Summary
Both αβ and γδ T cells develop in the thymus from a common progenitor. Historically distinguished by their T-cell receptor (TCR), these lineages are now defined on the basis of distinct molecular programs. Intriguingly, in many transgenic and knockout systems these programs are mismatched with the TCR type, leading to the development of γδ lineage cells driven by αβTCR and vice versa. These puzzling observations were recently explained by the demonstration that TCR signal strength, rather than TCR type per se, instructs lineage fate, with stronger TCR signal favoring γδ and weaker signal favoring αβ lineage fates. These studies also highlighted the ERK (extracellular signal regulated kinase)-Egr (early growth response)-Id3 (inhibitor of differentiation 3) axis as a potential molecular switch downstream of TCR that determines lineage choice. Indeed, removal of Id3 was sufficient to redirect TCRγδ transgenic cells to the αβ lineage, even in the presence of strong TCR signal. However, in TCR non-transgenic Id3 knockout mice the overall number of γδ lineage cells was increased due to an outgrowth of a Vγ1Vδ6.3 subset, suggesting that not all γδ T cells depend on this molecular switch for lineage commitment. Thus, the γδ lineage may in fact be a collection of two or more lineages not sharing a common molecular program and thus equipollent to the αβ lineage. TCR signaling is not the only factor that is required for development of αβ and γδ lineage cells; other pathways, such as signaling from Notch and CXCR4 receptors, cooperate with the TCR in this process.
Keywords: αβ T cells, γδ T cells, lineage choice, T-cell receptor, Id3, PLZF
Introduction
T-cell sublineages can be divided into two large classes: αβ and γδ T cells. It is believed that the choice between αβ and γδ fates is the first lineage decision made by progenitors after they commit to the T-cell lineage. Historically αβ and γδ lineages were defined by the T-cell receptor (TCR) type. CD4−CD8− [double negative (DN)] thymocytes rearrange three out of four TCR loci: Tcrb, Tcrg, and Tcrd. The cells at this stage are arrested in proliferation and require TCR expression to re-enter cell cycle. If a cell succeeds in an in-frame Tcrb rearrangement, it expresses TCRβ in a complex with the germline-encoded pre-TCRα (pTα) chain. Expression of this complex – pre-TCR – leads to a burst of proliferation, upregulation of the CD4 and CD8 coreceptors, silencing of Tcrg and initiation of Tcra rearrangement (which results in the excision of the Tcrd locus). If Tcra is productively rearranged, CD4+CD8+ [double positive (DP)] thymocytes express TCRαβ at the cell surface and can further differentiate, for instance towards CD4+ (‘helper’) or CD8+ (‘killer’) lineages. Progression through the DP stage is believed to be a hallmark of αβ lineage commitment.
Progenitors that productively rearrange Tcrg and Tcrd loci express the γδTCR at the cell surface. These cells likewise undergo a burst of proliferation, but in wildtype (wt) mice the majority of them avoid progression through the DP stage and egress to the periphery with a CD4−CD8− (or, more rarely, with CD4−CD8+ or CD4+CD8−) phenotype. As a common molecular program provides a firmer basis for lineage definition than the expression of a single receptor (TCR), the αβ and γδ lineages are currently defined on the basis of progression through the DP stage (αβ) or lack thereof (γδ lineage). As the rescue of the quiescent thymocytes depends on pre-TCR or γδTCR expression, these processes are termed β- and γδ- selection, respectively.
Over time it became clear that the correlation between TCR type and lineage fate was not always perfect (Table 1). Premature TCRαβ expression in DN thymocytes, which takes place in the majority of TCR-transgenic strains, leads to the appearance of TCRαβ+ cells that resemble γδ T cells in their surface phenotype and ability for rapid effector responses (1), that do not rearrange endogenous Tcra loci (2), and that do not progress through the DP stage, as revealed by fate mapping experiments (3). DP cells, however, are also present in TCRαβ transgenic animals even in the absence of pTα (4), suggesting that the αβTCR can drive both αβ and γδ lineage differentiation. Early TCRαβ expression is not merely a feature of TCR transgenic systems as it can also happen in wt mice due to rare premature Tcra rearrangements driven by the Tcrd enhancer (5).
Table 1.
Relationship between αβ/γδ lineages and TCR expression
In contrast, most TCRγδ transgenic mice studied so far generate some DP cells even when bred to a Rag (recombination-activating gene)-deficient background, albeit the frequency of DP cells is variable (6, 7). Furthermore, in TCR non-transgenic mice incapable of expression of either a pre-TCR or an αβTCR, such as TCRβ−/− (8, 9) and pTα−/−TCRα−/− (4), DP cells are likewise detected. There is evidence for the presence of a small population of TCRγδ-driven DP cells in wt mice (8). Thus although the pre-TCR drives exclusively differentiation of αβ lineage cells, both TCRαβ and TCRγδ are compatible with either fate. Interestingly, the pre-TCR seems to be a relatively novel evolutionary acquisition as pTα homologues, readily detected in all sequenced mammalian genomes, cannot be found in other vertebrates (10). Thus the ‘non-canonical’ relationship between TCR type and lineage fate observed in pTα-deficient animals and other knockout/transgenic systems may represent vestiges of once mainstream developmental pathways.
TCR signal strength as the lineage determining factor
If TCR type does not determine lineage fate but there is an obvious correlation between lineage choice and TCR composition in wt mice, what is the fate-determining factor? Two elegant studies suggested that TCR signal strength, rather than TCR type per se, determines the lineage choice (7, 12). Both studies took advantage of the fact that the γδTCR is compatible with both γδ and αβ lineage fates. On the one hand, the αβ (DP) compartment in γδTCR transgenic mice was enhanced when TCR signal strength was attenuated by CD3ζ hemizygocity (12) or lck deficiency (7). On the other hand, increased signal strength in TCR transgenic mice on CD5+/− or CD5−/− backgrounds led to a decrease in DP cell numbers. In the KN6 transgenic system, where transgenic TCR recognizes β2-microglobulin (β2m)-dependent major histocompatibility complex (MHC) class Ib molecules T10 and T22 (13, 14), β2m deficiency leads to a dramatic increase in DP cell numbers at the expense of DN TCRγδ+ cells with a mature phenotype (7). Thus, stronger TCR signal favors γδ and weaker signal favors αβ lineage development.
