Abstract
A risk score for AF has been developed by the Framingham Heart Study; however the applicability of this risk score, derived from whites, to predict new-onset AF in non-whites is uncertain. Therefore, we developed a 10-year risk score for new-onset AF using risk factors commonly measured in clinical practice using 14,546 individuals from the Atherosclerosis Risk in Communities study, a prospective community-based cohort of blacks and whites in the United States. During 10 years of follow-up, 515 incident AF events occurred. The following variables were included in the AF risk score: age, race, height, smoking status, systolic blood pressure, hypertension medication usage, precordial murmur, left ventricular hypertrophy, left atrial enlargement, diabetes, coronary heart disease, and heart failure. The area under the receiver-operating characteristics curve (AUC) of a Cox regression model including the previous variables was 0.78, suggesting moderately good discrimination. The point-based score developed from coefficients in the Cox model had an AUC of 0.76. This clinical risk score for AF in the ARIC cohort compared favorably with the Framingham Heart Study’s AF (AUC=0.68), CHD (AUC=0.63), and hard CHD (AUC=0.59) risk scores and the ARIC CHD risk score (AUC=0.58). In conclusion, we have developed a risk score for AF and have shown that the different pathophysiologies of AF and CHD limit the usefulness of a CHD risk score at identifying individuals at higher risk of AF.
Keywords: atrial fibrillation, risk factors
INTRODUCTION
Risk factors for AF include increasing age, male gender, obesity, hypertension, diabetes, and cardiac structural abnormalities, such as increased left ventricular wall thickness.1 While the relationship between some of the above mentioned risk factors with incident AF have been well studied on a population level, formulae for predicting a person’s individual risk of AF are scarce. A risk score for AF has been recently developed by the Framingham Heart Study.2 The discrimination of the Framingham risk score was good for whites (C statistic=0.78); however, its utility to predict 10-year risk of developing AF in non-whites is uncertain. This is particularly relevant given the lower risk of AF among blacks.3, 4 Therefore, we aimed to develop a risk score for predicting AF incidence based on risk factors that can be easily assessed in clinical practice using a cohort of blacks and whites, the Atherosclerosis Risk in Communities (ARIC) study.
METHODS
The ARIC study is a prospective cohort investigation aimed to identify risk factors for atherosclerosis and cardiovascular disease. ARIC recruited probability samples of adults aged 45–64 years from 4 U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland.5 Blacks were over-sampled from Forsyth County and exclusively sampled from Jackson. A total of 15,792 participants (8710 women, 4266 blacks) were enrolled from 1987 to 1989, and completed a home interview and clinic visit. Three triennial follow-up clinic visits were conducted (1990–92, 1993–95, 1996–98). In addition, participants are followed-up by annual telephone interviews (with a >93% response rate) and active surveillance of the ARIC community hospitals. The ARIC study was approved by institutional review boards at each participating center, and informed consent was obtained from participants at every clinic visit.
Electrocardiograms (ECGs) during the baseline visit were used to identify individuals with prevalent AF or atrial flutter for exclusion. Incident AF diagnoses within 10 years of the baseline exam were identified from 3 sources: ECGs performed during study follow-up visits through 1998, and hospital discharge records and death certificates through 10 years of follow-up.
