Abstract
Presynaptic biogenic amine transporters mediate reuptake of released amines from the synapse, thus regulating serotonin, dopamine and norepinephrine neurotransmission. Medications utilized in the treatment of depression, attention deficit-hyperactivity disorder and other psychiatric disorders possess high affinity for amine transporters. In addition, amine transporters are targets for psychostimulants. Altered expression of biogenic amine transporters has long been implicated in several psychiatric and degenerative disorders. Therefore, appropriate regulation and maintenance of biogenic amine transporter activity is critical for the maintenance of normal amine homoeostasis. Accumulating evidence suggests that cellular protein kinases and phosphatases regulate amine transporter expression, activity, trafficking and degradation. Amine transporters are phosphoproteins that undergo dynamic control under the influence of various kinase and phosphatase activities. This review presents a brief overview of the role of amine transporter phosphorylation in the regulation of amine transport in the normal and diseased brain. Understanding the molecular mechanisms by which phosphorylation events affect amine transporter activity is essential for understanding the contribution of transporter phosphorylation to the regulation of monoamine neurotransmission and for identifying potential new targets for the treatment of various brain diseases.
Keywords: Biogenic amines, Transporters, Regulation, Phosphorylation, Mental illness
1. Introduction
Overview of Monoamine Transporter Regulation: Neuronal communication is a complex and vital function of the organism. Effective communication in the brain requires precise and dynamic regulation of neurotransmitter concentrations. Many external signals in the form of neurotransmitters and hormones must be integrated and processed by neurons. Serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA) and noradrenaline/norepinephrine (NA/NE) are biogenic monoamine (MA) neurotransmitters synthesized in-vivo or de-novo from tryptophan and tyrosine respectively. Each amine controls distinct behavioral and physiological functions both in the central and peripheral nervous system. 5-HT modulates mood, aggression, motivation, appetite, sleep, cognition and sexual activity. Altered 5-HT signaling has been implicated in mental illnesses related to these biological processes (Coccaro, 1989; Compagnon et al., 1993; Owens & Nemeroff, 1994; Sellers et al., 1992). 5-HT also has important peripheral actions which include regulation of vasoconstriction, gastrointestinal and placental function. DA systems control motor function, mood, reward and cognition (Carlsson, 1987; Koob, 1998). Dysregulation of DA transmission is linked to attention deficit/hyperactivity disorder (ADHD), schizophrenia, addiction, Parkinson’s disease and Tourette’s syndrome (Bannon et al., 1995). NE controls arousal, mood, attention, stress-responsivenss and affective disorders (Klimek et al., 1997; Leonard, 1997; Ressler & Nemeroff, 1999; Schildkraut, 1965). NE is also the major neurotransmitter in postganglionic sympathetic synapses, and NE uptake sites and activity are compromised in cardiomyopathy, heart failure, hypertension and ischemia (Bohm et al., 1995; Esler et al., 1981; Imamura et al., 1996; Liang et al., 1989; Merlet et al., 1992; Schafers et al., 1998).
At the molecular level, MA signaling is dynamically regulated by a diverse set of macromolecules including biosynthetic enzymes, secretory proteins, ion channels, pre- and postsynaptic receptors and transporters. The presynaptically localized plasma membrane MA transporters for serotonin (SERT), dopamine (DAT) and norepinephrine (NET) exert dynamic spatial and temporal control of extracellular neurotransmitter concentration via re-uptake of released neurotransmitter from the synaptic cleft. SERT (SLC6A4), DAT (SLC6A3) and NET (SLC6A2) belong to one single gene (SLC6) family. MA transporters share a predicted structure of 12 transmembrane domains with intracellular cytoplasmic NH2 and COOH termini. This topology was recently confirmed by the high-resolution crystal structure of a bacterial homologue, LeuT, of the mammalian MA transporters (Yamashita et al., 2005).
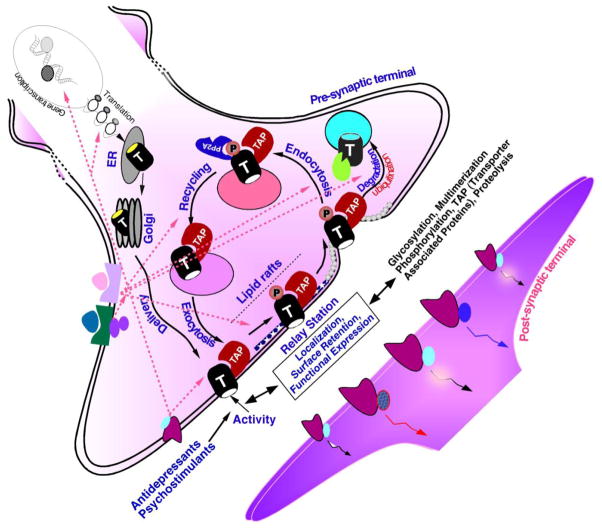
MA transporters are pharmacological targets for various clinically used antidepressants and psychostimulant drugs (Barker & Blakely, 1995; Jayanthi & Ramamoorthy, 2005). Mice lacking the gene encoding DAT, SERT or NET revealed that the mechanisms regulating amine biosynthesis, amine storage, receptor sensitivity and transporter expression are interdependent (Adriani et al., 2009; Bengel et al., 1998; Gainetdinov & Caron, 2003; Gainetdinov, Wetsel et al., 1999; Hall et al., 2002; Li et al., 2004; Rioux et al., 1999; Sora et al., 2001). The importance of MA transporters to the regulation of neurotransmitter signaling, to disease processes and that they are a target of several drugs of abuse highlights the importance of understanding the cellular and molecular mechanisms by which various signaling pathways modify these proteins and alter their properties and function. Recent advances in the field have provided a wealth of knowledge as to how the expression and functional properties of SERT, DAT and NET are regulated at the gene and protein level. Transporter regulation can occur via phosphorylation dependent and independent post-translational modifications. Post-translational modifications can a) change intrinsic transport activity, b) alter transporter turnover, c) regulate exocytic fusion of transporter containing vesicles with the plasma membrane and d) regulate sequestration of transporter from the plasma membrane by modulating endocytic machinery pathways. Alternatively, regulation of transporter can also occur through their association with other interacting proteins by phosphorylation dependent/or independent pathways (Eriksen et al., 2010).
This review describes one molecular mechanism/modification that alters several key properties of MA transporters, MA transporter phosphorylation. We review various studies that have examined the relationship between changes in transporter phosphorylation state, transporter expression and function. Some earlier aspects of monoamine transporter phosphorylation and regulation can be found in several excellent reviews (Eriksen et al., 2010; Melikian, 2004; Steiner et al., 2008; Torres & Amara, 2007; Torres et al., 2003;R. A. Vaughan, 2004; Zahniser & Doolen, 2001; Zahniser & Sorkin, 2004). We also review recent progress in the functional analyses of transporter gene variants identified in human diseases. These data have allowed investigators to begin to identify how phosphorylation affects transporter expression levels, transport properties and drug responses. The quest/potential for developing novel pharmacological applications will be strengthened in the future by defining the role of transporter phosphorylation and refining our search for compromised signals.
2. The serotonin transporter and its functional significance
The uptake of synaptic 5-HT through Na+/Cl−- dependent SERT is the principal process of terminating serotonergic neurotransmission. More than 15 different types of cell-surface receptors exist to transmit the specific actions of 5-HT on target cells (Glennon & Dukat, 1995), whereas a single gene (Ramamoorthy, Bauman et al., 1993) encoding the SERT appears responsible for extracellular 5-HT clearance (Blakely et al., 1991; Hoffman et al., 1991; Lesch, Wolozin, Murphy et al., 1993). In addition to serotonergic neurons, SERTs are expressed in peripheral tissue (Lesch, Wolozin, Murphy et al., 1993; Rudnick, 1977) including specialized cells of the gut (Gordon & Barnes, 2003), placenta (Balkovetz et al., 1989), lung (Paczkowski et al., 1996), adrenal chromaffin cells (Schroeter et al., 1997), blood lymphocytes (Faraj et al., 1994; Gordon & Barnes, 2003) and platelets (Carneiro & Blakely, 2006; Carneiro et al., 2008; Jayanthi et al., 2005). While clearance of synaptic and extra-synaptic 5-HT appears to be the principal function of SERT, certain cells, notably platelets, utilize SERT to acquire 5-HT from the extracellular environment for subsequent release; a function involved in the process of platelet activation (Cirillo et al., 1999; Musselman et al., 2002). Platelets and 5-HT neurons share many common properties, including vesicular monoamine transporters (VMAT), 5-HT release, pharmacological properties of SERT, identical SERT sequences, and 5-HT receptors (Owens & Nemeroff, 1994). Therefore, platelets have been widely used as a peripheral indicator of central 5-HT metabolism and SERT function (Wirz-Justice, 1988) in psychiatric disorders and vascular diseases in which 5-HT has been implicated (Meltzer et al., 1981). In the lung, SERTs efficiently clear plasma-borne 5-HT and regulate blood 5-HT levels with the aide of platelets.
Numerous SERT antagonists including the serotonin selective reuptake inhibitors (SSRIs), fluoxetine (Prozac™), paroxetine (Paxil™) citalopram (Celexa™) and sertraline (Zoloft™) are effective antidepressants. Documentation of altered SERT expression in various types of psychopathology indicates the importance of SERT expression in maintaining normal brain function (Murphy et al., 2004). Amphetamine derivatives such as fenfluramine, p-chloroamphetamine (PCA) and 3,4 –methylenedioxymethampetamine (MDMA or “Ecstasy”) are substrates for SERT (Rudnick & Wall, 1992). SERT in turn affords an access pathway to the cytoplasm for these drugs, where interactions with SERT and VMATs can, in concert, lead to nonvesicular 5-HT efflux (Fontana et al., 2009; Gobbi et al., 1997; Hilber et al., 2005; Seidel et al., 2005). SERT-knockout mice have provided unequivocal evidence that SERT is important for synaptic 5-HT clearance, SSRI recognition, MDMA induced hyperactivity and may contribute to the reinforcing effect of cocaine. In addition, these mice showed several compensatory changes in 5-HT levels, 5-HT synthesis and 5-HT receptor functions again highlighting the coordinated regulation of amine biosynthesis, amine storage, receptors sensitivity and transporter expression. Furthermore, these mice exhibit significant differences in basal peripheral physiology including gastrointestinal function, as well as overt behavior (Bengel et al., 1998; Li et al., 1999; Sora et al., 1998).