These studies firmly established the role of TCR signal strength in αβ versus γδ lineage choice. However, some data were compatible with lineage choice prior to TCR expression (so called pre-commitment) (15, 16). CD25+CD44+ immature thymocytes that do not yet express TCR can be subdivided on the basis of IL-7Rα expression. Although IL-7Rαhi and IL-7Rαlow cells were able to generated both lineages and in both cases γδ lineage cells were in the minority, IL-7Rαhi cells generated more γδ lineage cells than the IL-7Rαlow fraction (15). This result is compatible with the concept of pre-commitment; however, alternative explanations are possible. For instance, increased Tcrd rearrangement observed in the IL-7Rαhi fraction (15) can explain the increased ability to generate γδ lineage cells.
To reconcile the pre-commitment model with the obvious role of TCR signal strength in the development of αβ and γδ lineages, it was suggested that the TCR signal may ‘confirm’ the lineage choice made by a TCR-independent mechanism (17, 18): if the TCR signal strength matches the chosen lineage, then the cell continues to differentiate along the selected fate, whereas in case of a mismatch the development may be blocked and the cell eliminated. Indeed, it is easy to imagine that a strong TCR signal – for instance in the KN6 transgenic system in the presence of TCR ligand – deletes αβ pre-committed T cells, and these cells are rescued in the absence of the ligand thus restoring the DP compartment.
As it is impossible to distinguish between instructive and confirmatory roles of TCR in lineage choice in vivo or in bulk cultures, we followed the fate of single TCRγδ+ progenitors (19) in the OP9-DL1 coculture system (20) that can support the development of both αβ and γδ lineages (21). A substantial fraction of single immature TCRγδ+ thymocytes gave rise to DP cells when cultured on OP9-DL1 cells. However, when the progeny of the same TCRγδ-expressing cell which gave rise to DP cells received a strong TCR signal, they irreversibly acquired a mature γδ lineage phenotype (19). Thus γδ versus αβ fate choice can be made after TCR expression, an observation that supports an instructive role of TCR signaling in lineage commitment.
Strong TCR signal: is the ligand required?
As at least in case of KN6 TCR transgenic cells a ligand is required to prevent diversion of the cells to the αβ lineage (7), it is tempting to speculate that the same is true for other γδ lineage cells. However, information on endogenous γδTCR ligands and their role in development is extremely scarce. In fact KN6 ligands, MHC class Ib molecules T10 and T22, are the only endogenous molecules that were formally shown to interact with a murine γδTCR (13, 14, 22). For human γδ T cells, recognition of CD1c (23) and the stress-induced MHC-like molecule MICA (24, 25) by their TCRs was demonstrated; however, the role of these molecules in γδ lineage commitment could not be easily addressed. Many other endogenous molecules including but not restricted to isopentenyl pyrophosphate (26, 27), cardiolipin (28), surface-expressed F1-ATPase (29), the non-classical MHC molecule Qa-1(30), an insulin-derived peptide (31), and the Skint1 receptor (32, 33) were suggested as possible γδTCR ligands; however, formal evidence is still lacking. The role of these putative ligands in γδ T cell development, with the exception of Skint1, has not been addressed.
Skint1 was discovered by mapping the gene responsible for a defect in the differentiation of skin dendritic epidermal Vγ5Vδ1 T cells (DETCs) [here and below Vγ nomenclature, after Helig and Tonegawa (34)] in a substrain of FVB mice (32, 33). DETCs represent the first wave of fetal γδ T cells. They develop in the thymus around days 14-16 of embryonic development and home to the epidermis of the skin. In FVB/Tac mice, Vγ5Vδ1 cells were present in fetal thymi but did not acquire a mature phenotype and failed to populate the skin (their niche was taken over by other γδ T cells). The developmental block was rescued at the level of surface phenotype by crosslinking the TCR with an antibody, suggesting that the phenotype may be caused by the absence of the Vγ5Vδ1 TCR ligand expression (32). The defect was later mapped to the Skint1 gene, encoding a transmembrane protein that is expressed by thymic stroma and keratinocytes (33), supporting the idea that it may encode a TCR ligand. However, formal evidence for a Skint1 – Vγ5Vδ1 TCR interaction is still missing. It was also not tested whether some Vγ5Vδ1 cells were redirected to the αβ lineage in the absence of Skint1.
Some evidence supports the hypothesis that γδTCRs can generate ligand-independent signals as does the pre-TCR (35, 36). Indeed, although in the KN6 system, TCRγδ expressing cells were redirected to the αβ lineage when T10/T22 expression was abolished by β2m deficiency, in TCR non-transgenic mice comparable numbers of γδ T cells bound T22 tetramers in wt and β 2m−/− mice (37). However, it was noted that the only element of γδTCR that is required for binding of T10/T22 is Dδ2 (38), and interaction affinity between TCR and T10/T22 varies widely (39). Thus, it is possible that tetramer-binding cells in β2m−/− mice have TCRs different from those found in wt mice, are selected on ligands other than T10/T22, and merely cross-react with the T22 tetramers (40).
It is not clear whether the strong TCR signal required for γδ lineage commitment is always generated by an interaction with a cognate ligand or whether it can also result from ligand-independent signaling. Identification of endogenous γδTCR ligands or formal proof of ligand-independent signaling is required to address this question.
The problem of a ‘useful’ γδTCR repertoire
γδTCRs are potentially the most diverse antigen receptors, in part due to the possibility to utilize more than one D segment in the TCRδ CDR3 loop (41-43). Moreover, at least in the case of T10/T22-restricted γδTCRs, the TCRδ CDR3 loop is sufficient to recognize the ligand (22, 38, 39), a feature not shared by studied αβTCRs and reminiscent of antigen recognition by antibodies. This observation raises a question of how a ‘useful’ γδTCR repertoire is selected. Indeed, if a strong TCR signal is sufficient for γδ lineage differentiation, γδ lineage cells may theoretically be selected on any extracellular molecule present in the thymus.