All ARIC examination ECGs were recorded using MAC PC Personal Cardiographs (Marquette Electronics, Inc., Milwaukee, WI). A standard supine 12-lead resting ECG was recorded ≥ 1 hour after smoking tobacco or ingestion of caffeine at each clinic visit. ECGs were then transmitted by modem to the ARIC ECG Reading Center for computer coding. ECG recordings during follow-up that were computer coded as AF were visually re-checked by a cardiologist to confirm the diagnosis.6
Annual follow-up telephone calls were made to cohort participants in order to identify hospitalizations and deaths. In addition, ARIC community hospitals were surveyed for potential cardiovascular events. Hospital discharge ICD codes were recorded from all hospitalizations, and AF was identified by an ICD-9 discharge code of 427.31 or 427.32 among any of the discharge diagnoses. AF was also identified when any listed cause of death on a death certificate was coded as AF (ICD-9 code 427.3 or ICD-10 code I48). AF occurring simultaneously with heart revascularization surgery (ICD-9 code 36.X) or other cardiac surgery involving heart valves or septa (ICD-9 code 35.X) was not considered an incident event and follow-up was continued beyond that episode for incident AF not associated with cardiac surgery. Prior analysis within the ARIC cohort to determine the validity of hospital discharge diagnoses for AF reported 84% sensitivity and 98% specificity for the ascertainment of AF.3
Study participants were asked to fast for 12 hours before the clinic visit, during which a blood sample was obtained and a physical exam was performed. Blood collection and processing techniques for the ARIC study have been previously described.7 Enzymatic methods were used to measure total cholesterol (TC) and triglycerides (TG).8 High-density lipoprotein (HDL) cholesterol was measured enzymatically after dextran sulfate-Mg2+ precipitation of other lipoproteins.9 Low-density lipoprotein (LDL) cholesterol levels were estimated with the Friedewald formula for individuals with TG levels <400 mg/dL.10 In a scrub suit and without shoes, standing height and waist circumference (at the level of the umbilicus) were measured to the nearest centimeter. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared.
Race, smoking status, and drinking status were determined by self-report. The sports index for physical activity during leisure time ranged from 1 (low) to 5 (high), and was based on the questionnaire developed by Baecke et al.11 Blood pressures were measured 3 times in the sitting position after 5 minutes of rest using a random-zero sphygmomanometer, and the last 2 measurements were averaged. Participants were asked to bring all medications with them during clinic visits. A prescription bottle or self-report was used to determine cholesterol and blood pressure medication use.
The presence of a systolic or diastolic murmur was identified during the physical examination by a trained clinician using a stethoscope. A resting 12-lead ECG was used to define the P-R interval and presence of left ventricular hypertrophy (LVH) and left atrial enlargement (LAE). ECG-diagnosed LVH was considered present if the Cornell voltage was >28 mm in men or >22 mm in women.12 LAE by ECG was defined as a p-wave duration of ≥ 120 ms.13 A participant was categorized as diabetic if they had a fasting glucose ≥ 126 mg/dL (or non-fasting glucose of ≥200 mg/dL), reported a physician diagnosis of diabetes, or was currently taking medication for diabetes. Prevalent CHD at baseline included a history of myocardial infarction (MI), MI adjudicated from the baseline ECG, or history of coronary bypass or angioplasty. Prevalent HF was identified using the Gothenburg criteria14 or self-report of HF medication use in the past 2 weeks.
All analyses were conducted using SAS version 8.2 (SAS Institute, Cary, NC). For the development of this risk score, we considered the following variables at baseline: age (45 to <50 (reference), 50 to <55, 55 to <60, and 60 to 64 years), gender (male, female (reference)), race (black, white (reference)), BMI (<20, 20 to <25 (reference), 25 to <30, ≥30 kg/m2), height (<164 (reference), 164 to <173, ≥173 cm), waist circumference (<88/<102 (reference), ≥88/102 cm in men/women), sports score (<2.0, 2.0 to <3.0, 3.0 to <4.0, ≥4.0 (reference)), smoking status (current, former, never (reference)), drinking status (current, former, never (reference)), systolic blood pressure (<100, 100 to <120 (reference), 120 to <140, 140 to <160, ≥160 mmHg), diastolic blood pressure (<70, 70 to <80 (reference), 80 to <90, 90 to <100, ≥100 mmHg), hypertension medication usage (no (reference), yes), total cholesterol (<200 (reference), 200 to <240, ≥240 mg/dL), LDL cholesterol (<100 (reference), 100 to <130, 130 to <160, 160 to <190, ≥190 mg/dL), HDL cholesterol (<40, 40 to <60, ≥60 mg/dL (reference)), triglycerides (<150 (reference), 150 to <200, ≥200 mg/dL), cholesterol medication usage (no (reference), yes), precordial murmur (no (reference), yes), heart rate (<60, 60 to <90 (reference), ≥90 beats per minute (bpm)), P-R interval (<160 (reference), 160 to <200, ≥200 ms), LVH by ECG (no (reference), yes), LAE by ECG (no (reference), yes), diabetes (no (reference), yes), CHD (no (reference), yes), and HF (no (reference), yes). Continuous variables were categorized based on clinical criteria or on the distribution of the variable in the ARIC study.