Human SERT (hSERT) encodes a protein of 630 amino acids. Hydrophobicity analysis of the amino acid sequence predicts the presence of 12 hydrophobic transmembrane domains with cytoplasmic NH2 and COOH termini. Transmembranes 3 and 4 are separated by a large, hydrophilic loop that bears two canonical sites for N-linked glycosylation (Lesch, Wolozin, Estler et al., 1993; Ramamoorthy, Bauman et al., 1993). SERT is localized in the human chromosome 17q11.2 (Ramamoorthy, Bauman et al., 1993). It exists in homo-multimeric complexes (Chang et al., 1998; Chen et al., 1998; Jess et al., 1996; Kilic & Rudnick, 2000; Ramamoorthy, Leibach et al., 1993; Schmid, Just et al., 2001; Schmid, Scholze et al., 2001) and contains potential phosphorylation sites for several kinases (Hoffman et al., 1991; Miller & Hoffman, 1994; Ramamoorthy, Bauman et al., 1993).
2.1. Receptor-protein kinase mediated acute regulation of SERT activity and phosphorylation
Presynaptic receptor-second messenger-kinase/phosphatase linked pathways play a pivotal role in the regulation of SERT activity. The function of native and heterologously expressed SERT is rapidly inhibited in response to acute depletion of intracellular Ca2+, inhibition of calmodulin, CaMKII, Src-kinase, p38 MAPK and activation of PKC. On the other hand, increased intracellular Ca2+, activation of NOS/cGMP and MAPK pathways stimulate SERT activity. Presynaptic receptor-mediated regulation of SERT has been documented (summarized in Table 1). Activation of adenosine receptors (AR), 5-HT1B, histamine receptors (H3R) and BDNF/TrkB stimulates 5-HT uptake (Benmansour et al., 2008; Daws et al., 1999; Daws et al., 2000; Launay et al., 1994; Matheus et al., 2009; Steiner et al., 2009; Zhu, Hewlett, Feoktistov et al., 2004; Zhu, Hewlett, Francis et al., 2004), whereas α2 adrenergic receptor stimulation reduces uptake of 5-HT (Ansah et al., 2003). The contribution of several of these kinase cascades to SERT regulation and phosphorylation has been demonstrated and a close temporal correlation between amine transport and surface expression has been documented (Jayanthi et al., 1994; Jayanthi et al., 2005; Qian et al., 1997; Ramamoorthy, 2002; Ramamoorthy & Blakely, 1999; Ramamoorthy et al., 1998; Samuvel et al., 2005). For example, activation of PKC and PKA, or inhibition of PP2Ac, increases SERT basal phosphorylation in HEK-293-hSERT cells (Ramamoorthy et al., 1998). Time course studies indicate that the decrease in 5-HT transport parallels that of SERT surface expression. Thus, SERT phosphorylation by the PKC-dependent pathway triggers SERT internalization and reduces 5-HT uptake. Phosphorylation of the intracellular SERT pool may slow down the recycling of SERT or redirect SERT trafficking events which contribute to changes in surface expression. Alternatively, SERT may be phosphorylated at the cell surface providing a signal for internalization, a change in SERT catalytic properties, or both. These events may occur but at different time frames. Recent efforts have brought into sharper focus the time-dependent molecular events associated with PKC-mediated regulation of SERT that is constitutively expressed in platelets (Jayanthi et al., 2005). Jayanthi et al demonstrated that diminished SERT catalytic activity occurs prior to an enhancement of SERT endocytic rate that ultimately reduces cell surface SERT expression. The authors provided evidence that PKC activation regulates SERT in a biphasic manner. The initial phase (1 – 5 min) of 5-HT uptake inhibition occurs independently of trafficking whereas the later phase (5 – 60 min) is associated with enhanced endocytosis. The biphasic inhibition of SERT is accompanied by sequential phosphorylation of plasma membrane resident SERT such that phosphorylation of serine (Ser) residues precedes that of threonine (Thr) residue(s). It has been suggested that the initial phosphorylation on Ser residues may be responsible for changes in the intrinsic properties and/or silencing of SERT, and that Thr phosphorylation may trigger internalization of phosphorylated SERT (Jayanthi et al., 2005).
Table 1.
Summary of kinase, receptor, substrates and antagonists on SERT regulation
| Regulators | Effects on SERT | References |
|---|---|---|
| Protein kinases | ||
| PKC | Activation: Decreased SERT Vmax and surface SERT. Increased SERT endocytosis and phosphorylation. Biphasic effects in platelets: During initial phase, PKC phosphorylates SERT on serine residue(s) and inhibits 5-HT uptake, decreases Vmax and 5-HT affinity without affecting surface SERT. Later phase, PKC phosphorylates SERT on both serine and threonine residues, enhances SERT internalization, inhibits 5-HT uptake decreased Vmax with out effecting 5-HT affinity. | (Anderson & Horne, 1992; Jayanthi et al., 2005; Oz et al., 2010; Qian et al., 1997; Ramamoorthy & Blakely, 1999; Ramamoorthy et al., 1998) |
| p38 MAPK | Inhibition: Decreased SERT Vmax and Km. Decreased SERT basal phosphorylation with or with out changes in surface SERT proteins. | (Oz et al., 2010; Samuvel et al., 2005; Zhu et al., 2005; Zhu, Hewlett, Feoktistov et al., 2004) |
| PKG | Activation: Increased 5-HT uptake and SERT Vmax with no effect on 5-HT Km. Trafficking dependent and/or independent. Phosphorylation of SERT-Thr276 site is required for PKG to stimulate SERT. | (Ramamoorthy et al., 2007; Zhu, Hewlett, Feoktistov et al., 2004) |
| CaMKII | Inhibition: Decreased 5-HT uptake. | (Jayanthi et al., 1994) |
| Tyrosine kinase | Inhibition: Decreased 5-HT-uptake and Vmax. | (Helmeste & Tang, 1995; Zarpellon et al., 2008) |
| Phosphatases | ||
| PP2A | Inhibition: Decreased 5-HT uptake and increased SERT phosphorylation. Associates with SERT. | (Bauman et al., 2000; Ramamoorthy et al., 1998) |
| Receptors | ||
| AR3 | Activation: Increased 5-HT uptake and SERT Vmax with no effect on Km. Trafficking dependent and independent mechanisms through PKG, p38 MAPM and PP2A pathways. | (Miller & Hoffman, 1994; Zhu et al., 2007) |
| H3R | Activation: Increased 5-HT uptake through NO/PKG pathway. | (Launay et al., 1994) |
| α2–AR | Activation: Decreased 5-HT uptake and Km with no effect on SERT Vmax through Ca2+ influx via voltage-sensitive Ca2+ channels. | (Ansah et al., 2003) |
| IL1β | Activation: Increased 5-HT uptake and decreased Km, no effect on SERT Vmax through p38 MAPK activation. | (Zhu et al., 2006) |
| TNF- | Activation: Increased 5-HT uptake, decreased Km and increased Vmax through p38 MAPK activation. | (Zhu et al., 2006) |
| 5-HT1B | Antagonists inhibit 5-HT clearance. | (Daws et al., 2000) |
| BDNF | Activation: Increased 5-HT uptake | (Benmansour et al., 2008) |
| Substrates | ||
| 5-HT | Upregulates surface SERT and attenuates PKC-dependent SERT phosphorylation and surface down regulation. | (Ramamoorthy & Blakely, 1999; Whitworth et al., 2002) |
| AMPH | Increases SERT basal phosphorylation through p38 MAPK pathway. | (Ramamoorthy & Blakely, 1999) |
| Fenfluramine | Attenuates PKC-dependent SERT phosphorylation and surface down regulation. | (Ramamoorthy & Blakely, 1999) |
| Antagonists | ||
| Paroxitine, Citalopram, Imipramine, and Cocaine | Attenuate PKC-dependent SERT phosphorylation and surface down regulation. | (Ramamoorthy & Blakely, 1999) |
In contrast to a PKC-dependent regulation of SERT trafficking and phosphorylation, mitogen-activated protein kinases (MAPK) activate SERT in midbrain synaptosomes and heterologous cell models. Among MAPK pathways, inhibition of p38 MAPK either pharmacologically or by specific siRNAs-mediated knockdown inhibits SERT activity. In contrast, extracellular signal-regulated kinase (ERK1/2) or c-Jun N-terminal Kinase 1 (JNK) inhibition is without effect (Oz et al., 2010; Samuvel et al., 2005). Furthermore, activation of p38 MAPK either by over expressing a constitutively active form of MAP kinase kinase 3b, an upstream kinase activator of p38 MAPK, exposure to aniosmycin, an activator of p38 MAPK, or adenosine receptor (AR3) activation stimulates SERT activity (Samuvel et al., 2005; Zhu et al., 2005; Zhu, Hewlett, Feoktistov et al., 2004; Zhu et al., 2007). The degree to which p38 MAPK regulates SERT surface expression is controversial, with evidences both supportive of trafficking dependent and independent mechanisms. Zhu and coworkers (Zhu, Hewlett, Feoktistov et al., 2004) showed that short term incubation with the p38 MAPK inhibitor, SB203580, blocks AR3 mediated increases in 5-HT uptake in RBL-2H3 cells and in CHO cells transfected with SERT and AR3. However, SB203580 had no effect on AR3 mediated increases in SERT surface expression. However, studies by Samuvel et al. demonstrated that p38 MAPK inhibition affects SERT insertion into the plasma membrane (Samuvel et al., 2005). Although an explanation for the discrepancy between these two studies remains unclear, many factors such as time of treatment, cell type used, differential expression of p38 MAPK isoforms in the cell model systems used, SERT expression levels and the SERT distribution ratio between plasma membrane and intracellular pool all could contribute to the observed differences. Interestingly, basal SERT phosphorylation is inhibited following p38 MAPK inhibition suggesting that constitutively active p38 MAPK is involved in maintaining SERT basal phosphorylation, expression and activity (Samuvel et al., 2005). The findings that PKC activation increases SERT internalization and phosphorylation whereas inhibition of p38 MAPK reduces basal SERT phosphorylation and plasma membrane insertion suggests that p38 MAPK and PKC must act on different phospho-sites. If direct phosphorylation is involved in both processes, then these kinases may target SERT at different cellular locations. Furthermore, PKC and p38 MAPK mediated SERT basal phosphorylation may differentially impact SERT trafficking and catalytic activity. Future efforts directed towards the identification of the specific sites of PKC and p38 MAPK mediated phosphorylation will illuminate the cellular and molecular basis of SERT regulation by these kinases. P38 MAPK is induced by stress. Numerous studies have demonstrated altered extracellular 5-HT concentrations, 5-HT synthesis/metabolism, 5-HT receptor signaling cascades, SERT binding sites and serotonergic neuronal firing in the brain in response to stressful stimuli (Chaouloff et al., 1999). Thus, adjustments in 5-HT neurotransmission may allow an appropriate behavioral response to stress and SERT regulation by p38 MAPK may provide a novel presynaptic mechanism by which appropriate synaptic 5-HT levels are maintained during stressful conditions.