In αβ T-cell development, MHC restriction of the TCR repertoire is imposed at least in part by CD4 and CD8 coreceptors that sequester the pool of available lck (44). Absence of both coreceptors leads to the availability of ‘free’ lck and rescues some αβ T-cell development in MHC-deficient animals due to positive selection of non-MHC-restricted αβ T cells (44). This selection in the absence of coreceptors resembles γδ T-cell development, as γδ T cells do not progress through the CD4+CD8+ stage.
Vγ5Vδ1 and Vγ6Vδ1 TCRs of γδ T cells developing in fetal thymi have restricted diversity due to the lack of expression of terminal deoxytransferase (TdT) (required for the generation of junctional diversity) and to the restricted use of Vγ gene segments in embryonic thymocytes. However, this explanation is not valid for γδ T cells generated later in ontogeny. Thus, whether and how positive selection in the thymus leads to the generation of a ‘useful’ TCRγδ repertoire is not clear at present.
The ERK-Egr-Id3 axis in αβ versus γδ lineage choice
The fact that TCR signal strength determines lineage fate implies the presence of a molecular switch capable of transforming an analog TCR signal strength into a binary lineage choice. The original work on the role of TCR signal strength in lineage commitment (7, 12) hinted that the ERK (extracellular signal regulated kinase)-Egr (early growth response)-Id3 (inhibitor of differentiation 3) axis may play such a role: ERK was more phosphorylated in TCRγδ- than in pre-TCR-expressing thymocytes (12) and in KN6 thymocytes in the presence of the ligand (7). Correspondingly, the Egr transcription factors and their target, inhibitor of E protein function Id3, were induced in KN6 cells more strongly when the ligand was present (7).
Enforced expression of Egr1 disrupted the development of αβ lineage cells from the β2m−/− KN6 fetal thymic organ cultures (7). Likewise, it interfered with the development of αβ lineage cells from wt but not Id3−/− progenitors – with a compensatory increase in TCRγδ+ cells (7). As the Egr family consists of several members with overlapping functions, the authors further focused on the role of Id3 in αβ versus γδ lineage commitment. To this end, they bred KN6 TCR transgenic mice to the Id3−/− background. In the absence of Id3, large numbers of αβ lineage (DP) cells were generated even when the γδTCR ligand was expressed (45), suggesting that Id3 upregulation was required for γδ lineage commitment. Moreover, retroviral overexpression of Id3 in the absence of TCR (in Rag-deficient thymocytes) was sufficient to confer to them the ability to secrete IFNγ (45). In TCR non-transgenic mice, Id3 deficiency led to a dramatic decrease in Vγ5 T cells in the skin and in Vγ4 T cells in the spleen. It was previously reported that the enforced expression of Id3 disrupts αβ but not γδ T-cell development (46).
Id proteins negatively regulate the function of the E protein family transcription factors. They can heterodimerize with E proteins but lack a DNA-binding domain and thus function in a dominant-negative fashion (47). Inhibition of E protein functions is also believed to be required for αβ lineage development, and E47 deficient Rag−/− thymocytes bypass the β-selection checkpoint (48, 49). Thus, inhibition of E protein activity seems to be required for the differentiation of both lineages. It was suggested that the level of this inhibition may define the lineage fate, with stronger induction of Id3 by stronger TCR signal resulting in γδ lineage differentiation (45).
How can an analog signal from TCR result in binary lineage choice? Wiest and colleagues (40) suggest that the duration of the signal rather signal intensity per se may play a role. It was previously reported that in Swiss 3T3 fibroblasts, transient ERK activation led to upregulation of the immediate early gene c-Fos but fails to stabilize c-Fos protein by phosphorylation, whereas when signaling was sustained and active ERK was still present when the c-Fos protein was produced, it stabilized c-Fos by phosphorylation (50). It is conceivable that ERK activation is sustained in presence of the ligand in the KN6 system and this in turn leads to stabilization of the immediate early gene Egr1 (40). Indeed, the level of Egr1 upregulation in the presence of the ligand was about threefold on the level of mRNA but was almost 25-fold on the protein level (40), providing a potential mechanism for amplification of gradual differences in the signal into a near on/off switch.
The striking observation that the ERK-Egr-Id3 axis is involved in γδ lineage commitment in the KN6 system, and that in the absence of Id3 the cells are redirected to the αβ lineage, suggests that this is the molecular switch that determines αβ versus γδ lineage choice.
Id3-independent γδ T cells
While Id3 expression was required for γδ lineage development in the KN6 system and for the development of Vγ5 and Vγ4 cells in wt animals (45), the overall numbers of γδ T cells in TCR non-transgenic Id3-deficient mice were increased due to an outgrowth of a Vγ1Vδ6.3 population (45, 51, 52). These cells had previously been shown to have an unusual surface phenotype (Thy1low, some NK1.1+ and CD4+) and are unique among γδ T cells in their ability to produce IL-4 together with IFNγ (53). As these features are characteristic of NKT cells, these Vγ1Vδ6.3/Vδ6.4 γδ T cells are often referred to as γδ NKT cells (53, 54).
Recently others (55, 56) and we (57) demonstrated that these cells express the transcription factor PLZF (promyelocytic leukemia zinc finger), previously shown to be required and sufficient for the acquisition of innate-like properties by NKT cells (58, 59). Like NKT cells, Vγ1Vδ6.3 cells from PLZF-deficient mice are incapable of rapid cytokine responses, suggesting that this transcription factor plays similar roles in NKT and Vγ1Vδ6.3 functional maturation.
