65 to 100 | 65 to 92 | 65 to 100 | 65 to 92 | |
| 64 to 69 | 1747 (30) | 1047 (33) | 606 (26) | 94 (25) | |
| 70 to 74 | 1671 (28) | 924 (29) | 634 (28) | 113 (30) | |
| 75 to 79 | 1421 (24) | 724 (23) | 613 (27) | 84 (22) | |
| ≥ 80 | 1033 (18) | 493 (15) | 448 (19) | 92 (24) | |
| BMI (kg/m2) | |||||
| Mean (SD) | 27.4 (3.8) | 27.3 (3.7) | 27.4 (4) | 27.7 (3.9) | .06 |
| Range | 17.2 to 50.7 | 17.9 to 46.7 | 17.2 to 50.7 | 17.5 to 40.2 | |
| Race | .39 | ||||
| White | 5258 (90) | 2841 (89) | 2080 (90) | 337 (88) | |
| African American | 235 (4) | 124 (4) | 86 (4) | 25 (7) | |
| Asian | 186 (3) | 116 (4) | 61 (3) | 9 (2) | |
| Hispanic | 124 (2) | 71 (2) | 47 (2) | 6 (2) | |
| Other | 69 (1) | 36 (1) | 27 (1) | 6 (2) | |
| PASE score | < .0001 | ||||
| Mean (SD) | 147.1 (68.1) | 486.4 (69.2) | 141.8 (66) | 134.6 (67.2) | |
| Range | 0 to 358 | 0 to 152.4 | 0 to 426.7 | 0 to 358.0 | |
| Medical history | |||||
| Arthritis or gout | 2791 (48) | 1401 (44) | 1179 (51) | 211 (55) | < .0001 |
| Benign prostatic hyperplasia |
2867 (49) | 1207 (38) | 1379 (60) | 281 (73) | < .0001 |
| Diabetes | 637 (11) | 334 (10) | 246 (11) | 326 (15) | .06 |
| Dizziness | 1471 (25) | 635 (20) | 691 (30) | 145 (38) | < .0001 |
| Fall in prior 12 months |
1231 (21) | 567 (18) | 557 (24) | 107 (28) | < .0001 |
| Heart attack | 813 (14) | 380 (12) | 361 (16) | 72 (19) | < .0001 |
| Hypertension | 2534 (43) | 1299 (41) | 1043 (45) | 192 (50) | < .0001 |
| Prostate cancer | 695 (12) | 387 (12) | 259 (11) | 49 (13) | .69 |
| Stroke | 333 (6) | 156 (5) | 139 (6) | 38 (10) | .0002 |
| Use of urological medications |
|||||
| Finasteride | 177 (3) | 62 (2) | 94 (6) | 21 (9) | < .0001 |
| Alpha-blocker | 896 (15) | 281 (9) | 485 (21) | 130 (34) | < .0001 |
| Anticholinergic | 66 (1) | 17 (0.5) | 38 (2) | 11 (3) | < .0001 |
As measured by the American Urological Association Symptom Index [18]
Men with more severe urinary symptoms tended to be older (P< .0001), had lower physical activity scores (P< .0001), and were more likely to report: arthritis or gout (P< .0001), benign prostatic hyperplasia (P< .0001), recent episodes of dizziness (P< .0001), a fall in the previous 12 months (P< .0001), heart attack (P< .0001), hypertension (P< .0001), stroke (P = .0002), and use of urological medications (P< .0001) (Table 1).
Risk of falls: overall lower urinary tract symptoms
During 1 year of follow-up, 1489 (25%) men reported at least 1 fall and 694 (12%) reported recurrent (≥ 2) falls. The unadjusted 1-year cumulative incidence of any fall varied by symptom severity: 22% for mild, 27.0% for moderate, and 37.4% for severe. The unadjusted cumulative incidences of recurrent falls were 9%, 14%, and 22%, respectively.
The adjusted 1-year cumulative incidences of any fall and of recurrent falls were significantly higher among men with moderate or with severe urinary symptoms (Table 2). Compared to men with mild symptoms, the multiply-adjusted risk of any fall was significantly increased by 11% among those with moderate and by 33% among those with severe symptoms; the multiply-adjusted risk of recurrent falls was significantly increased by 21% among those with moderate and by 63% among those with severe symptoms (Table 2).