Activation of AR3 in RBL cells, or in CHO-1 cells transfected with SERT and ARs stimulates 5-HT uptake through PKG activation (Miller & Hoffman, 1994; Zhu, Hewlett, Feoktistov et al., 2004). Nitric oxide (NO) stimulation of SERT expressed in HeLa and COS cells requires the cGMP pathway (Kilic et al., 2003). In platelets, histamine receptor activation stimulates 5-HT uptake via NO/PKG activation (Launay et al., 1994). Cyclic GMP analogs also stimulate 5-HT uptake in these model systems (Miller & Hoffman, 1994; Zhu, Hewlett, Feoktistov et al., 2004; Zhu, Hewlett, Francis et al., 2004). Although studies from multiple laboratories have shown that PKG activation enhances 5-HT uptake, the issue of whether there is surface redistribution of SERT following PKG activation is both complex and controversial. It appears that under certain experimental conditions, PKG mediated SERT regulation can occur through alternative pathways. Furthermore, SERT regulation or trafficking in response to PKG may differ depending on cell type. For example, exposure of HeLa cells transiently expressing hSERT to 8-Br-cGMP resulted in increased 5-HT uptake and surface binding to SERT (Zhu, Hewlett, Feoktistov et al., 2004; Zhu, Hewlett, Francis et al., 2004). In RBL-2H3 and CHO-1 cells co-expressing SERT and AR3, activation of AR3 increased 5-HT uptake and SERT surface expression via activation of PKG and p38 MAPK (Zhu, Hewlett, Feoktistov et al., 2004; Zhu, Hewlett, Francis et al., 2004). Interestingly, inhibition of either p38 MAPK or PP2 blunted AR3 stimulation of SERT activity, but not the stimulation of SERT surface density. The authors of this study proposed that AR3 stimulates SERT activity via a PKG dependent enhancement of surface SERT and a separate p38 MAPK dependent enhancement of SERT intrinsic activity. Thus, PKG and p38 MAPK may work in concert to maintain a balance between the number and catalytic state of surface SERT. In contrast to these reports, studies from other laboratories demonstrated trafficking independent SERT upregulation by PKG activation (Kilic et al., 2003; Miller & Hoffman, 1994). The discrepancies between studies are not understood.
The studies described above clearly indicate that several kinases regulate SERT activity and that regulation can be trafficking dependent or independent. These kinases also trigger SERT phosphorylation. Activation of PKG phosphorylates endogenously expressed SERT in rat midbrain as well as human SERT expressed in CHO-1 cells (Ramamoorthy et al., 2007). It would not be surprising if transporters use phosphorylation as a trigger for transporter regulation. Proof of such regulation requires the identification of kinase-specific site(s) within SERT and the demonstration of loss of kinase-mediated SERT regulation and phosphorylation when the putative site is mutated into non-phosphorylatable amino acids such as alanine. Recently, Ramamoorthy’s group identified a phosphorylation site in SERT and demonstrated that phosphorylation of Thr-276 is required for cGMP-mediated SERT regulation (Ramamoorthy et al., 2007). Interestingly, this study showed that cGMP-stimulated phosphorylation of native SERT occurs only on Thr residues. Mutation of SERT Thr-276 to alanine abolished cGMP- mediated stimulation of 5-HT transport and SERT phosphorylation. Phosphorylation of Thr-276 results in addition of a negative charge that might influence SERT activity. If this hypothesis is correct, then, substitution of Thr-276 with aspartic acid (Asp), (which in its ionized form carries a negative charge) would mimic PKG effects on 5-HT uptake. Indeed mutation to Thr-276 to Asp, which would mimic phosphorylation, increased 5-HT uptake to a level equal to that of cGMP-stimulated 5-HT uptake in wild-type SERT expressing cells, and uptake was no longer sensitive to cGMP. These findings provide the first identification of a phosphorylation site in SERT and demonstrate a direct link that PKG phosphorylates SERT on Thr-276 leading to increased 5-HT uptake.
In addition to the role Ser/Thr protein kinases play in the regulation of SERT, evidence suggests that a family of tyrosine protein kinases also regulate SERT activity and phosphorylation. Several structurally distinct tyrosine kinase inhibitors such as genistein, herbimycin A and 2,5-dihydroxycinnamate inhibit 5-HT uptake in platelets (Helmeste & Tang, 1995; Zarpellon et al., 2008). Recently, Zarpellon and coworkers provided evidence that Src-family tyrosine kinases regulate SERT function and SERT phosphorylation on tyrosine residues (Zarpellon et al., 2008). In human platelets, while the Src kinase inhibitors PP2 and SU6656 inhibit 5-HT uptake and phosphorylation of tyrosine residues of SERT, the tyrosine protein phosphatase inhibitor, pervanadate, increases SERT activity and tyrosine phosphorylation of SERT. In addition, using Src-specific peptide and an in vitro kinase assay the authors documented the presence of Src-kinase activity in SERT immunoprecipitates from human platelets. Likewise, blots from these SERT immunoprecipitates revealed the presence of Src kinase suggesting that SERT and Src exist as a complex (Zarpellon et al., 2008). Clearly, further studies identifying the tyrosine residue(s) within SERT and the role of tyrosine phosphoryation in Src-tyrosine kinase mediated SERT regulation are warranted.
3. The norepinephrine transporter and its functional significance
NET clears NE released into the synaptic cleft in a Na+/Cl− dependent manner (Bonisch & Bruss, 2006; Iversen, 1971, 1978; Pacholczyk et al., 1991; Trendelenburg, 1991). NET is a target for the treatment of mood and cognitive disorders (Blier, 2001; Bonisch & Bruss, 2006). NET is also a target for psychostimulants, including cocaine and amphetamines (Binda et al., 2006; Jayanthi et al., 2002; Justice et al., 1998; Pacholczyk et al., 1991). NET is selectively expressed on NE nerve terminals, thereby, enabling spatial and temporal control of the actions of NE (Foote et al., 1983; Moore & Bloom, 1979). NET is also expressed in peripheral tissue (e.g., adrenal glands, vas deferens and placenta (Jayanthi et al., 2002; Schroeter et al., 2000; Sung et al., 2003). Altered NET function is associated with attention, mood and cardiovascular disorders (Esler et al., 2006; Haenisch et al., 2008; Hahn et al., 2008; Hahn et al., 2005; Kim et al., 2006; Klimek et al., 1997; Rumantir et al., 2000; Shannon et al., 2000). Human NET, which is localized to chromosome 16q13-q21 (16q12.2) (Brüss et al., 1993; Gelernter et al., 1993), is a 617 amino acid protein containing 12 transmembrane domains with cytoplasmic amino- and carboxy terminals. A large extracellular loop between transmembrane domains 3 and 4 contains glycosylation sites. Several canonical sites for protein kinases can be found in sequences of cytoplasmic domains (Binda et al., 2006; Jayanthi et al., 2002; Justice et al., 1998; Pacholczyk et al., 1991). Alternative splicing has been reported to regulate NET expression and function (Kitayama et al., 2001). This occurs at 3′-flanking coding and noncoding regions, resulting in different carboxy terminals. When expressed in HEK-293 cells, these isoforms of NET showed reduced functional activity and surface expression. NET knock out mice display altered seizure susceptibility and opiate/cocaine sensitivities, as well as maladaptive responses to social and cardiovascular stressors, The phenotype of these animals underscores the importance of NET in normal physiology and behavior (Ahern et al., 2006; Bohn et al., 2000; Haller et al., 2002; Keller et al., 2006; Keller et al., 2004; Mitchell et al., 2006; Xu et al., 2000). NET also effectively clears DA in brain regions (e.g. prefrontal cortex) where DAT expression is low or absent suggesting the physiological significance of NET in controlling DA transmission and DA-induced behavior (Madras et al., 2005; Moron et al., 2002; Siuta et al.). NET function is highly regulated (Bonisch & Bruss, 2006; Mandela & Ordway, 2006) (summarized in Table 2). NET control of NE signaling is, thus, an important homeostatic mechanism and its dysregulation by psychostimulants is thought to contribute to the behavioral and neurochemical effects of psychostimulants (Binda et al., 2006; Dipace et al., 2007)and chronic stress (Miner et al., 2006).