Of the 15,792 ARIC participants, we excluded those who were not of black or white race (N=48), blacks from Minneapolis and Washington County (N=55), prevalent AF (N=37) or missing AF status (N=244) at baseline, those with unreadable ECGs (N=85), and those with missing values for any variable in our final risk score (N=777). Person-years of follow-up were computed from the baseline exam until a first AF diagnosis, death, loss to follow-up, or a follow-up of 10 years, whichever came first. Univariate associations of AF with potential risk factors were run first using Cox proportional hazards models. Significant (p<0.10) risk factors from the univariate models were then pooled into 1 multivariate Cox model and a backwards stepwise elimination was used to identify significant (p<0.10) predictors in our multivariate model. All possible interactions of risk factors with age and race were then tested. Interaction tests between risk factors identified in our multivariate model and log of follow-up time confirmed the proportional hazards assumption was met.
Once the final Cox model was determined, we followed the method used by the Framingham Heart Study15 to calculate points associated with each level of our risk factors and to determine the 10-year probability of developing AF by point total. We calculated a score for all participants in our dataset by calculating a point total based on the risk score. The discrimination of both the Cox regression model and the actual point-based risk score was estimated using the area under the receiver-operating characteristics curve (AUC).16 The calibration, a measure of goodness of model fit, was assessed by comparing the observed and predicted number of AF events in deciles of predicted risk, as calculated by the Grønnesby-Borgan chi-square statistic.17 We also calculated a point-based score for participants using the Framingham Heart Study’s AF,2 CHD,18 and hard CHD19 risk scores, as well as ARIC’s CHD risk score,20, 21 in order to estimate how well these risk scores predict AF in comparison to our newly developed AF risk score.
Finally, 1000 bootstrap samples were generated, sampling individuals with replacement, in order to compare our Cox regression model to the point-based score and to conduct an internal validation of our risk score. Bootstrapping methods provide more stable estimates with lower bias compared to other methods of internal validation.22 However, since we used the same cohort to generate the 1000 bootstrap samples for validation of our risk score as we used to develop the risk score, we adjusted our AUC obtained for the internal validation for optimism.23
RESULTS
After exclusions, 14,546 individuals remained at risk of AF. During 10 years of follow-up, 515 incident AF events occurred. The baseline characteristics of the study sample, along with age-, race-, and sex-adjusted hazard ratios for AF by potential risk factor category, are presented in Table 1. The final risk score model included the following variables: age, race, height, systolic blood pressure, hypertension medication use, smoking status, precordial murmur, LVH and LAE by ECG, diabetes, CHD, and HF. An interaction with race and LVH, along with interactions of diabetes and CHD with age were also found. Table 2 lists all risk factors in our risk score, along with points derived for each category. The oldest age group (60–64) had the highest points assigned of any risk factor category. Blacks were given a point value of −4, indicating a lower risk of developing AF compared to whites. The presence of ECG-diagnosed LVH increased the probability of developing AF in whites, but not blacks. Also, diabetes and CHD were associated with AF in younger individuals, but not among the oldest in our cohort.
Table 1.