Id3 independence of the PLZF-positive subset may in part be explained by the fact that these cells – similarly to αβ NKT cells (60) – express a high level of Id2, another Id family member (T.K., H.v.B., unpublished observations). Id2 was previously reported to be a direct PLZF target in myeloid cells (61). However, in Rag1−/− Vγ1Vδ6.4 TCR transgenic mice, which normally generate large numbers of PLZF+ γδ T cells (57), PLZF deficiency did not alter the balance between αβ (DP) and γδ lineage cells, suggesting that PLZF and its regulation of Id2 were dispensable for γδ lineage commitment of Vγ1Vδ6.4 cells (T.K., H.v.B., manuscript in preparation). As neither PLZF nor Id3 are required for γδ lineage commitment of Vγ1Vδ6.3/Vδ6.4 cells, molecular mediators of this process are yet to be identified.
The fact that the role of TCR signal strength was initially established for the Id3-dependent γδ lineage (7) raises the question of whether strong TCR signal is also required for the commitment of Id3-independent γδ T cells. As both in γδ (Vγ1Vδ6.3/Vδ6.4 cells) and in αβ (NKT cells) compartments PLZF expression is associated with a very restricted combination of TCR chains and as both cell types are believed to undergo agonist selection (62), we decided to test if PLZF can be induced by strong TCR signal. We were able to induce PLZF in polyclonal immature TCRγδ+ thymocytes in cell culture by antibody-mediated TCR crosslinking in the presence (but not in the absence) of OP9 stroma (57), suggesting that PLZF may indeed be induced in vivo by agonist selection. Moreover, the conditions of these cultures were identical to those used in our experiments with lineage diversion of immature TCRγδ+ thymocytes (see above) (19), suggesting that PLZF+ γδ T cells also require strong TCR signal for lineage commitment.
Although PLZF was readily induced by TCR crosslinking in culture, it was not upregulated in vivo by the presence of the TCR ligand in HY and KN6 TCR transgenic mice (T.K., H.v.B., unpublished observations) suggesting that additional stimuli were required. NKT cells are selected on other thymocytes (63) and require costimulatory signal from SLAM (signaling lymphocytic activating molecule) receptors that in turn require the adapter molecule SAP (SLAM-associated protein) (64). In the absence of SAP, NKT cell development is blocked (65). As Vγ1Vδ6.3 cell numbers are also reduced in SAP-deficient mice, albeit not as dramatically as numbers of NKT cells (55, 57), it is conceivable that PLZF+ γδ T cells are likewise selected on other thymocytes and that some costimulatory interaction in the course of this selection is required for PLZF induction. This interaction, however, is unlikely to be mediated by SLAM receptors, as PLZF is induced in SAP-deficient Vγ1Vδ6.3 cells, though not to the levels observed in wt cells (55).
Intriguingly, Vγ1Vδ6.3 T cells are increased in numbers not only in Id3 knockouts but also in mice deficient for the tyrosine kinase Itk (56, 66), the transcription factor KLF2 (67), as well as in mice with a mutated adapter molecule SLP-76 (SH2 domain-containing lymphocyte phosphoprotein of 76 kDa)(55). At least for KLF2 (67) and Itk (66) deficient cells, the mechanism of the increase in the Vγ1Vδ6.3 compartment was cell autonomous, as inferred from experiments with mixed bone marrow chimeras. Itk and SLP-76 are important for TCR signaling, and Id3 is induced by TCR signaling. These facts were used as an argument against PLZF induction in vivo by a strong TCR signal (55). However, if TCR signals have nothing to do with PLZF upregulation, it is difficult to explain why its expression correlates with two TCR types, the Vα14 TCR of invariant NKT cells and the Vγ1Vδ6.3/Vδ6.4 TCR, and why PLZF expression can be induced by anti-TCR antibodies. One possibility is that some branches of TCR signaling (i.e. those that require Itk and tyrosines at positions 112, 128, and 145 in SLP-76) indeed inhibit PLZF induction (or expansion of PLZF+ cells), whereas others promote it. An alternative explanation, suggested by Lauritsen and colleagues (45), is that some of the Vγ1Vδ6.3 cells may be deleted by a very strong TCR signal and are rescued when TCR signaling is somewhat attenuated. Indeed, the junctional composition of TCR chains in Id3−/− (52) Vγ1Vδ6 cells when compared to wt cells (53, 68) seems to be overlapping but distinct. This finding may suggest that some cells that are normally deleted escape elimination in Id3 knockouts.
Does a single γδ lineage exist?
The notion that some γδ T cells strictly require Id3 for lineage commitment and are diverted to the αβ lineage in its absence (45) while others do not require Id3 and even increase in number in Id3 knockouts (45, 51, 52, 55) raises a question of whether such an entity as the γδ lineage exists. Indeed, there is an important difference in how we currently define αβ and γδ lineages.
αβ lineage cells are defined on the basis of a well-characterized molecular program, associated with the progression through the DP stage, some parts of which, including upregulation of CD4 and CD8 coreceptors and transcription factor RORγt (69), are documented to take place in all cells of the αβ lineage. DP cells can be viewed as common uncommitted progenitors of all αβ sublineages (although some suggestive evidence for pre-commitment to the CD8αα IEL lineage does exist) (70).
The γδ lineage is defined on the basis of acquisition of functional maturity without progression through the DP stage. A common molecular program, characteristic of all developing γδ lineage cells, has not been identified so far. The only pan-γδ T-cell marker identified to date is the γδTCR itself. Although several molecules including sox13 (16) and ICER (71) were suggested for this role, some γδ subsets do not express these molecules (19, and authors’ unpublished observations). However, markers for γδ sublineages can be readily identified (72, 73).
This is probably not surprising, as most αβ lineage cells in wt mice are driven by ligand-independent pre-TCR signaling, and the αβTCR is assembled only at the DP stage. Thus all TCR (and antigen presenting cell)-specific signals are received by the cell only after the common αβ lineage program has brought the cell to the DP stage. On the contrary, as there is no pre-TCR analogue in γδ T-cell development, a cell is likely to receive signals specific for a particular γδ TCR once it is expressed on the cell surface. This may lead to an execution of ‘sublineage-specific’ programs right at the transition through the TCR controlled checkpoint. Thus, it is conceivable that multiple equipollent γδ lineages diverge from each other and from the αβ lineage at the first TCR-controlled checkpoint.