Table 2.
Summary of kinase, receptor, substrates and antagonists on NET regulation
| Regulators | Effects on NET | References |
|---|---|---|
| Protein kinases | ||
| PKC | Activation: Decreased NET Vmax, unaltered NE Km, decreased surface NET proteins, increased NET endocytosis and increased NET phosphorylation. Phosphorylation of Thr258/Ser259 is required for PKC-linked NET down regulation. Translocates NET from lipid-rafts to non-lipid rafts. | (Apparsundaram, Galli et al., 1998b; Apparsundaram, Schroeter et al., 1998; Bönisch et al., 1998; Jayanthi et al., 2006; Jayanthi et al., 2004) |
| P38 MAPK | Inhibition: Decreased NET Vmax and NE Km. | (Apparsundaram et al., 2001) |
| CaMKII | Inhibition: Blunted Ca2+-induced stimulation of NE uptake, AMPH-induced NET down regulation. | (Uchida et al., 1997, 1998) |
| Tyrosine kinase | Inhibition: Decreased NET Vmax, unaltered Km. | (Apparsundaram et al., 2001) |
| PI-3 kinase | Inhibition: Reduced NE uptake and decreased surface NET. | (Apparsundaram et al., 2001) |
| Akt-1 | Decreased Akt-Ser473 phosphorylation increased NE uptake and NET functional expression | (Siuta et al., 2010) |
| Phosphatases | ||
| PP2A | Inhibition: Reduced NE uptake and increased NET phosphorylation. | (Bauman et al., 2000, Jayanthi, 2004 #8329) |
| Receptors | ||
| AT1 | Activation: Increased NET activity and surface expression through activation of PI-3 kinase and MAPK. | (Savchenko et al., 2003; Yang & Raizada, 1999) |
| NK1 | Activation: Decreased NET Vmax, unaltered NE Km, decreased surface NET proteins and increased NET phosphorylation. | (Jayanthi et al., 2006) |
| mAChRs | Activation: Decreased NET Vmax, unaltered NE Km, decreased surface NET proteins through intracellular Ca2+ and PKC-dependent/independent pathways. | (Apparsundaram, Galli et al., 1998a) |
| Insulin | Increased NE uptake and NET Vmax, unaltered NE Km and surface NET. Ca2+, PP2A, tyrosine kinase, Akt, PI-3 kinase and p38 MAPK activities are involved. | (Apparsundaram et al., 2001; Boyd et al., 1985; Figlewicz, Bentson et al., 1993) |
| Substrates | ||
| NE | Long term treatment of NE down regulates NET protein. | (Zhu et al., 1998) |
| AMPH | Decreased surface NET. Ca2+ and CaMKII– dependent Syntaxin 1A interaction with NET and Rab 11 is implicated in AMPH- mediated effects. Decreased surface NET via increasing NET endocytosis, Mutations of Thr-258/Ser-259 block AMPH induced NET down regulation. | (Annamalai et al., 2010; Dipace et al., 2007; Matthies et al., 2010) |
| Desipramine | Decreased NE uptake and NET binding sites. | (Zhu et al., 1998; M. Y. Zhu et al., 2000) |
3.1. Receptor-protein kinase mediated acute regulation of NET activity and phosphorylation
PKC-activation inhibits NET function. Down regulation of function involves sequestration of NET protein from the plasma membrane (Apparsundaram, Schroeter et al., 1998; Jayanthi et al., 2004) In transformed SK-N-SH cells, which endogenously express NET, activation of muscarinic acetylcholine receptors by the agonist methacholine also results in NET down-regulation. Moreover, inhibition of PKC by antagonists abolishes PKC-mediated effects but produces only partial blockade of methacholine-mediated effect. PKC activation and pathways dependent on mobilization of Ca2+ stores are involved in muscarinic receptor-mediated NET regulation (Apparsundaram, Galli et al., 1998a). Together these data suggest the existence of PKC-independent regulatory pathways that maintain NET surface expression and/or intrinsic transporter catalytic activity (summarized in Table 2). Jayanthi et al. provided key evidence for lipid raft-mediated endocytosis as the mechanism of NET down-regulation by PKC (Jayanthi et al., 2004). Subsequent studies by the same group identified a Thr-258/Ser-259 trafficking motif linked to substance P-mediated activation of neurokinin 1 receptor (NK1R-PKC) -induced NET down-regulation and phosphorylation (Jayanthi et al., 2006). Only Ser-259 was identified as a potential PKC site. However, phosphoamino acid analysis showed phospho-Ser and phospho-Thr residues following PKC activation (Jayanthi et al., 2006). Mutation of both Thr-258 and Ser-259 sites was required for abolition of PKC-mediated inhibition of NE transport. Mutation of Thr-258 and Ser-259 sites blocked PKC-induced phosphorylation to a significant extent (60%), but not completely. In addition, the Thr-258Ala/Ser-259Ala mutant showed enhanced basal phosphorylation suggesting that phosphorylation of this motif may influence other sites (Jayanthi et al., 2006). Other sites including Thr-19, Thr-30 and Thr-58, Ser-502, Ser-579, Thr-580 and Ser-583 are not involved in PKC-mediated NET regulation. Although Ser-259 appears to be the direct site of action of PKCε. However, based on in vitro phosphorylation assays, Ser-259 phosphorylation is not required for PKC-mediated NET down-regulation. Thr-258Ala mutation partly blunted both PKC-mediated phosphorylation and transporter down-regulation. However, the fact that the double mutation (258Ala/Ser-259Ala) partially eliminates PKC-induced phosphorylation and completely blocks NET down-regulation/internalization suggests a possible relationship between phosphorylation of the Thr-258/Ser-259 motif and transporter internalization. It is possible that PKC-mediated Thr-258 phosphorylation may have a modulatory role in NET regulation, and, in concert with Ser-259 phosphorylation, dictates NET endocytosis (Jayanthi et al., 2006).
The existence of physical complexes containing biogenic amine transporters and PP2Ac proteins has been established (Bauman et al., 2000) suggesting that modulation of transporter/phosphatase association is involved in regulated transporter phosphorylation and trafficking. In addition to NET phosphorylation, the presynaptic factor, syntaxin 1A is known to bind the N-terminal domain of NET. Truncation at the N-terminal domain of human NET disrupts NET/syntaxin 1A association and limits the ability of PKC (phorbol ester treatment) to downregulate NET function (Sung et al., 2003). Future studies exploring the idea that transporter association with these partners, per-se regulates transporter phosphorylation and function will aid our understanding of how these two mechanisms are inter-related.
Diverse biologic stimuli including neuronal activity, peptide hormones, and trophic factors have been implicated in NET regulation. Insulin regulates NE transport (Apparsundaram et al., 2001; Boyd et al., 1985; Boyd et al., 1986; Figlewicz, Bentson et al., 1993; Figlewicz, Szot et al., 1993) and MAPKs differentially regulate NET function by trafficking dependent and independent mechanisms (Apparsundaram et al., 2001). PP2Ac blockers are also known to abolish insulin-mediated NET regulation (Apparsundaram et al., 2001). Raizada and colleagues demonstrated that acute angiotensin II (AngII) treatments elicit a rapid increase in NE transport in cultured brainstem neurons (Boyd et al., 1985; Lu et al., 1996; Sumners & Raizada, 1986). Later studies from Blakely’s group using a newly developed ectodomain NET antibody, mouse brainstem and superior cervical ganglion neurons (SCG) primary cultures, further confirmed that Ang II treatments increase surface NET and activity rapidly (Savchenko et al., 2003). In addition, when neuronal activity was mimicked by KCl-evoked depolarization, increased surface NET expression was seen. Although the involvement of phosphatidylinositol 3-kinase (PI-3 kinase) and MAPK following long-term AngII receptor activation has been demonstrated (Yang & Raizada, 1999), future studies are warranted to elucidate the pathways by which acute AngII receptor activation alters NET surface expression and delineate the convergence between these and those engaged by depolarization-evoked increases in neuronal activity. Recent studies using neuronal Rictor Null mice established that Akt (protein kinase B)-linked NET regulation contributes to altered DA-transmission and schizophrenia-like behaviors (Siuta et al., 2010). Rictor Null mice exhibit diminished Akt-Ser473 phosphorylation and enhanced NET functional expression within the cortex. NE levels are elevated and lower DA content is lower in the cortex. Interestingly, the NET blocker, nisoxetine reverses hypodopaminergia condition and schizophrenia-linked behaviors (Siuta et al., 2010). Thus, NET activities regulate NE as well as DA homeostasis, thereby, affecting both NE and DA-linked behaviors.
4. The dopamine transporter and its functional significance
DAT is a major regulators of DA neurotransmission. DAT activity requires Na+ and Cl− to clear synaptic DA. Human DAT. which is located in chromosome 5p15.3 (Giros et al., 1992), encodes 620 amino acids with 12 putative transmembrane domains and a large second extracellular loop with several putative N-glycosylation sites. Both amino- and carboxy-terminals are intracellular with several predicted protein kinase sites (Kilty et al., 1991; Shimada et al., 1991). DAT is primarily expressed in DA neurons. As observed with other MA transporters, it is also found in peripheral systems including lymphocytes (Amenta et al., 2001). DAT-knockout mice display increased extracellular DA concentrations relative to wild types as well as altered synthesis of DA and other amines (Giros et al., 1996). DA receptor function and expression is also abnormal (Fauchey et al., 2000; Gainetdinov, Jones et al., 1999; Ghisi et al., 2009; Jones et al., 1999; Ralph et al., 2001). DAT knock out mice are hyperactive, exhibit dwarfism, cognitive problems, sleep dysregulation, alterations in gut motility, skeletal abnormalities and behavioral inflexibility (Caron & Gainetdinov, 2010; Gainetdinov & Caron, 2003; Giros et al., 1996).