Baseline Prevalences of Potential Atrial Fibrillation Risk Factors and Hazard Ratios for Atrial Fibrillation, ARIC 10-Year Follow-Up
| Risk Factor | AF cases (N) | Hazard Ratio (95% CI)* | |
|---|---|---|---|
| Age (years) | |||
| 45 to <50 | 3897 (26.8%) | 46 | 1.00 (ref) |
| 50 to <55 | 3771 (25.9%) | 95 | 2.09 (1.47–2.97) |
| 55 to <60 | 3556 (24.5%) | 129 | 2.91 (2.08–4.07) |
| 60 to 64 | 3322 (22.8%) | 245 | 5.94 (4.34–8.15) |
| Race | |||
| Black | 3864 (26.6%) | 83 | 0.60 (0.47–0.76) |
| White | 10682 (73.4%) | 432 | 1.00 (ref) |
| Gender | |||
| Male | 6506 (44.7%) | 325 | 1.92 (1.60–2.30) |
| Female | 8040 (55.3%) | 190 | 1.00 (ref) |
| Body mass index (kg/m2) | |||
| <20 | 469 (3.2%) | 14 | 1.22 (0.70–2.13) |
| 20 to <25 | 4316 (29.7%) | 121 | 1.00 (ref) |
| 25 to <30 | 5730 (39.4%) | 200 | 1.12 (0.89–1.40) |
| ≥>30 | 4026 (27.7%) | 179 | 1.78 (1.41–2.24) |
| Height (cm) | |||
| <164 | 4892 (33.6%) | 114 | 1.00 (ref) |
| 164 to <173 | 4671 (32.1%) | 142 | 1.28 (0.97–1.69) |
| ≥173 | 4983 (34.3%) | 259 | 1.92 (1.38–2.67) |
| Waist (cm) | |||
| <88/102 (women/men) | 6792 (46.7%) | 209 | 1.00 (ref) |
| ≥88/102 (women/men) | 7751 (53.3%) | 305 | 1.55 (1.29–1.86) |
| Systolic blood pressure (mmHg) | |||
| <100 | 1315 (9.0%) | 23 | 0.82 (0.53–1.28) |
| 100 to <120 | 6180 (42.5%) | 153 | 1.00 (ref) |
| 120 to <140 | 4852 (33.4%) | 197 | 1.42 (1.15–1.76) |
| 140 to <160 | 1648 (11.3%) | 103 | 2.16 (1.67–2.79) |
| >≥160 | 551 (3.8%) | 39 | 2.63 (1.83–3.78) |
| Diastolic blood pressure (mmHg) | |||
| <70 | 5326 (36.6%) | 173 | 0.93 (0.75–1.15) |
| 70 to <80 | 5227 (35.9%) | 178 | 1.00 (ref) |
| 80 to <90 | 2890 (19.9%) | 114 | 1.24 (0.98–1.57) |
| 90 to <100 | 790 (5.4%) | 34 | 1.53 (1.06–2.22) |
| ≥100 | 312 (2.2%) | 16 | 2.02 (1.20–3.41) |
| Hypertension medication use | 4446 (30.6%) | 276 | 2.55 (2.13–3.04) |
| Total cholesterol (mg/dL) | |||
| <200 | 5410 (37.5%) | 182 | 1.00 (ref) |
| 200 to <240 | 5432 (37.7%) | 205 | 1.02 (0.83–1.24) |
| > 240 | 3575 (24.8%) | 127 | 0.95 (0.75–1.19) |
| LDL cholesterol (mg/dL) | |||
| <100 | 2257 (15.9%) | 64 | 1.00 (ref) |
| 100 to <130 | 4045 (28.5%) | 137 | 1.03 (0.77–1.39) |
| 130 to <160 | 4239 (29.8%) | 160 | 1.05 (0.78–1.40) |
| 160 to <190 | 2377 (16.7%) | 94 | 1.03 (0.75–1.41) |
| >190 | 1291 (9.1%) | 40 | 0.88 (0.59–1.30) |
| HDL cholesterol (mg/dL) | |||
| <40 | 3861 (26.8%) | 215 | 1.76 (1.34–2.30) |
| 40 to <60 | 6730 (46.7%) | 212 | 1.15 (0.89–1.49) |
| >60 | 3828 (26.5%) | 87 | 1.00 (ref) |
| Triglycerides (mg/dL) | |||
| <150 | 14054 (97.4%) | 489 | 1.00 (ref) |
| 150 to <200 | 227 (1.6%) | 12 | 1.28 (0.72–2.27) |
| >200 | 139 (1.0%) | 13 | 2.61 (1.51–4.53) |
| Cholesterol medication use | 422 (2.9%) | 22 | 1.20 (0.78–1.84) |