Different requirements for Notch signaling in αβ and γδ lineage development
In addition to TCR signaling, other pathways also regulate the transition through the β-/γδ-selection checkpoint. Notch signaling, important throughout T-cell development, was initially thought to determine the αβ versus γδ lineage fate (74, 75). However, later studies demonstrated that rather than instructing lineage choice, Notch signaling was absolutely required for αβ lineage development, whereas γδ lineage cells were relatively Notch-independent (6, 21, 76). It was shown that Id3 expression by γδ lineage cells was necessary and sufficient for this relative Notch independence (45). Interestingly, downregulation of E47 was blocked in pre-TCR- but not in γδTCR-expressing cells in the absence of Notch signaling (45), and it was suggested that weak pre-TCR signals required Notch signaling to counteract E protein activity, whereas strong signals from the γδTCR did not (45).
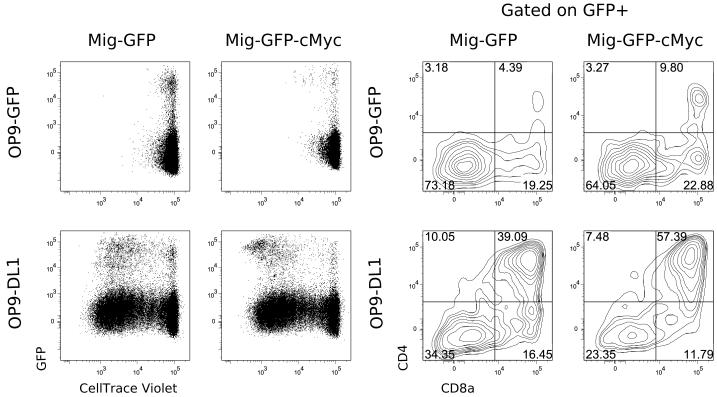
Although Notch1 expression as well as expression of its targets quickly diminishes after β-selection (76), several studies – both in culture (6, 21, 77) and in vivo (78) – demonstrated that Notch signaling is absolutely crucial at and after the β-selection checkpoint. The exact role of Notch at this stage of T-cell development, however, remains poorly understood. Ciofani et al. (79) demonstrated that Notch signaling was required for the survival of pre-β-selected cells, as Rag-deficient DN3 thymocytes quickly disappeared from cultures in the absence of Notch ligands but persisted in cultures where Notch ligands were present. As pre-β-selection DN3 cells do not proliferate, this result clearly indicates that Notch is required for the survival of DN3 cells. To address the role of Notch signaling beyond cell survival, it is crucial to compensate the survival defect. In one such attempt, Ciofani et al. (77) utilized constitutively active protein kinase C overexpression that increased cell yield from DN3 cultures in the absence of Notch ligands but did not lead to DP progression. However, neither cell proliferation nor the ability of surviving cells to differentiate to DP in secondary cultures were addressed in these experiments, making it difficult to conclude that Notch was crucial for differentiation per se. To address these issues, we performed a similar experiment with vav-bcl2 transgenic (80) DN3a thymocytes. The bcl2 transgene efficiently rescued the survival of thymocytes in the absence of Notch signaling (Fig. 1A). Although the cells cultured on OP9-GFP (without Notch ligand) showed diminished proliferation (Fig. 1C), some of them did progress to the DP stage (Fig. 1B). This may be the consequence of a sufficient level of Notch signaling having been received in vivo. To overcome this problem, we further sorted undifferentiated (CD4−CD8−) undivided cells from these cultures and subcultured them for another four days in the presence or absence of Notch ligands. Cells cultured for the first four days on OP9-GFP readily proliferated and progressed to the DP stage after transfer to OP9-DL1. The same cells cultured on OP9-GFP showed no signs of proliferation or differentiation (Fig. 1B,C). Thus, we conclude that Notch signaling is directly required for proliferation and differentiation towards the αβ lineage.
Fig. 1. Notch signaling is required for differentiation and proliferation of αβ T-cells.
(A). A vav-bcl2 transgene compensates for the survival defect in the absence of Notch signaling. 105 vav-bcl2 transgenic (blue) or wt (red) DN3a cells were plated on OP9-DL1 cells in the presence of 1 μM of γ-secretase inhibitor to block Notch signaling. On days 4 and 8, cells were harvested, and live cells were counted. (B, C). vav-bcl2 transgenic DN3a cells were plated on OP9-DL1 or OP9-GFP monolayer as indicated. On day 4, cells were analyzed for CD4/CD8 expression (B) and CFSE dilution (C). Undivided CD4−CD8− cells were sorted, plated on OP9-DL1 or OP9-GFP monolayer as indicated, and analyzed again four days later.
Several direct Notch targets relevant to T-cell development were identified over last years. This includes pTα (81), c-Myc (82), and IL-7Rα (83). IL-7Rα signaling inhibits progression to the DP stage (84). Indeed, in OP9-DL1 cocultures, the presence of IL-7 delays progression to the DP stage, and addition of antibodies blocking the IL-7 receptor reverses this effect but does not alter differentiation of cells cultured in the absence of exogenous IL-7 (T.K., H.v.B., unpublished observations). IL-7 signaling is therefore not required for β-selection. We tested whether ectopic expression of pTα and c-Myc in presence of the vav-bcl2 transgene was sufficient to drive DP progression in the absence of Notch ligands. To this end, we crossed vav-bcl2 transgenic mice to mice that express pTα ectopically under the lck promoter and infected thymocytes from these animals with a retrovirus encoding c-Myc. DN3a thymocytes ectopically expressing bcl2, pTα, and c-Myc differentiated normally on OP9-DL1 monolayers but were still incapable of proliferation and progression to the DP stage in the absence of Notch ligands (Fig. 2). Thus our knowledge of Notch targets, relevant in αβ lineage differentiation is incomplete and further studies are required to identify the missing parts of this pathway.
Fig. 2. Ectopic expression of c-Myc, bcl2 and pTα is insufficient to compensate for the absence of Notch signaling.