The DAT is a well-established target of various psychoactive compounds including cocaine, amphetamines and PCP derivatives. Whereas cocaine and methylphenidate are DAT activity blockers and inhibit DA uptake, amphetamine and methamphetamine (METH) are substrates that are transported by DAT and that trigger DA release via a DAT-dependent mechanism. Neurotoxins such as 6-hydroxydopamine and MPP+ enter DA neurons by the activity of DAT and produce DA neuronal damage. Altered DAT expression and function has been documented in human cocaine and METH addicts and in Parkinson’s disease (Mash et al., 2002; Volkow et al., 2001; Weintraub et al., 2005). The dependence on DAT for normal DA clearance and signaling suggests that DAT dysfunction may contribute to various brain disorders associated with dysregulation of DA transmission including schizophrenia, affective disorders and addiction. Data from animal models, human neuroimaging and genetic studies suggest altered DAT availability or function in attention deficit hyperactivity disorder (ADHD) and two ADHD medications, Ritalin™ and Adderall™ target DAT (Logan et al., 2007; Volkow et al., 2001). The DAT coding variant Ala-559Val identified in ADHD is associated with abnormal DAT function and regulation (Mazei-Robison & Blakely, 2005; Mazei-Robison et al., 2008). Decreased mesocorticolimbic DA transmission is implicated in certain symptoms of depression (Nestler & Carlezon, 2006) and medications with proven antidepressant efficacy in humans (e.g., bupropion, nomifensine, amineptine) are DA uptake inhibitors. Thus normal regulation and expression of DAT is important for maintaining DA homoeostasis.
4.1. Receptor-protein kinase mediated acute regulation of DAT activity and phosphorylation
As described above for SERT and NET, the function of native and heterologously expressed DAT is rapidly altered in response to acute activation and/or inhibition of several protein kinases including PKA, PKC, PI-3 kinase, ERK1/2, Akt, CaMKII, cyclin-dependent kinase (Cdk 5), tyrosine kinases and protein phosphatase PP1/PP2Ac (Bolan et al., 2007; Carvelli et al., 2002a; Foster et al., 2003; Hoover et al., 2007; Melikian, 2004; Moron et al., 2003; Sorkina et al., 2005;R. A. Vaughan, 2004). In addition, G-protein coupled receptor (GPCR) and non-GPCR receptor regulation of DAT has been documented (Bolan et al., 2007; Granas et al., 2003; Hoover et al., 2007; Page et al., 2001; Savchenko et al., 2003; Yang et al., 1997; Zapata et al., 2007)(summarized in Table 3).
Table 3.
Summary of Kinase, receptor, substrates and antagonists on DAT regulation
| Regulators | Effects on DAT | References |
|---|---|---|
| Protein kinases | ||
| PKC | Activation: Decreased DAT Vmax, unaltered DA Km, decreased surface DAT proteins, increased DAT endocytosis and increased straital DAT phosphorylation on N-tail serines. Regulates AMPH-induced DA efflux. Triggers DAT degradation in some models and associates with DAT. Residues 587 – 596 located at DAT-carboxy terminal is required for basal and PKC-stimulated DAT internalization. | (Boudanova et al., 2008; M. Y. Chang et al., 2001; R. Chen et al., 2009; Daniels & Amara, 1999; Holton et al., 2005; Johnson, Guptaroy et al., 2005; Melikian & Buckley, 1999; Vaughan et al., 1997; S. J. Zhu et al., 1997) |
| ERK1/2 | Inhibition: Decreased DAT Vmax, unaltered DA Km and decreased surface DAT proteins. | (Moron et al., 2003) |
| CaMKII | Associates with DAT, facilitates N-tail DAT phosphorylation and mediates AMPH-induced DAT mediated DA efflux. | (Fog et al., 2006) |
| Akt | Involved in insulin and AMPH-mediated DA efflux. | (Garcia et al., 2005; Wei et al., 2007) |
| PI-3-kinase | Inhibition: Decreased DAT Vmax, unaltered DA Km and decreased surface DAT proteins. | (Carvelli et al., 2002b; Lute et al., 2008) |
| Cdk5 | Inhibition: Decreased DAT Vmax with out altering surface DAT. | (Price et al., 2009) |
| Tyrosine kinase | Inhibition: Decreased DAT Vmax and decreased surface DAT proteins. | (Doolen & Zahniser, 2001) |
| Phosphatases | ||
| PP2A | Inhibition: Decreased DA uptake and increased DAT phosphorylation. | (Bauman et al., 2000) |
| PP1 | Dephosphorylates phospho-DAT, effects on DAT functional properties are not known. | (Foster et al., 2003) |
| Receptors | ||
| D2R | Activation: Increased DAT activity and surface expression through activation of ERK1/2. Associates with DAT. | (Lee et al., 2007; Mayfield & Zahniser, 2001a, 2001b) |
| D3R | Activation: Regulates DAT in a biphasic manner through activation of ERK1/2 and PI-3 kinase: Earlier activation (1–3 min) increases DAT activity, surface DAT and DAT exocytosis. Prolonged activation (30 min) decreases DAT activity, surface DAT and DAT exocytosis, increased DAT endocytosis. | (Zapata et al., 2007) |
| NK1R | Activation: Decreased DAT Vmax, unaltered DA Km, decreased surface DAT proteins and increased DAT phosphorylation. | (Granas et al., 2003) |
| KOR | Activation: Increase DA-uptake | (Thompson et al., 2000) |
| mGluR5 | Activation: Decreased DAT Vmax, PKC and/or CaMKII inhibitors prevent mGluR5 effect, phosphatase inhibitor augments mGluR5-effect. | (Page et al., 2001) |
| TrkB | Activation: Increased DAT Vmax, DA Km and surface DAT through MAPK and PI-3 kinase. | (Hoover et al., 2007) |
| Insulin | Increased DA-uptake and surface DAT. Requires Akt, PI-3 kinase activity. | (Garcia et al., 2005; Wei et al., 2007; Williams et al., 2007a) |
| Substrates | ||
| DA | DA down regulates surface DAT in a PKC dependent manner. | (Chi & Reith, 2003; Richards & Zahniser, 2009) |
| AMPH | Affects in a biphasic manner: Increased surface DAT at early exposure followed by decreased surface DAT. Increased DAT phosphorylation that is sensitive to cocaine and other DAT antagonists. Akt, PKC, PI-3 kinase, CaMKII and Syn 1A are involved in AMPH-mediated DA efflux and phosphorylation. Insulin attenuates AMPH- effect. | (Binda et al., 2008; Cervinski et al., 2005; Fog et al., 2006; Garcia et al., 2005; Gorentla & Vaughan, 2005; Johnson, Guptaroy et al., 2005; Khoshbouei et al., 2004; Saunders et al., 2000) |
| Antagonists | ||
| Cocaine | Increased DAT Vmax and surface expression. Regulates DAT- serine phosphorylation and DAT-PP2A association. | (Daws et al., 2002; Little et al., 2002; Ramamoorthy et al.,; Samuvel et al., 2008) |
| GBR 12909 | Decreased basal and PKC-stimulated DAT phosphorylation. Prevents PKC-mediated DAT down regulation. | (Gorentla & Vaughan, 2005) |
The most thoroughly studied kinase is PKC. Studies in various cell lines transfected with DAT have shown that activation of PKC by phorbol esters, such as phorbol 12-myristate 13-acetate (PMA) decreases dopamine transport capacity (M. Y. Chang et al., 2001;R. Chen et al., 2009; Daniels & Amara, 1999; Doolen & Zahniser, 2002; Eriksen et al., 2009; Melikian & Buckley, 1999; Reith et al., 1997; Sorkina et al., 2005;S. J. Zhu et al., 1997). A similar down-regulation has also been reported in synaptosomal preparations (Copeland et al., 1996; Foster et al., 2008; Foster et al., 2002; Vaughan et al., 1997). DAT down-regulation in response to PKC activation has been attributed to dynamin-dependent endocytosis (Daniels & Amara, 1999; Eriksen et al., 2009; Melikian & Buckley, 1999; Saunders et al., 2000; Sorkina et al., 2005) although an initial rapid trafficking-independent inactivation of the transporter might occur at the plasma membrane (Mazei-Robison & Blakely, 2005) as reported for SERT (Jayanthi et al., 2005). While recycling of internalized DAT was shown in PC12 cells, the internalized DAT was subject to lysosomal degradation in HeLa and MDCK cells (Daniels & Amara, 1999; Melikian & Buckley, 1999). Using PC12 cells and site-directed mutagenesis, the importance of DAT-carboxy terminal residues 587 – 596 in basal and PKC-stimulated internalization has been documented (Boudanova et al., 2008; Holton et al., 2005). DAT ubiquitination is also a critical step for PKC-dependent DAT endocytosis. Miranda et al. showed that DAT is constitutively ubiquitinated and that ubiquitination is enhanced in response to PMA stimulation (Miranda et al., 2005). The ubiquitination was dependent on the presence of lysines (Lys) located in the intracellular DAT N-terminus (Lys-19, Lys-27, Lys-35) and mutation of these residues to arginine abolished ubiquitination and phorbol ester stimulated DAT down-regulation (Miranda et al., 2007). Interestingly, ubiquitination was most apparent in endosomes suggesting that ubiquitination may be a signal for endocytosis. Evidence from both in-vitro and ex-vivo studies have demonstrated that DAT exists in a phosphorylated form and basal DAT phosphorylation is stimulated by PKC activation and phosphatase inhibition (Cervinski et al., 2005; Granas et al., 2003; Huff et al., 1997; Vaughan et al., 1997). Thus, both PKC and PP1/2A/PP1 play a pivotal role in the turnover of DAT phosphorylation.