| Sports Exercise | |||
| <2.0 | 4102 (28.3%) | 143 | 1.17 (0.77–1.78) |
| 2.0 to <3.0 | 6295 (43.4%) | 222 | 1.06 (0.71–1.59) |
| 3.0 to <4.0 | 3408 (23.5%) | 122 | 0.92 (0.61–1.40) |
| >4.0 | 696 (4.8%) | 27 | 1.00 (ref) |
| Smoking status | |||
| Never | 6059 (41.6%) | 150 | 1.00 (ref) |
| Former | 4692 (32.3%) | 202 | 1.27 (1.02–1.59) |
| Current | 3795 (26.1%) | 163 | 1.69 (1.35–2.12) |
| Alcohol drinking status | |||
| Never | 3618 (25.0%) | 101 | 1.00 (ref) |
| Former | 2743 (18.9%) | 126 | 1.25 (0.95–1.63) |
| Current | 8132 (56.1%) | 285 | 0.97 (0.76–1.24) |
| Heart rate (beats per minute) | |||
| <60 | 3601 (24.8%) | 136 | 1.00 (0.82–1.22) |
| 60 to <90 | 10588 (72.8%) | 355 | 1.00 (ref) |
| >90 | 357 (2.4%) | 24 | 2.03 (1.34–3.06) |
| P-R Interval (ms) | |||
| <160 | 6297 (43.3%) | 200 | 1.00 (ref) |
| 160 to <200 | 6897 (47.4%) | 247 | 1.04 (0.86–1.25) |
| >200 | 1351 (9.3%) | 68 | 1.41 (1.07–1.86) |
| Left ventricular hypertrophy | 325 (2.2%) | 27 | 2.73 (1.84–4.06) |
| Left atrial enlargement | 5654 (38.9%) | 277 | 1.61 (1.35–1.92) |
| Precordial murmur | 1083 (7.5%) | 69 | 1.92 (1.49–2.47) |
| Diabetes mellitus | 1741 (12.0%) | 110 | 1.87 (1.51–2.32) |
| Coronary heart disease† | 688 (4.7%) | 74 | 2.21 (1.71–2.84) |
| Heart failure | 670 (4.6%) | 64 | 3.03 (2.32–3.95) |
Hazard ratios are adjusted for sex, race, and continuous age.
Coronary heart disease includes a history of myocardial infarction, myocardial infarction adjudicated from the baseline electrocardiogram, or history of coronary bypass or angioplasty.
Table 2.
Points Assigned to Atrial Fibrillation Risk Factor Categories, ARIC
| Risk Factor | Points |
|---|---|
| Age (years) | |
| 45 to <50 | 0 |
| 50 to <55 | 3 |
| 55 to <60 | 4 |
| 60 to 64 | 8 |
| Race | |
| Black | −4 |
| White | 0 |
| Height (cm) | |
| <164 | 0 |
| 164 to <173 | 1 |
| ≥173 | 4 |
| Systolic blood pressure (mmHg) | |
| <100 | −1 |
| 100 to <120 | 0 |
| 120 to <140 | 1 |
| 140 to <160 | 2 |
| ≥160 | 3 |
| Hypertension medication use | 3 |
| Smoking status | |
| Never | 0 |
| Former | 1 |
| Current | 3 |
| Precordial murmur | 2 |
| Left atrial enlargement | 2 |
| Left ventricular hypertrophy | |
| white race | 4 |
| black race | 0 |
| Diabetes mellitus | |
| age 45 to <50 | 4 |
| age 50 to <55 | 4 |
| age 55 to <60 | 1 |
| age 60 to 64 | 0 |
| Heart failure | 2 |
| Coronary heart disease | |
| age 45 to <50 | 5 |
| age 50 to <55 | 3 |
| age 55 to <60 | 3 |
| age 60 to 64 | 0 |
The 10-year predicted probability of developing AF by total risk score is presented in Table 3. Individuals scoring ≤11 points had a 10-year predicted probability of developing AF of <5%, whereas those scoring ≥19 points had >24% predicted probability of developing AF in 10 years.
Table 3.