Table 2.
Association of urinary symptoms* with 1-year risk of falls among men ≥ 65 years: the MrOS study
| Any fall |
≥ 2 falls |
|||||
|---|---|---|---|---|---|---|
| Urinary Symptom Category | Urinary Symptom Category | |||||
| None/Mild | Moderate | Severe | None/Mild | Moderate | Severe | |
| Unadjusted RR (95% CI) |
ref | 1.26 (1.15–1.38) |
1.68 (1.45–1.94) |
ref | 1.49 (1.29–1.73) |
2.37 (1.90–2.95) |
| Age-adjusted RR (95% CI) |
ref | 1.23 (1.12–1.34) |
1.62 (1.40–1.87) |
ref | 1.44 (1.24–1.67) |
2.21 (1.78–2.76) |
| Multivariable RR† (95% CI) |
ref | 1.11 (1.01–1.22) |
1.33 (1.15–1.53) |
ref | 1.21 (1.05–1.40) |
1.63 (1.31–2.02) |
| P value | .02 | < .001 | .01 | < .001 | ||
As measured by the American Urological Association Symptom Index [18]
Includes adjustment for age, history of falls, history of dizziness, mobility limitation, and number of narrow-walk trials completed
The RR estimates in the urinary symptom categories remained virtually unchanged after adjustments for histories of prostate cancer and benign prostatic hyperplasia during the variable selection process, indicating that these conditions were not confounders (data not shown). In addition, we repeated the analyses after restricting the analytic cohort in 2 different ways: first, to men with no history of prostate cancer (n = 5163); second, to men with no history of falls in the 12 months before baseline (n = 4641). In these analyses, the associations were similar to those shown in Table 2. When men with prevalent prostate cancer were excluded, the multivariable RRs (95% CI) of any fall were 1.09 (0.99 – 1.21) for moderate and 1.25 (1.04 – 1.49) for severe symptoms. When men with a fall history were excluded, the multivariable RRs (95% CI) of any fall were 1.19 (1.05 – 1.34) for moderate and 1.35 (1.09 – 1.67) for severe symptoms.
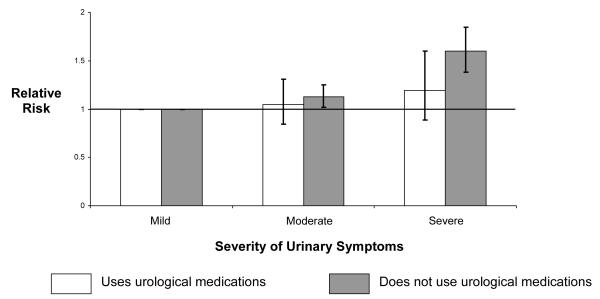
Use of urological medications
Since urinary symptoms were associated with increased incidence of falls, we hypothesized that use of urological medications, which diminish urinary symptoms, would attenuate this risk. To test this hypothesis, we stratified participants by use of urological medications and repeated the analyses. Effect estimates were similar for those who used urological medications—alpha-blockers, finasteride, or uroselective anticholinergics—compared to those who did not (Figure 1).
Figure 1.
Association of urinary symptom severity* with 1-year risk of any fall within strata of prescription urological medication† use.
*As measured by the American Urological Association Symptom Index [18]
†5-alpha reductase inhibitors, alpha-blockers, and urinary antispasmodics
Risk of falls: individual lower urinary tract symptoms
To determine the relation of fall risk with specific urinary tract symptoms, we entered each symptom of the AUA-SI into a separate multivariable model. The strongest predictors were urinary urgency, defined as the sensation of needing to urinate within 2 hours of previously urinating (with a 31% increased risk of any fall and 59% increased risk of recurrent falls, respectively, for individuals reporting that symptom as occurring at least half the time); the need to push or strain to initiate urination (with a 24% and a 60% increased risk, respectively); and nocturia, defined as arising at least 4 times per night to urinate (with a 23% and a 42% increased risk, respectively) (Table 3).