Several other kinase pathways affect DAT function and surface expression. Inhibitors of PI-3 kinase or Akt, as well as over-expression of a dominant negative Akt mutant in heterologous cells, decrease DAT activity and surface levels (Carvelli et al., 2002a; Garcia et al., 2005). A physiological role of this pathway in regulating DAT levels was supported by studies in rats in which insulin was depleted using the diabetogenic agent, streptozotocin. Following insulin depletion, Akt function and DAT surface expression were markedly reduced (Williams et al., 2007b). Other studies (Carvelli et al., 2002a; Moron et al., 2003) have demonstrated that DAT transport properties are sensitive to modulation of MAPK modulation. Pharmacological blockade of constitutively active MAPK reduces DAT activity and surface levels (Moron et al., 2003). However, changes in surface expression are not always associated with kinase-induced alterations in DAT activity. For example, Cdk5 inhibition reduces DAT activity without affecting the levels of surface DAT protein (Price et al., 2009). Another study provided evidence that PKC induced internalized DAT can be recycled back to the cell surface by PKA-dependent mechanisms (Pristupa et al., 1998).
D2 and D3 DA receptors (D2R, D3R) are located on DA neurons. Although their role in the presynaptic regulation of DA release is well recognized, early studies examining their contribution to DAT regulation yielded disparate results. Using voltammetry to monitor instantaneous rates of DA clearance or electrophysiology, several studies showed that D2R/D3R agonists increase DAT function whereas a downregulation of function occurs in response to receptor antagonists or gene deletion (Dickinson et al., 1999; Mayfield & Zahniser, 2001a; Meiergerd et al., 1993). However, radioligand uptake studies using tritiated DA revealed no such effects. Recent works by Shippenberg and colleagues have provided an explanation for this seeming paradox. Using confocal microscopy in conjunction with the fluorescent DAT substrate, 4-[4-(diethylamino)-styryl]-N-methylpyridinium iodide (ASP+) to monitor DAT activity in real time or tritiated tyramine in uptake studies, these investigators showed that activation of either D2R or D3R is sufficient to upregulate DAT function in heterologous expression systems (Bolan et al., 2007; Mayfield & Zahniser, 2001a; Zapata et al., 2007). However, these effects were not observed in [3H]DA uptake studies. Importantly, and in contrast to DA, ASP+ and tyramine do not bind to or activate DA receptors. Thus, by using these substrates the measurement of basal DAT activity is not confounded by substrate-induced receptor activation, thereby, enabling detection of agonist-evoked increases in transporter function. These investigators went on to show that the short form of the D2R, which is located on DA nerve terminals, is associated with DAT and its activation increases transporter activity by an ERK1/2 dependent mechanism. Using co-immunoprecipitation and GST fusion protein pull-down experiments in striatal tissue extracts, Liu and co-workers provided evidence that D2Rs directly interacts with DAT (Lee et al., 2007). Interestingly, the D3R also regulates DAT trafficking and DA uptake. However, this effect varies as a function of the duration of receptor activation and appears to involve multiple kinase cascades. Thus, transient receptor activation (1–3 min) of D3R increased DA uptake via ERK1/2 and PI-3 Kinase-dependent mechanisms. DAT cell surface expression and the rate of DAT exocytosis were also increased. In contrast, prolonged (30 min) D3R activation reduced DA uptake and DAT cell surface expression. This was accompanied by a decreased rate of DAT exocytosis and an increased rate of endocytosis. Together, these findings demonstrate that D2R and D3R regulate DA transmission by affecting DAT function as well as DA release. Insulin increases DA uptake and DAT cell surface levels through activation of PI-3 Kinase and Akt. Non-GPCRs can also regulate DAT function and expression. BDNF stimulates DA uptake and surface expression through activation of TrkB-receptor linked PI-3 kinase and ERK1/2 activation (Hoover et al., 2007). Systemic administration of the endocannabinoid, anandamide, increases extracellular DA levels in the nucleus accumbens, a brain region implicated in mediating the abuse liability of various psychoactive drugs (Solinas et al., 2006). Although these effects were initially attributed to alterations in DA release evidence that anandamide and other cannabinoids inhibit DA uptake in native tissue and heterologous expression systems has been presented (Chen et al., 2003; Price et al., 2007). A recent study has shown that the interaction of anandamide with DAT occurs via a cannabinoid receptor-independent mechanism and is associated with a redistribution of DAT from the plasma membrane to the cytosol (Oz et al., 2009). However, there is no direct evidence demonstrating that modulation of DAT phosphorylation by these kinases, receptors or receptor linked kinase cascades is a prerequisite for their effects on DAT trafficking and function. Another study from Page et al has shown that activation of metabotropic glutamate receptors (mGluR5) down regulates DA uptake through the activation of both PKC and CaMKII in striatal synaptosomes (Page et al., 2001).
DAT phosphorylation was reviewed extensively by Foster et al (Foster et al., 2006). DAT-Ser phosphorylation was identified using p-Ser antibody (Vrindavanam et al., 1996). It is, however, important to note that the cytoplasmic domains of DAT contain putative consensus sites for several protein kinases (Kilty et al., 1991; Shimada et al., 1991). Mutatagenesis (Chang et al., 2001; Lin et al., 2003) studies revealed phosphorylation sites(s) Ser-7 for PKC, Thr-62, Ser-581 and Thr-612 for PI-3 kinase and Ser-12 and Thr-595 for ERK1/2. Using enzymatic cleavage, phospho-peptide mapping, amino acid analysis and truncation studies Vaughan and coworkers (Foster et al., 2002) identified a cluster of six serines located at the distal end of the DAT N-tail that are phosphorylated following PKC activation or phosphatase inhibition (Cervinski et al., 2005; Foster et al., 2002; Granas et al., 2003). Another cluster of serines and threonines located at the DAT N-tail more proximal to the membrane contain consensus motifs for PKC, PKA, and proline-directed kinases (Gorentla et al., 2009). DAT C-terminal Ser and Thr residues have been suggested as potential phosphorylation sites (Chang et al., 2001) for PKC, PKA, and proline-directed kinases (Gorentla et al., 2009). Whether these kinases directly phosphorylate DAT is unclear. While the cluster of N-tail serines are phosphorylated by PKC (Foster et al., 2002), elimination of PKC-mediated N-tail DAT phosphorylation by mutagenesis and truncation failed to prevent PKC mediated DAT internalization suggesting that N-terminal phosphorylation of DAT is not essential for internalization (Chang et al., 2001; Granas et al., 2003). Proline-directed kinases (e.g. RK1/2, p38 MAPK, and JNK) phosphorylated N-DAT on the proximal Thr residue. These kinases require a proline immediately following the phosphoacceptor site. Vaughan and colleagues verified the presence of pThr on the N-terminal tail of DAT and identified Thr-53 as the phosphorylation site for ERK1/2 using recombinantly expressed N- peptide domain of rDAT and heterologously expressed DAT in LLCPK1 cells (Gorentla et al., 2009). These findings are also supported by the fact that MEK inhibitor U0126 is able to inhibit DAT phosphorylation (Lin et al., 2003). However, the functional implication of DAT Thr-53 and other phosphorylation sites is yet to be determined. Furthermore, using recombinantly expressed N- and C-terminal peptide domains of rDAT (N-DAT and C-DAT) and in vitro phosphorylation and dephosphorylation assays, Gorentla et. al estabilished that PKCα, PKA, PKG, and CaMKII were most efficacious in phosphorylating N-DAT serines. Other kinases (PKCβI, PKCβII, PKCγ, and Akt) had less of an effect. The lower probability for these kinases to act on DAT, could be due to the absence of DAT-interacting proteins or other in-vivo conditions that are absent in the in vitro preparation used (Gorentla et al., 2009). PKCβII and Akt regulate DAT (Garcia et al., 2005; Johnson, Guptaroy et al., 2005), but regulation may be due to phosphorylation of targets upstream of DAT rather than by direct phosphorylation. DAT substrates and antagonists regulate DAT phosphorylation (described below under activity dependent MA transporter phosphorylation).