Predicted 10-Year Risk of Atrial Fibrillation by Risk Score, ARIC
| Score | Predicted Risk |
|---|---|
| ≤1 | <1% |
| 2 to 6 | 1% |
| 7 to 8 | 2% |
| 9 | 3% |
| 10 to 11 | 4% |
| 12 | 6% |
| 13 | 7% |
| 14 | 9% |
| 15 | 11% |
| 16 | 13% |
| 17 | 16% |
| 18 | 20% |
| ≥19 | >24% |
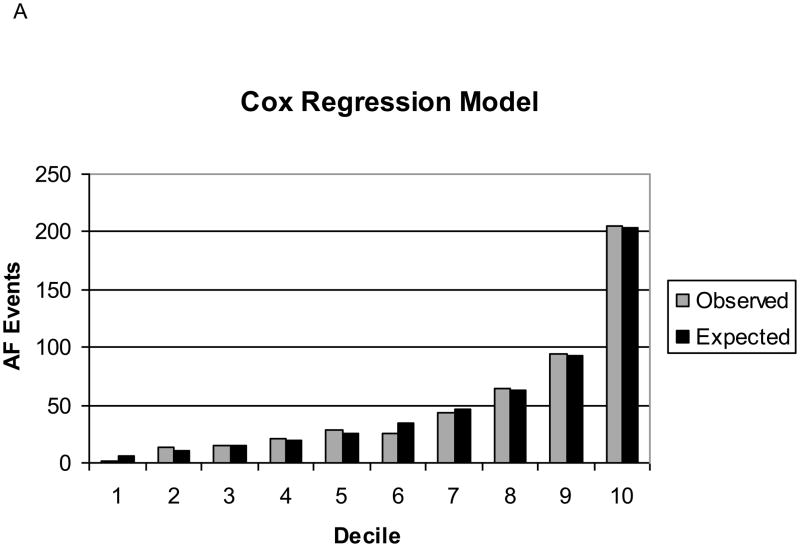
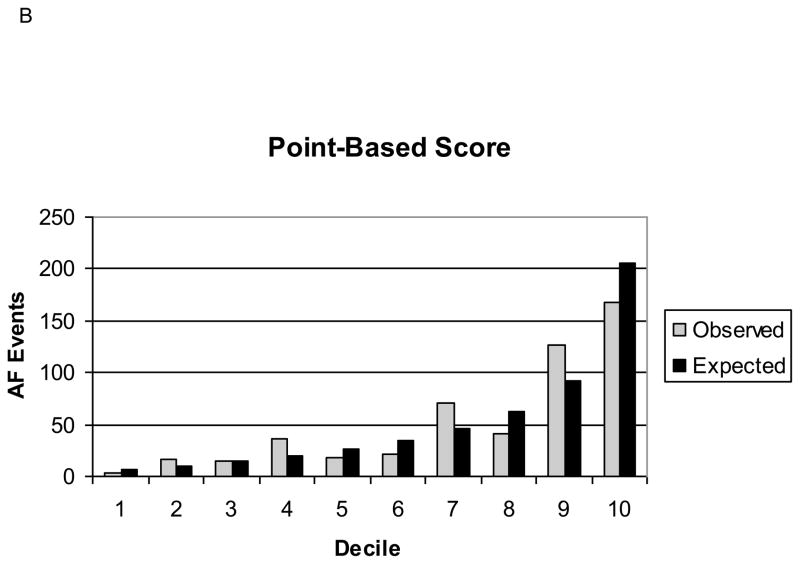
Our final Cox regression model had an AUC of 0.78, indicating good discrimination. The predicted number of AF events in the 10-year risk deciles were similar to the observed events (Grønnesby-Borgan χ2=6.71, p=0.67). The point-based score had an AUC of 0.76, which was statistically significantly lower than the Cox regression model. However, the calibration of the point-based score was still good (Grønnesby-Borgan χ2=10.00, p=0.35). Figure 1 depicts the observed and expected AF events by decile of predicted risk for both the Cox regression model and the point-based score. The internal validation of our risk score, based on 1000 bootstrap samples adjusted for optimism, revealed an AUC of 0.77 (95% CI, 0.75–0.78) for both the Cox regression model and for the point-based score, indicating that our score would perform well in individuals from populations similar to the ARIC cohort. Individual regression coefficients for the Cox regression model are reported in Supplemental Table 1.
Figure 1. Observed and Expected Atrial Fibrillation Events by Decile of Predicted Risk, ARIC.
A: Cox regression model.
B: Point-based score.