DN thymocytes from lck-pTα/vav-bcl2 double transgenic mice were spin-infected with c-Myc encoding retrovirus or empty vector on OP9-DL1 monolayer. DN3a thymocytes were sorted 2 days after infection, labeled with CellTrace Violet and plated on OP9-GFP (top row) or OP9-DL1 (bottom row). Proliferation of GFP+ and GFP− cells (A) and CD4/CD8 expression on GFP+ cells (B) was assessed after 4 days of culture.
The role of CXCR4 signaling in αβ lineage development
Both pre-TCR and Notch signaling are required for αβ lineage development, but are not sufficient to drive the cell beyond the β-selection checkpoint, as DP cells do not develop in a feeder-free system in presence of recombinant Notch ligands (85, 86). The chemokine receptor CXCR4 was recently shown to be required for transition through the β-selection checkpoint (87, 88). Somatic CXCR4 deficiency has a multi-organ embryonic lethal phenotype (89), and the thymic phenotype of the first floxed CXCR4 deficiency (90) was perplexing: a complete block at the DN1 stage prior to the expression of lck-Cre. Recently, however, CXCR4 was conclusively identified as a non-redundant actor in β-selection, serving not only as a chemoattractant but also as a developmental cue. The two effects could only be uncoupled in the reductionist single-environment OP9-DL1 culture system.
An independently generated floxed CXCR4 allele showed only a partial block at β-selection when bred to lck-Cre (87), with pre-selection thymocytes appearing lost in the medulla, failing to home to the subcapsular zone and undergoing increased apoptosis. CXCR4-deleted early thymocytes were also impaired in DP progression in vitro, indicating that in addition to directional signals, CXCR4 signaling promotes some combination of survival, proliferation, and differentiation during β-selection. A complementary approach revealed that downstream of CXCR4, the phosphoinositide 3-kinase (PI3K) subunit isoforms p110γ and p110δ were both required for optimal DP progression (88). In a key experiment, the combination of recombinant CXCL12, IL-7, Flt3L and Notch ligand Dll4 for the first time allowed for limited progression of DN3 cells to DP in a stromal cell-free system. This key step in the molecular dissection of cell surface receptors in β-selection is, however, not the last. Other G-protein-coupled receptors may be involved, as treatment of OP9-DL1 cultures with pertussis toxin blocks DP progression completely (T.K. and H.v.B., unpublished data).
β-selection: pushing the cell off the ledge?
One aspect of the β-selection checkpoint is the seeming ease with which it can be bypassed. It is not surprising that activation of TCR signaling even in the absence of TCR, e.g. by expression of constitutively active lck (91), leads to DP progression. It is also not surprising that the deletion of the phosphatase PTEN (phosphatase and tensin homolog), which counteracts PI3K (92), or the ectopic co-expression of the costimulatory molecule CD28 and its ligand B7-2 (93), which can activate PI3K, bypasses the β-selection checkpoint, as PI3K activity is involved in pre-TCR and CXCR4 signaling (88). PI3K phosphorylates PIP2 (phosphatidylinositol-4,5-bisphosphate) to form PIP3 (phosphatidylinositol-3,4,5-trisphosphate), which in turn is required for positioning of the Akt kinase at the plasma membrane. Expression of constitutively active Akt, which does not require PIP3 for membrane localization, also leads to β-selection bypass (79). Inhibition of E protein activity, which is in part mediated by Id3, is also a part of β-selection process (47). The deletion of E47, that also leads to checkpoint bypass (48), can likewise mimic an aspect of physiological pre-TCR signaling. Similarly, β-selection was bypassed in mice deficient for the transcription factor Ikaros (94). It is less obvious why the overexpression of intracellular Notch1 rescinds the necessity for TCR signaling (95). However, here one can argue that as pre-TCR and Notch signaling cooperate in driving the cell past the checkpoint, their targets may overlap, and thus a supraphysiological Notch signal may activate the expression (or repression) of targets that normally require cooperation between the two pathways. It is more difficult to explain why activation of the Wnt signaling pathway by expression of constitutively active β-catenin also drives DP progression in the absence of pre-TCR (96). At the time these experiments were performed, it was thought that canonical Wnt signaling, which requires the stabilization of β-catenin, was crucial for normal T-cell development (97). However, later it was shown that β-catenin deletion led to a mild thymic phenotype (98) or no phenotype at all (99) even in the absence of its homolog γ-catenin (100, 101), suggesting that Wnt signaling, or at least its canonical branch, is not vital for T-cell development. β-selection is also bypassed under other conditions, including the NOD genetic background [DP cells are present in NOD.Rag−/− mice (102)] and in mice deficient for the serine/threonine kinase Pim-1 (103); however, it is unclear which signaling pathways are responsible here. Even the expression of the SV40 large T antigen (which counteracts p53 and Rb and thus suppresses apoptosis and activates cell proliferation) in Rag- deficient thymocytes led to the development of CD4+CD8+ lymphoma (the phenotype of non-malignant thymocytes was not addressed in this study) (104). Consistent with these observations, mice deficient in both Rag2 and p53 have a significant DP population in the thymus (105), albeit the mechanism for that is unclear. Similarly, p53 deficiency rescues the partial block in β-selection caused by CD3γ deficiency (106), although this effect is visible in 6-week-old mice but not in 10-day-old mice, consistent with a slow accumulation of initially rare DP cells due to improved survival.
It is possible that these signaling pathways – even those which are dispensable for normal T-cell development – all have a common set of downstream targets that are specifically required in DN3 to DP progression. However, it is also conceivable that rather than activating an αβ T-cell-specific transcription program directly, they merely drive a change in some basic cell state (e.g. in cell cycle or metabolism), which is sufficient to induce DP progression. In such a scenario, a DN3 cell would be already poised to become a DP cell, and a change in a cell state (e.g. cell cycle entry) would trigger the progression.
In OP9-DL1 cultures, the differentiation of DN3a cells correlates well with proliferation, as only those cells that have divided several times begin to upregulate CD4 and CD8 (Fig. 3). Cell proliferation per se can be required, for instance, for the dilution of a factor that blocks differentiation (such as E47) and/or be required for chromatin remodeling.