4.2 Altered DAT Phosphorylation; an Animal Model of Addiction
While cocaine inhibits both SERT, and NET, several lines of evidence have shown that cocaine produces its primary reinforcing effects by binding to DAT and blocking the reuptake of DA into presynaptic terminals, thereby potentiating DA neurotransmission in mesocorticolimbic reward pathways (Koob et al., 1998; McFarland & Kalivas, 2001; Ritz et al., 1987; Robinson & Berridge, 1993). The importance of DAT to DA homeostasis and the reinforcing effects of cocaine is supported by a number of studies of cocaine-induced behavior, amine synthesis, storage, release, DA receptor expression changes resulting from constitutive gene deletion, as well as changes described in human cocaine addicts (Fumagalli et al., 1998; Giros et al., 1996; Jones et al., 1998; Rocha et al., 1998; Sora et al., 2001; Sora et al., 1998). Long-term changes in DAT levels, kinetics, or regulation would be expected to greatly influence spontaneous and drug-induced behaviors. Ramamoorthy and coworkers addressed potential changes in the regulation and phosphorylation of DAT using the rat-cocaine self-administration model of drug reinforcement. Their study provided evidence that environmental conditions experienced during abstinence from protracted drug use impact the nature of abstinence-induced changes in dynamic DAT regulation (Ramamoorthy et al., 2010; Samuvel et al., 2008). Specifically, in rats with a history of cocaine self-administration followed by three weeks of abstinence in which animals are not re-exposed to the environment associated with drug administration, cocaine-experienced rats exhibited elevated DAT activity and Vmax in both the caudate putamen (CPu) and nucleus accumbens (NAcc) (Samuvel et al., 2008). Furthermore, increased surface DAT density, a higher level of DAT-PP2Ac association along with decreased levels of p-Ser DAT phosphorylation were observed in the CPu, but not in the NAcc. Increased DA affinity was evident only in the NAcc (Samuvel et al., 2008). By contrast, in rats with a history of repeated cocaine self-administration followed by three weeks of daily extinction trials in which they were placed in the self-administration environment but drug was not available, DA uptake was significantly higher in the CPu relative to saline controls. In spite of elevated DAT activity in this region, total and surface DAT density and the DAT-PP2Ac interaction remained unaltered, although p-Ser- DAT phosphorylation was elevated. In contrast to the CPu, DAT activity, levels of total and surface DAT, p-Ser-DAT phosphorylation, and DAT-PP2Ac interactions remain unchanged in NAcc (Ramamoorthy et al., 2010). These studies demonstrate that abstinence from cocaine produces marked and persistent alterations in DAT function, expression and catalytic properties. Furthermore, following re-exposure to stimuli associated with drug administration alterations in DAT-trafficking and catalytic regulatory cascades differ from those that occur in their absence. Further understanding of key signaling pathways involved in cocaine-induced DAT neuroplasticity may aide in the development of environmentally based interventions for addiction, as well as pharmacotherapies that restore DAT function.
5. Activity-dependent regulation of monoamine transporter phosphorylation
Increasing evidence indicates that MA transporters are regulated by their substrates. This feedback loop provides a mechanism by which changes in extracellular neurotransmitter concentrations can rapidly modulate neurotransmitter transport capacity. Transporter substrates are known to regulate transporter function and expression (Chi & Reith, 2003; Gulley & Zahniser, 2003; Ramamoorthy & Blakely, 1999). Ramamoorthy and Blakely provided evidence that SERT substrates such as 5-HT, amphetamines, and fenfluramine control PKC-dependent SERT phosphorylation and surface redistribution (Ramamoorthy & Blakely, 1999). The inhibitory effect of 5-HT occurs in the presence of 5-HT receptor antagonists, suggesting that the effect of 5-HT on PKC-mediated SERT phosphorylation is not dependent on 5-HT-receptor activation. Furthermore, like 5-HT transport, 5-HT effects on PKC-induced SERT phosphorylation requires extracellular Na+ and Cl− and can be blocked by SERT antagonists, SSRIs and cocaine. Intriguingly, PKC induced decreases in cell surface SERT density can be abolished by the presence of 5-HT during PKC activation. Together, these findings suggest that SERT, in the presence of actively transporting substrates, reduce the opportunity for PKC-linked SERT phosphorylation and transporter internalization. In addition, d-amphetamine in the presence of Na+ and Cl− increases basal SERT phosphorylation (Ramamoorthy & Blakely, 1999). Interestingly, p38 MAPK inhibition by PD169316, not only inhibited SERT basal phosphorylation but also blunted the ability of d-amphetamine to influence phosphorylation suggesting that p38 MAPK governs the influence of d-amphetamine on basal phosphorylation and cell surface expression (Samuvel et al., 2005). Interestingly, amphetamines substitute for 5-HT in suppressing PKC-mediated SERT phosphorylation. This action could override homeostatic transporter sequestration processes and contribute to psychostimulant sensitization by increasing the number of psychostimulant targets available to a subsequent stimulus. On the other hand, non-permeant SERT ligands including SSRIs and cocaine, that prevent 5-HT permeation, block the effect of 5-HT. Thus SSRIs may have therapeutic utility in disease states, not only by preventing 5-HT uptake but also by shifting the cellular distribution of SERT.
In striking contrast to SERT, the DAT substrates DA and AMPH decrease DA uptake and cell surface DAT in a PKC dependent manner (Gorentla & Vaughan, 2005; Richards & Zahniser, 2009). However, amphetamine regulates DAT in a biphasic manner. DAT cell surface delivery is increased with in 30 sec treatment of amphetamine and returned to control levels at 2.5 min. At later time points, DAT internalization is increased (Johnson, Furman et al., 2005). A recent study demonstrated that METH is more potent than AMPH in inducing DAT-mediated DA efflux and DA efflux induced by the DAT substrate, METH as well as by AMPH is sensitive to PKC and CaMKII inhibition (Goodwin et al., 2009). Vaughan and coworkers reported that METH decreases DAT function in parallel with an increase in DAT basal phosphorylation in both striatal tissue and the LLC-PK1 cells (Cervinski et al., 2005). Interestingly, the DAT inhibitor, cocaine, and the PKC inhibitor, BIM, prevent METH effects on DAT phosphorylation. The authors also reported that removal of the N-terminal tail serine-cluster abolished both basal, PKC and METH induced DAT phosphorylation but not PKC- or METH induced transporter down regulation. Interestingly, mutation of PKC-mediated N-tail DAT phosphorylation sites (Ser-2, Ser-4, Ser-7, Ser-12 and Ser-13) into Ala blunts AMPH induced DAT-mediated DA efflux without affecting DA uptake (Khoshbouei et al., 2004). Substitution of Ser-7 and Ser-12 to Asp so as to mimic phosphorylation restored DA efflux to that of wild types. The authors proposed that phosphorylation of N-tail serine residues shifts DAT to a conformation that favors AMPH-induced DA efflux without affecting DA uptake. CaMKII has been implicated in AMPH-induced DA efflux (Fog et al., 2006). Recent studies from the Gether laboratory have provided evidence that CaMKIIa binds to the DAT C-tail facilitating phosphorylation of N-terminal Ser residues and triggering AMPH-induced DAT-mediated DA efflux (Fog et al., 2006). Furthermore, studies from Galli and coworkers established the requirement of DAT-Syn1A interactions for CaMKII to facilitate AMPH evoked DAT-mediated DA efflux (Binda et al., 2008). In this study, blocking CaMKII activity decreased the AMPH- induced DAT-Syn1A association and DA efflux. Interestingly, the DAT-Syn1A interaction promotes transporter channel-like activity which has been implicated in AMPH-induced DA efflux (Binda et al., 2008; Carvelli et al., 2008; Dipace et al., 2007; Sung et al., 2003). Thus, DAT-CaMKII interactions modulate DAT phosphorylation and facilitate DA efflux by regulating DAT-Syn1A interactions. However, whether CaMKII triggers N-tail DAT phosphorylation Ser residues in intact cell models is unknown. Insulin has been shown to oppose AMPH induced-decreases in DAT surface expression by inhibiting Akt, a kinase downstream of PI-3 kinase (Carvelli et al., 2002a; Garcia et al., 2005; Lute et al., 2008). AMPH causes a time-dependent decrease in the activity of Akt, and this effect is blocked by the DAT inhibitor cocaine, suggesting that AMPH must interact with DAT to inhibit Akt. The ability of AMPH to decrease Akt activity was blocked by CaMKII inhibition suggesting that DAT activity mediates the amphetamine-induced inhibition of Akt through a CaMKII-dependent mechanism (Wei et al., 2007). AMPH-mediated effects on DAT properties have been discussed in a previous review (Fleckenstein et al., 2007) and the reader is also referred to a very recent review of AMPH-induced reverse transport and trafficking of DAT and NET (Robertson et al., 2009).
Substitution of Thr-62 to Ala or Asp in DAT results in reduced Vmax and lower Km for DA. Interestingly mutation of Thr-62 to Asp causes higher basal DA efflux (Guptaroy et al., 2009). Because Thr-62 is a potential canonical site for PKC, PKG and PKA, Gnegy and coworkers suggest that phosphorylation of DAT Thr-62 may favor DAT to an inward-facing conformation during the transition cycle between inward- and outward-facing conformations (Guptaroy et al., 2009). In the view of high conservation of Thr-62 residue with SERT (Thr-81) and NET (Thr-58), Gnegy and coworkers further suggest that perhaps, the phosphorylation of Thr at this position in SERT and NET influence amine efflux and influx through regulating transporter protein conformational switch (Guptaroy et al., 2009). However, recent studies from Sitte and colleagues reported that while mutation of Thr-81 residue in SERT into Ala diminishes PCA stimulated 5-HT efflux, substituting Thr-81 to Asp also diminishes PCA stimulated 5-HT efflux and failed to rescue from the effect of Thr-81Ala mutation. Furthermore the authors also document that substitution of Thr-58 in NET and Thr-62 in DAT to Ala or Asp resulted in the failure to observe amphetamine-induced amine efflux. The authors have proposed that Thr-81 in SERT and similar positions in NET and DAT may support a network of interactions of N-terminus with internal loops lining the inner vestibule which in turn influences conformational cycle between inward- and outward-facing conformations (Sucic et al.). However, N-tail DAT-Thr-62 or SERT-Thr-81 or NET-Thr-58 phosphorylation in intact cell models and their role in conformational changes or transporter functional regulation remain to be examined. Recently, it was postulated that modification of Thr-276 in SERT via PKG-dependent phosphorylation alters the TM5 conformation that leads to increased catalytic activity (Zhang et al., 2007). It is possible that conformational changes resulting from phosphorylation or dephosphorylation of specific residue(s) or motif may dictate the ability of AMPH to influence transporter trafficking and efflux with or without the influence of transporter interacting proteins.