Table 3.
Associations of individual urinary symptoms* with 1-year risk of falls among men ≥ 65 years: the MrOS study†
| Fall Outcomes |
||||||
|---|---|---|---|---|---|---|
| Any fall |
≥ 2 falls |
|||||
| Symptom Frequency |
Symptom Frequency |
|||||
| Symptom | Not at all | < 1/2 the time | ≥1/2 the time | Not at all | < 1/2 the time | ≥1/2 the time |
| Sense of incomplete emptying |
||||||
| Multivariable RR (95% CI) |
ref | 1.07 (0.97–1.18) |
1.19 (1.06–1.33) |
ref | 1.17 (1.01–1.37) |
1.39 (1.16–1.66) |
| Frequency |
||||||
| Multivariable RR (95% CI) |
ref | 1.12 (1.00–1.26) |
1.17 (1.04–1.33) |
ref | 1.22 (1.02–1.47) |
1.25 (1.02–1.53) |
| Stop and start during urination |
||||||
| Multivariable RR (95% CI) |
ref | 1.07 (0.97–1.17) |
1.10 (0.98–1.23) |
ref | 1.22 (1.05–1.42) |
1.26 (1.05–1.51) |
| Urgency |
||||||
| Multivariable RR (95% CI) |
ref | 1.18 (1.07–1.31) |
1.31 (1.17–1.47) |
ref | 1.27 (1.08–1.49) |
1.59 (1.33–1.89) |
| Weak urinary stream |
||||||
| Multivariable RR (95% CI) |
ref | 1.06 (0.96–1.16) |
1.08 (0.97–.21) |
ref | 1.29 (1.10–1.52) |
1.20 (1.00–1.42) |
| Push or strain to start urination |
||||||
| Multivariable RR (95% CI) |
ref | 1.03 (0.93–1.15) |
1.24 (1.06–1.46) |
ref | 1.15 (0.98–1.35) |
1.60 (1.27–2.02) |
| Nocturia (times per night) | 0-1 | 2-3 | 4-5 | 0-1 | 2-3 | 4-5 |
|
|
|
|||||
| Multivariable RR (95% CI) |
ref | 1.05 (0.96–1.16) |
1.23 (1.08–1.41) |
ref | 1.11 (0.95–1.28) |
1.42 (1.16–1.74) |
As measured by the American Urological Association Symptom Index [18]
Includes adjustment for age, history of falls, history of dizziness, mobility limitation, and number of narrow-walk trials completed
Discussion
Understanding the links between urinary symptoms and falls in men is a salient public health issue. The high prevalence of lower urinary tract symptoms in the older male population—a prevalence that increases steadily by decade of age—underscores the relevance of this problem, particularly in an era of rapidly increasing male longevity.[26] In this large cohort of community-dwelling men aged 65 years and older we observed that, in the first year following assessment of urinary symptoms, men with moderate or severe symptoms were at increased risk of falls compared to those with mild symptoms. The magnitude of risk was greater for recurrent falls. The symptoms most strongly associated with falls were urinary urgency, difficulty initiating urination, and nocturia.
These results confirm the observations of prior studies suggesting a connection between urinary symptoms and falls in community-dwelling men. In a cohort of ambulatory, elderly men in the US, those who reported nocturia at least twice nightly were significantly more likely to report a history of having fallen within the previous year; the likelihood was greater in those who reported > 3 episodes of nocturia nightly.[12] In another US cohort, urinary incontinence was associated with increased history of falls.[15] In a US managed care organization, patients diagnosed with overactive bladder were significantly more likely to fall than controls.[13] Finally, in a multivariable analysis of participants in the Longitudinal Aging Study Amsterdam, self-reported urinary incontinence was positively associated both with any fall and with recurrent falls.[11]
Our study possesses several important characteristics that distinguish it from previous work in this field. First, our study is unique in that it is the first to employ a validated instrument for analysis of urinary symptom data within the context of fall assessment. The AUA-SI is a reliable instrument that is one of the most commonly utilized measures of lower urinary tract symptoms in clinical practice. As part of its best practice guidelines, the American Urological Association recommends its routine use in the clinical evaluation of patients with lower urinary tract symptoms and suspected benign prostatic hyperplasia.[27] Moreover, numerous previous epidemiological studies have employed the AUA-SI as a primary outcome measure for assessing urinary symptoms in population-based studies. The relatively straightforward design of the AUA-SI questionnaire—1 page and 7 questions—lends itself well to routine use in clinical practice. Indeed, given the high prevalence of lower urinary tract symptoms in older men, and the fact that many men with moderate to severe symptoms do not mention these symptoms to their providers, it is reasonable to consider evaluation of older men with the AUA-SI not only to identify and treat bothersome lower urinary tract symptoms (with lifestyle modifications, medications, or surgery), but to identify men at heightened risk for falls.