In addition to developing a risk score for AF in the ARIC cohort, we calculated scores for all participants based on the Framingham Heart Study’s AF,2 CHD,18 and hard CHD19 risk scores, as well as ARIC’s CHD risk score,20, 21 to determine whether these previously published risk scores predict 10-year risk of AF as well as our AF risk score (Table 4). The Framingham AF risk score predicted AF in the ARIC cohort (AUC=0.68), although it had better discrimination for AF in whites compared to blacks. Finally, the 10-year probability of developing AF was not predicted well by the Framingham CHD, Framingham hard CHD, or ARIC CHD risk scores.
Table 4.
Comparison of Prediction of Atrial Fibrillation in the ARIC Cohort using Various Risk Scores from the ARIC study and the Framingham Heart Study
| Score | AUC | Risk Factors in Score |
|---|---|---|
| ARIC AF | age, race, height, systolic blood pressure, hypertension medication, smoking status, heart murmur, left ventricular hypertrophy, left atrial enlargement, diabetes, heart failure, coronary heart disease | |
| Cox model | 0.78 | |
| Point-based score | 0.76 | |
| Framingham AF18 | age, gender, body mass index, systolic blood pressure, hypertension medication, P-R interval, cardiac murmur, heart failure | |
| Overall | 0.68 | |
| Whites | 0.69 | |
| Blacks | 0.65 | |
| Framingham CHD33 | age, gender, total or low-density lipoprotein cholesterol,* high-density lipoprotein cholesterol, systolic and diastolic blood pressure, diabetes, current smoking | |
| Overall | 0.63 | |
| Whites | 0.63 | |
| Blacks | 0.66 | |
| Framingham Hard CHD34 | age, gender, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure,hypertension medication, current smoking | |
| Overall | 0.59 | |
| Whites | 0.60 | |
| Blacks | 0.59 | |
| ARIC CHD35,36 | 0.58 | age, race, gender, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, hypertension medication, current smoking |
AF=atrial fibrillation; CHD=coronary heart disease; AUC=area under the receiver-operating characteristics curve.
The Framingham CHD score uses either total cholesterol or LDL cholesterol. In this table, we report AUC’s using total cholesterol in our replication of the Framingham CHD score; AUC’s were 0.62 overall, 0.62 in whites, and 0.66 in blacks when using LDL cholesterol.
DISCUSSION
A 10-year risk score for incidence of AF was developed using risk factors commonly measured in clinical practice in a prospective bi-racial community-based cohort. The risk score had good discrimination, with AUC’s of 0.78 for the Cox regression model and 0.76 for the point-based score. As expected, in comparison to the Framingham AF risk score,2 our risk score better predicted who would develop AF in the ARIC cohort. In addition, the prediction of AF using the Framingham18, 19 and ARIC20, 21 CHD risk scores was not good, suggesting an AF-specific score is required to predict individual risk of AF.
Of interest, we found the incidence of AF was higher in whites compared to blacks. Yet, most risk factors predicted AF similarly by race. Unlike the Framingham AF score, we did not find P-R interval or BMI to predict AF risk. However, we additionally found height, smoking status, ECG-diagnosed LVH and LAE, diabetes, and CHD to predict incidence of AF. ECG-diagnosed LVH increased the risk of AF in whites, but did not appear to contribute to 10-year incidence of AF in blacks. A clear explanation for this racial difference is not evident; however, it is possible that the diagnosis of LVH by ECG results in more false positives in blacks.24 It also appeared that diabetes and CHD contributed more to AF risk in younger individuals. This may reflect more serious disease among individuals diagnosed at younger ages or fewer competing risk factors, resulting in a greater proportion of AF risk attributed to diabetes or CHD among young individuals.
The Framingham 10-year risk score for CHD is widely used clinically and has been shown to predict CHD well in other cohorts within the US, Australia, and New Zealand.25 However, we have shown that CHD risk scores are not effective at predicting AF risk. CHD and AF share some common risk factors, such as hypertension, diabetes, and obesity, but others, such as lipids, seem important in the development of CHD only. This highlights the importance of a separate risk score to predict AF and, potentially, the need to develop different preventive interventions.