Fig. 3. Link between proliferation and differentiation.
Wt DN3a thymocytes were sorted, labeled with CFSE and cultured on OP9-DL1 monolayers. After 4 days cells were harvested, stained for CD4 and CD8α and analyzed by FACS. CD4 and CD8α fluorescence was summed electronically. Arrows indicate cells that went through 3 or 4 divisions.
Concluding remarks
The molecular mechanisms governing the first TCR-controlled checkpoint, the choice between αβ and γδ lineages, are starting to come into focus. The analog TCR signal is digitized by the Erk-Egr-Id3 axis, with strong signal instructing the γδ and weak signal instructing the αβ fate. These findings, however, add an unexpected twist to the model, as some γδ T cells are independent of Id3 and express PLZF, which drives a unique NKT-like phenotype. Thus, under the ill-defined γδ umbrella, upon close inspection, may lie a cluster of full-fledged developmental lineages with discrete molecular programs, producing peripheral γδ T cells with distinct functions.
The lack of known ligands for most γδTCRs remains the main obstacle in the definition of distinct γδ lineages. The absence on most γδ T cells of known lck-interacting coreceptors, which in the αβ lineage mediate class I or class II restriction by sequestering the limiting lck, suggests that γδTCRs, like BCRs, may recognize many geometric shapes on an antigen; this hypothesis is supported by the first crystal structure of a γδTCR in complex with its ligand. The presence in some γδ T cells of unknown lck-sequestering coreceptors cannot be excluded; these could recognize any ligand and drive the restriction of some γδ T cells, not necessarily to an MHC-like molecule.
While γδ thymocytes are relatively independent of Notch signaling, αβ lineage cells die rapidly in its absence. By compensating the survival defect using transgenic bcl2, we show here that Notch signaling is absolutely required not only for the survival but also for the proliferation and differentiation of developing αβ thymocytes. Recent work identifies another receptor-ligand pair, CXCR4 and CXCL12, as contributors to β-selection via the PI3K cascade, although it remains to be determined which combination of survival, proliferation, and differentiation this signal promotes. Importantly, this work presents a reconstitution of the thymic environment permissive for β-selection in a stroma-free culture using recombinant ligands.
While the biochemical reconstitution of β-selection had proven difficult until now, a wide variety of both relevant and irrelevant genetic abnormalities, such as the deletion of E47 or Ikaros, allow cells to bypass this checkpoint. These findings lead us to the hypothesis that the DN3 cell is already poised on the brink of DP-ness, requiring a generic stimulus—such as entry into the cell cycle, which is downstream of many pathways known to bypass β-selection—to realize its fate.
Acknowledgements
We are grateful to Valentina Schmidt for technical assistance. We thank Professor Anthony Capobianco for providing c-Myc retrovirus and Professor Jerry Adams for vav-bcl2 transgenic mice. These studies were supported by National Institutes of Health Grants R01 A145846 and R01 A151378.
References
- 1.Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gammadelta lineage T cells. J Exp Med. 2000;192:537–548. doi: 10.1084/jem.192.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno L, Fehling HJ, von Boehmer H. The alpha beta T cell receptor can replace the gamma delta receptor in the development of gamma delta lineage cells. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- 3.Egawa T, Kreslavsky T, Littman DR, von Boehmer H. Lineage diversion of T cell receptor transgenic thymocytes revealed by lineage fate mapping. PLoS ONE. 2008;3:e1512. doi: 10.1371/journal.pone.0001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buer J, Aifantis I, DiSanto JP, Fehling HJ, von Boehmer H. Role of different T cell receptors in the development of pre-T cells. J Exp Med. 1997;185:1541–1547. doi: 10.1084/jem.185.9.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aifantis I, Bassing CH, Garbe AI, Sawai K, Alt FW, von Boehmer H. The E delta enhancer controls the generation of CD4− CD8− alphabetaTCR-expressing T cells that can give rise to different lineages of alphabeta T cells. J Exp Med. 2006;203:1543–1550. doi: 10.1084/jem.20051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbe AI, Krueger A, Gounari F, Zuniga-Pflucker JC, von Boehmer H. Differential synergy of Notch and T cell receptor signaling determines alphabeta versus gammadelta lineage fate. J Exp Med. 2006;203:1579–1590. doi: 10.1084/jem.20060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haks MC, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kang J, Coles M, Cado D, Raulet DH. The developmental fate of T cells is critically influenced by TCRgammadelta expression. Immunity. 1998;8:427–438. doi: 10.1016/s1074-7613(00)80548-8. [DOI] [PubMed] [Google Scholar]
- 9.Mombaerts P, et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 10.Kreslavsky T, Gleimer M, von Boehmer H. alphabeta versus gammadelta lineage choice at the first TCR-controlled checkpoint. Curr Opin Immunol. 2010;22:185–192. doi: 10.1016/j.coi.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 12.Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Bonneville M, et al. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci USA. 1989;86:5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy DB, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse γδ T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 15.Kang J, Volkmann A, Raulet DH. Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med. 2001;193:689–698. doi: 10.1084/jem.193.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melichar HJ, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 17.Narayan K, Kang J. Molecular events that regulate alphabeta versus gammadelta T cell lineage commitment: old suspects, new players and different game plans. Curr Opin Immunol. 2007;19:169–175. doi: 10.1016/j.coi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Garbe AI, von Boehmer H. TCR and Notch synergize in alphabeta versus gammadelta lineage choice. Trends Immunol. 2007;28:124–131. doi: 10.1016/j.it.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 21.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 23.Spada FM, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 27.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Born WK, et al. Hybridomas expressing gammadelta T-cell receptors respond to cardiolipin and beta2-glycoprotein 1 (apolipoprotein H) Scand J Immunol. 2003;58:374–381. doi: 10.1046/j.1365-3083.2003.01315.x. [DOI] [PubMed] [Google Scholar]