Second, the MrOS cohort is especially well suited for this type of analysis, as it is specifically designed to identify and prospectively track risk factors for incident falls in older men living in the community.
Finally, in our analyses we were able to evaluate several potential confounders—including physical performance measures, prescription medications (urological and others), and history of falls—to an extent that prior studies were unable to achieve. Even after adjustment for confounding factors, a significant association of symptom severity with fall risk remained, which underscores the robustness of our findings. In this cohort, we did not discern a significant difference in fall risk between those taking medications for urinary symptoms (alpha-blockers, finasteride, or anticholinergics) and those not taking medications. While it is possible that use of urological medications does not decrease fall risk, there are several other potential explanations for this finding. First, and most likely, successful medical treatment in highly symptomatic men may have improved their symptoms, shifted them into lower strata of fall risk, and thus obscured beneficial effects of intervention. Second, the study may have been underpowered to address this question. Third, since lower urinary tract symptoms have multiple etiologies, the medications may not have been applied selectively enough to have achieved an adequate treatment effect. For example, treatment of overactive bladder symptoms with prostate selective medications alone will not necessarily lead to improvement in lower urinary tract symptoms. Finally, since alpha-blockers and anticholinergics carry slightly increased risks of dizziness and syncope [28] and cognitive dysfunction, [29] respectively, it is possible that an increased fall risk independently conferred by these medications superseded any beneficial effects.
In fact, a limitation of this analysis is the inability to determine with confidence whether treatment of moderate or severe urinary symptoms would attenuate the fall risk. Although alpha-blocker use was not associated with falls in a prior study, [30] whether or not identification of urinary symptoms and subsequent intervention with medications or surgery would decrease fall risk remains unclear. While further observational cohort studies are possible, a more definitive answer to this question may lie in the performance of a randomized clinical trial. Another possible study limitation is the fact that MrOS is composed of relatively healthy volunteers, which potentially hinders the generalizability of the findings. However, if anything, a healthy cohort strengthens the external validity of these findings within the general population, since the majority of community-dwelling elderly males are relatively healthy compared to less independent, age-matched peers.
We posit several mechanisms by which urinary symptoms could trigger falls in older men, many related to adaptive behaviors. Urinary urgency—whereby uncoordinated bladder contractions produce strong, frequently overwhelming desires to urinate—may generate urge incontinence and prompt impulsive, risk-generating motions toward the toilet in order to avoid social embarrassment. Similarly, by generating the need for additional micturition episodes, urinary frequency potentially magnifies risk exposure. The individual symptom most strongly associated with increased risk in our analysis was difficulty in initiating urination. Use of the Valsalva maneuver—which symptomatic men often employ to increase abdominal pressure and thus assist in bladder emptying—may potentially initiate a syncopal episode through vasovagal mechanisms.[31] In addition, many men employ the Valsalva maneuver by sitting on the toilet to urinate, thus increasing the complexity of movements required for bladder empyting. Nocturia may disrupt sleep patterns, induce sleep deprivation, and diminish daytime wakefulness;[32] moreover, nocturia potentially increases risk exposure by prompting affected individuals to repeatedly rise from a recumbent position and navigate darkened environments.