Our study has several limitations. First, some AF events may have been missed because the majority of events were ascertained by hospital discharge records. However, since incidence rates of AF in ARIC are similar to those from other cohorts3 and associations of genetic risk factors and AF in ARIC are similar to those found in cohorts relying more on study exam ECGs for identification of AF events,26 we believe the underascertainment of AF events was likely minimal. Second, our risk score was developed in a bi-racial cohort, and although our risk score may be useful for black and white patients, it may not be useful for individuals of other racial or ethnic backgrounds. Third, the limited age range of our population at baseline, 45–64 years of age, limits the generalizability of our risk score to older individuals. Fourth, only 1 measure of AF risk factors was used to develop our risk score. Misclassification of exposure as risk factors change over follow-up may result in poorer prediction of AF, and a risk score that accounts for changes in risk factors over time may better predict AF risk. Fifth, the addition of genetic variants or biomarkers may have improved the ability of our risk score to correctly classify those at greatest risk of developing AF; however, we did not include these variables because they are less likely to be consistently measured in primary care. Finally, the external validation of our risk score using other cohorts may have provided better information on how well our risk score predicts AF in other populations.
We have developed a clinical risk score for AF that may be appropriate for risk prediction in blacks and whites. In addition, we have shown that the different pathophysiologies of AF and CHD limit the usefulness of a CHD risk score at identifying individuals at higher risk of AF. Thus, adoption of a new risk score for predicting incident AF, in addition to a CHD risk score, may be clinically useful to identify those patients who are also at risk of developing AF.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. AMC was supported by NHLBI grant T32-HL-007779. This study was further supported by American Heart Association grant 09SDG2280087 and NHLBI grants RC1HL099452 and RC1HL101056.
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Manuscript Number AJC-D-10-01326
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. European Society of Cardiology Committee for Practice Guidelines. European Heart Rhythm Association. Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soliman EZ, Goff DC., Jr Paradox of racial distribution of atrial fibrillation. J Natl Med Assoc. 2008;100:447–448. doi: 10.1016/s0027-9684(15)31282-7. [DOI] [PubMed] [Google Scholar]
- 5.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 6.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thrombosis & Haemostasis. 1989;61:15–19. [PubMed] [Google Scholar]
- 8.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 11.Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 12.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 13.Goldberger AL. In: “Chapter 221. Electrocardiography” (Chapter) Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Anonymous Harrison’s Principles of Internal Medicine; p. 17e. http://www.accessmedicine.com/content.aspx?aID=2871872. [Google Scholar]
- 14.Eriksson H, Caidaul K, Larsson B, Ohlson L, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 16.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–3486. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 17.Grønnesby JK, Borgan Ø. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Chambless LE, Duncan BB, Gilbert AC, Pankow JS Atherosclerosis Risk in Communities Study Investigators. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care. 2003;26:2777–2784. doi: 10.2337/diacare.26.10.2777. [DOI] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Marantz PR, Devereux RB, Kligfield P, Alderman MH. Left ventricular hypertrophy in black and white hypertensives. Standard electrocardiographic criteria overestimate racial differences in prevalence. JAMA. 1992;267:3294–3299. [PubMed] [Google Scholar]
- 25.Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007 May;153:722. 731. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, Schnabel RB, Bis JC, Boerwinkle E, Sinner MF, Dehghan A, Lubitz SA, D’Agostino RB, Sr, Lumley T, Ehret GB, Heeringa J, Aspelund T, Newton-Cheh C, Larson MG, Marciante KD, Soliman EZ, Rivadeneira F, Wang TJ, Eiriksdottir G, Levy D, Psaty BM, Li M, Chamberlain AM, Hofman A, Vasan RS, Harris TB, Rotter JI, Kao WH, Agarwal SK, Stricker BH, Wang K, Launer LJ, Smith NL, Chakravarti A, Uitterlinden AG, Wolf PA, Sotoodehnia N, Kottgen A, van Duijn CM, Meitinger T, Mueller M, Perz S, Steinbeck G, Wichmann HE, Lunetta KL, Heckbert SR, Gudnason V, Alonso A, Kaab S, Ellinor PT, Witteman JC. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.