- 29.Scotet E, et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Vidovic D, Roglic M, McKune K, Guerder S, MacKay C, Dembic Z. Qa-1 restricted recognition of foreign antigen by a gamma delta T-cell hybridoma. Nature. 1989;340:646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Jin N, Nakayama M, O’Brien RL, Eisenbarth GS, Born WK. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J Autoimmun. 2010;34:478–484. doi: 10.1016/j.jaut.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 33.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 35.Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, Grassi F. Different initiation of pre-TCR and gammadeltaTCR signalling. Nature. 2000;406:524–527. doi: 10.1038/35020093. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki S, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 37.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin S, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 39.Adams EJ, Strop P, Shin S, Chien YH, Garcia KC. An autonomous CDR3delta is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by gammadelta T cells. Nat Immunol. 2008;9:777–784. doi: 10.1038/ni.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S-Y, Stadanlick J, Kappes DJ, Wiest DL. Towards a molecular understanding of the differential signals regulating αβ/γδ T lineage choice. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.04.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 43.Konigshofer Y, Chien YH. Gammadelta T cells - innate immune lymphocytes? Curr Opin Immunol. 2006;18:527–533. doi: 10.1016/j.coi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Van Laethem F, et al. Deletion of CD4 and CD8 coreceptors permits generation of αβ T cells that recognize antigens independently of the MHC. Immunity. 2007;27:735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Lauritsen JPH, et al. Marked induction of the helix-loop-helix protein Id3 promotes their T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blom B, et al. Disruption of αβ but not of γδ T cell development by overexpression of the helix-loop-helix protein Id3 in committed T cell progenitors. EMBO J. 1999;18:2793–2802. doi: 10.1093/emboj/18.10.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 48.Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2a protein activity. J Exp Med. 2001;194:733–746. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy LO, Smith S, Chen R-H, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 51.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gammadelta lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, Kee BL. Inhibitor of DNA binding 3 limits development of murine SLAM-associated adaptor protein-dependent “innate” gammadelta T cells. PLoS ONE. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 54.Lees RK, Ferrero I, MacDonald HR. Tissue-specific segregation of TCRgamma delta+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR alphabeta+NKT cells. Eur J Immunol. 2001;31:2901–2909. doi: 10.1002/1521-4141(2001010)31:10<2901::aid-immu2901>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Alonzo ES, et al. Development of promyelocytic zinc finger and ThPOK-expressing innate γδ T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184:1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci USA. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreslavsky T, et al. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci USA. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monticelli LA, et al. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proc Natl Acad Sci USA. 2009;106:19461–19466. doi: 10.1073/pnas.0908249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doulatov S, et al. PLZF is a regulator of homeostatic and cytokine-induced myeloid development. Genes Dev. 2009;23:2076–2087. doi: 10.1101/gad.1788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–6520. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 63.Wei DG, et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi Q, et al. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odumade OA, Weinreich MA, Jameson SC, Hogquist KA. Kruppel-like factor 2 regulates trafficking and homeostasis of gammadelta T cells. J Immunol. 2010;184:6060–6066. doi: 10.4049/jimmunol.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azuara V, Lembezat MP, Pereira P. The homogeneity of the TCRdelta repertoire expressed by the Thy-1dull gammadelta T cell population is due to cellular selection. Eur J Immunol. 1998;28:3456–3467. doi: 10.1002/(SICI)1521-4141(199811)28:11<3456::AID-IMMU3456>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 69.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 70.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Pennington DJ, et al. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 72.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 73.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanigaki K, et al. Regulation of αβ/γδ T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 75.Washburn T, et al. Notch Activity Influences the αβ versus γδ T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 76.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Ciofani M, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 78.Maillard I, et al. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203:2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 80.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reizis B, Leder P. Direct induction of T lymphocyte-specific gene expression by the mammalian Notch signaling pathway. Genes Dev. 2002;16:295–300. doi: 10.1101/gad.960702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weng AP, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Garcia S, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J Exp Med. 2009;206:779–791. doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Q, Erman B, Park JH, Feigenbaum L, Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORgammat: impact on thymocyte development. J Exp Med. 2004;200:797–803. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 87.Trampont PC, et al. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11:162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond β-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 90.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 91.Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. An activated Ick transgene promotes thymocyte development in rag-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 92.Hagenbeek TJ, et al. The Loss of PTEN Allows TCR αβ Lineage Thymocytes to Bypass IL-7 and Pre-TCR–mediated Signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams JA, et al. Regulated costimulation in the thymus is critical for T cell development: dysregulated CD28 costimulation can bypass the pre-TCR checkpoint. J Immunol. 2005;175:4199–4207. doi: 10.4049/jimmunol.175.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winandy S, Wu L, Wang JH, Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J Exp Med. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michie AM, et al. Constitutive Notch signalling promotes CD4 CD8 thymocyte differentiation in the absence of the pre-TCR complex, by mimicking pre-TCR signals. Int Immunol. 2007;19:1421–1430. doi: 10.1093/intimm/dxm113. [DOI] [PubMed] [Google Scholar]
- 96.Gounari F, et al. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 97.Staal FJ, et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 99.Cobas M, et al. β-Catenin Is Dispensable for Hematopoiesis and Lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeannet G, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of -catenin and γ-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 101.Koch U, Wilson A, Cobas M, Kemler R, MacDonald HR, Radtke F. Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 102.Yui MA, Rothenberg EV. Deranged early T cell development in immunodeficient strains of nonobese diabetic mice. J Immunol. 2004;173:5381–5391. doi: 10.4049/jimmunol.173.9.5381. [DOI] [PubMed] [Google Scholar]
- 103.Jacobs H, et al. Pim1 reconstitutes thymus cellularity in interleukin 7– and common γ chain–mutant mice and permits thymocyte maturation in Rag− but not Cd3γ-deficient mice. J Exp Med. 1999;190:1059–1068. doi: 10.1084/jem.190.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao M-J, et al. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol Cell Biol. 1998;18:3495–3501. doi: 10.1128/mcb.18.6.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang D, Lenardo MJ, Zuniga-Pflucker JC. p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J Exp Med. 1996;183:1923–1928. doi: 10.1084/jem.183.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haks MC, Krimpenfort P, van den Brakel JH, Kruisbeek AM. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11:91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]