In summary, this prospective study indicates that moderate and severe urinary symptoms increase the risk of falls—particularly recurrent falls—among community-dwelling older men and that the increased risk is independent of other risk factors including history of falls, physical performance, and prescription medication use. Our observations suggest that falls should be included as endpoints in clinical trials of treatment for lower urinary tract symptoms. Moreover, because of the serious consequences of falls, the results of this study may justify the routine assessment of urinary symptoms with a validated questionnaire in the primary care of older, community-dwelling men.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
References
- 1.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173(4):1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R, Kiemeney L, Lee C, UrEpik Study Group The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92(4):409–14. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 3.Kupelian V, Wei JT, O’Leary MP, Kusek JW, Litman HJ, Link CL, McKinlay JB, BACH Survery Investigators Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166(21):2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BC, Wilt TJ, Fink HA, Lambert LC, Marshall LM, Hoffman AR, Beer TM, Bauer DC, Zmuda JM, Orwoll ES. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68(4):804–9. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Trueman P, Hood SC, Nayak US, Mrazek MF. Prevalence of lower urinary tract symptoms and self-reported diagnosed ‘benign prostatic hyperplasia’, and their effect on quality of life in a community-based survey of men in the UK. BJU Int. 1999;83(4):410–5. doi: 10.1046/j.1464-410x.1999.00966.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, O’Leary MP, Puppo P, Robertson C, Giuliano F. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44(6):637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760–6. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342–54. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 9.Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: prevalence and associated factors. Age Ageing. 1988;17(6):365–72. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337(18):1279–84. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 11.Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol. 2001;54(8):837–44. doi: 10.1016/s0895-4356(01)00349-3. [DOI] [PubMed] [Google Scholar]
- 12.Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc. 1992;40(12):1217–20. doi: 10.1111/j.1532-5415.1992.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 13.Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy. 2005;25(4):511–9. doi: 10.1592/phco.25.4.511.61033. [DOI] [PubMed] [Google Scholar]
- 14.Morris V, Wagg A. Lower urinary tract symptoms, incontinence and falls in elderly people: time for an intervention study. Int J Clin Pract. 2007;61(2):320–3. doi: 10.1111/j.1742-1241.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 15.de Rekeneire N, Visser M, Peila R, Nevitt MC, Cauley JA, Tylavsky FA, Simonsick EM, Harris TB. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(6):841–6. doi: 10.1046/j.1365-2389.2003.51267.x. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association Symptom Index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 19.Barnboym E, Ahrens A, Roehrborn CG. Effect of scrambling on the short-term reliability of the American Urological Association Symptom Index. Urology. 1999;53(3):568–73. doi: 10.1016/s0090-4295(98)00552-4. [DOI] [PubMed] [Google Scholar]
- 20.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1558–63. doi: 10.1016/s0022-5347(17)36967-7. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Blackwell TL, Mangione CM, Bowman PJ, Whooley MA, Bauer DC, Schwartz AV, Hanlon JT, Nevitt MC, Study of Osteoporotic Fractures Research Group Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50(10):1629–37. doi: 10.1046/j.1532-5415.2002.50453.x. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control Public Health and Aging: Trends in Aging—United States and Worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–6. [PubMed] [Google Scholar]
- 27.AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170(2 Pt 1):530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 28.Chapple CR. A Comparison of varying alpha-blockers and other pharmacotherapy options for lower urinary tract symptoms. Rev Urol. 2005;7(Suppl 4):S22–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Staskin DR, MacDiarmid SA. Using anticholinergics to treat overactive bladder: the issue of treatment tolerability. Am J Med. 2006;119(3 Suppl 1):9–15. doi: 10.1016/j.amjmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Hall GC, McMahon AD. Comparative study of modified release alpha-blocker exposure in elderly patients with fractures. Pharmacoepidemiol Drug Saf. 2007;16(8):901–7. doi: 10.1002/pds.1402. [DOI] [PubMed] [Google Scholar]
- 31.Gray C, Ward JF, Sands JP. Syncope from increased ventricular response in atrial fibrillation during voiding: a new indication for surgical management in benign prostatic hyperplasia. J Urol. 1999;161(2):606–7. [PubMed] [Google Scholar]
- 32.Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. Neurourol Urodyn. 2008;27(1):34–9. doi: 10.1002/nau.20484. [DOI] [PubMed] [Google Scholar]