In LLC-PK1 cells stably expressing DAT, several DAT blockers including cocaine, mazindol and methylphenidate have no effect on basal or PKC-induced DAT phosphorylation. However, another DAT blocker, GBR 12909, suppresses both (Gorentla & Vaughan, 2005). Consistent with its effects on phosphorylation, GBR 12909 prevents PKC-induced DAT down regulation whereas other DAT blockers are without effect. Cocaine mediated upregulation of DA uptake and surface DAT has also been documented (Daws et al., 2002; Little et al., 2002). These observations indicate that substrates and inhibitors have distinct effects on DAT phosphorylation and expression, and indicate distinct molecular mechanisms of action. Although, several cellular candidates that are involved in DAT mediated DA influx and efflux have been identified, the influence of multiple signaling cascades in the regulation of DAT which is occupied with substrates and/or inhibitors remains to be determined.
NET proteins are down regulated following acute and chronic treatment (3 days) with either NE and AMPH or the NET uptake inhibitor, desipramine, perhaps through changes in protein expression and/or NET turnover (Zhu et al., 1998). Amphetamine but not cocaine treatment decreased the number of NET binding sites providing further evidence that NET ligands affect transporter expression (Zhu et al., 2000). In the CAD, catecholaminergic cell line, a CaMKII dependent (KN93-sensitive) NET-SYN1A association has been implicated in mediating acute AMPH-mediated NET downregulation. Deletion of amino acids 28-47 from the N–terminus accelerates AMPH-mediated decrease in surface NET through a CaMKII-dependent increase in NET-SYN1A interaction (Dipace et al., 2007). However, a similar decrease in surface NET was achieved when CAD cells expressing wild type-hNET or hNETΔ28–47 were exposed to AMPH for 60 min. How amino acids located in positions 28–47 regulate these processes is unknown. Intriguingly, in human placental trophoblast, HTR cells, mutation of the PKC-sensitive motif Thr-258/Ser-259 prevented acute AMPH-mediated NET downregulation and endocytosis (Annamalai et al., 2010) but did not perturb cocaine mediated NET upregulation (Jayanthi et al., 2010). The NET-specific blocker DESIPRAMINE prevented AMPH-induced NET downregulation. However, inhibition of PKC, CaMKII, p38 MAPK or depletion of Ca2+ was without effect. Although, the results are consistent in both CAD and HTR cells with regard to acute AMPH exposure decreasing surface NET density, it is reasonable to speculate that the underlying molecular mechanisms linked to AMPH-mediated NET downregulation may be distinctly different in CAD and HTR cells and perhaps specific to the CNS and periphery. Recently, using ex vivo rat cortical preparations and mouse superior cervical ganglion neurons, Matthies et al demonstrated that acute AMPH exposure triggers NET to accumulate in recycling endosomes in a Rab-11 dependent manner (Matthies et al., 2010). Thus studies exploring the effects of substrates and inhibitors on NET phosphorylation and functional expression are just emerging.
Collectively these studies indicate the potential for endogenous amine substrates and psychostimulants (substrates or blockers) in concert with other regulatory cascades to modulate the retention time of MA transporters at the plasma membrane via activity dependent-transporter phosphorylation. It is also possible that state of transporter phosphorylation may modulate how endogenous and psychoactive compounds interact with transporters. Substrate translocation or inhibitor occupancy may influence protein equilibrium to establish a conformational set to favor and/or hinder transporter phosphorylation or transporter association with other regulators. Thus, control of transporter cell surface expression by endogenous amines may provide a unique homeostatic mechanism in the neuron to fine-tune transport capacity to match the changing demands imposed by fluctuation in synaptic amine concentrations that occur in response to multiple signals. It is important to note that while SERT substrates induce neurotransmitter clearance in the synapse by increasing surface transporters, the opposite occurs for DAT. The physiological relevance of this divergent regulation has yet to be determined. Substrate mediated amine transporter regulation not only serves to regulate the level and duration of available synaptic amines to receptors, but also regulates the neuronal accumulation of neurotoxins such as METH, MDMA, MPTP and 6-hydroxydopamine leading to nerve terminal damage and neurodegeneration.
6. Role of protein phosphatases in the regulation of monoamine transporters
The state of a phosphoprotein is the balance between phosphorylation and dephosphorylation rates catalyzed by protein kinases and protein phosphatases. While research from different laboratories has provided much information regarding the role of protein kinases in the regulation of MA transporter functional expression and phosphorylation, very little is known about MA-transporter dephosphorylation and its role in regulating transporter function. The PP1/PP2A inhibitor, okadaic acid (OK) down regulates SERT, DAT and NET activity (Bauman et al., 2000; Foster et al., 2003). Treatment with OK or another potent PP1/PP2A inhibitor, calyculin A, results in rapid increase in SERT phosphorylation in parallel with the decrease in 5-HT uptake (Bauman et al., 2000; Ramamoorthy et al., 1998). Similarly these inhibitors also promote DAT phosphorylation and functional down regulation in striatal synaptosome preparations (Vaughan et al., 1997). Studies have shown that PP2Ac and phosphatase activity present with MA transporter as a complex (Bauman et al., 2000). Interestingly, the associations can be regulated by kinases. In addition, Foster et al established that PP1 dephosphorylates phospho-DAT in striatum and cell preparations (Foster et al., 2003). While it is not known whether MAPK phosphates regulate DAT phosphorylation, Amara’s group demonstrated that MAPK phosphates, MKP3 functions as a positive regulator of DAT by stabilizing DAT at the cell surface. The latter effect is due to interference with dynamin-dependent internalization and a delay in DAT degradation (Mortensen et al., 2008). It has been proposed that the association of PP2Ac or the activity of PP1 may govern the stoichiometry of amine transporter phosphorylation as well as the duration of the phosphorylated form of the transporter. Thus, coordinated phosphorylation and dephosphorylation provides an important trigger for transporter protein expression and trafficking.
7. Role of lipid rafts in the regulation of monoamine transporters
Cholesterol depletion reduces SERT, NET and DAT activity. Recent studies provided evidence for lipid raft localization and raft-mediated internalization of SERT, NET and DAT (Adkins et al., 2007; Foster et al., 2008; Jayanthi et al., 2004; Magnani et al., 2004; Matthies et al., 2009; Samuvel et al., 2005; Scanlon et al., 2001). PKC activation stimulates lipid raft-mediated internalization of native NET expressed in placental trophoblasts (Jayanthi et al., 2004). Interestingly, PKC-, but not p38 MAPK- mediated SERT regulation in rat brain involves raft-mediated distribution (Samuvel et al., 2005). The presence of NET and SERT in lipid rafts suggests that signaling machinery specific to lipid rafts may be linked to PKC-mediated transporter down regulation. Similar to SERT and NET, the presence of DAT in lipid-rafts has been demonstrated in striatal tissue and cell models (Adkins et al., 2007; Foster et al., 2008). Using fluorescence correlation spectroscopy, fluorescence recovery after photobleaching and biochemical approaches Adkins et al demonstrated that depletion of cholesterol or disrupting the cytoskeleton increased lateral mobility of DAT, suggesting that association of the DAT with lipid microdomains in the plasma membrane and/or the cytoskeleton serves to regulate both the lateral mobility of the transporter and its transport capacity (Adkins et al., 2007). It is interesting to note that Foster et al described that while activation of PKC did not alter DAT distribution between lipid rafts and non-rafts, the majority of PKC stimulated phosphorylation occurs at DAT located in lipid rafts (Foster et al., 2008).
Raft- associated sorting has been proposed to underlie several cellular processes including signal transduction, protein sorting and membrane trafficking (Chamberlain & Gould, 2002). GPCRs, tyrosine kinase receptors, several channel proteins, many components of GPCR signal transduction proteins including adenylyl cyclase, Akt, PLC, activated PKC, PP2Ac, non-receptor tyrosine kinases, and other signaling molecules such as syntaxin 1A (a SNARE protein), alpha-synuclein and the PKC binding protein PICK1 are associated with lipid rafts (Chamberlain & Gould, 2002; Simons & Toomre, 2000). The localization of receptors that regulate MA transporter function and potential transporter-interacting proteins as well as transporters in lipid raft microdomains, raises the possibility that lipid rafts may act as morphological “conveyers” of signal transduction by placing various signal transduction molecules near the transporter molecule. For example, transporter-interacting proteins may “guide” targeting of the transporter to lipid rafts, and phosphorylation of a “motif/site” within the transporter may act as a “signal” for fostering protein-protein interactions and redistribution. Thus, protein redistribution from plasma membrane microdomains may be one of the several mechanisms by which synaptic plasticity and neurotransmitter homeostasis are maintained (Parton & Richards, 2003; Simons & Ikonen, 1997). More interestingly, antidepressants and antipsychotic drugs are colocalized in raft-like domains, suggesting that lipid-raft localized amine transporters SERT and NET as well as 5-HT3 receptors may functionally interact with antidepressants and antipsychotic drugs therein, and this interaction may play a role in the pharmacological effect of these drugs (Eisensamer et al., 2005).
8. Dysregulation of Amine Transporters in Human Disease
Several lines of evidence suggest that variants of the MA- transporter gene may play important roles in a number of neuropsychiatric disorders. Systematic screening for DNA sequence variations in the coding regions of SERT, NET and DAT uncovered several amino acid changes in these proteins. We emphasize here those variations exhibiting abnormal regulation. Comprehensive details of these coding variants can be found in the original publications (Carneiro et al., 2009; Hahn et al., 2008; Hahn & Blakely, 2007; Hahn et al., 2005; Hahn et al., 2003; Lin & Uhl, 2003; Mazei-Robinson & Blakely, 2006; Mazei-Robison et al., 2008; Ozaki et al., 2003; Prasad et al., 2009; Prasad et al., 2005; Sutcliffe et al., 2005).
8.1. The effect of SERT coding variants on SERT regulation in disease states
A recent search for sequence variants in the SLC6A4 gene, encoding SERT, uncovered 15 variants in genomic DNA from a population of 450 individuals in the DNA Polymorphism Discovery Resource database (Ozaki et al., 2003). Of these, six were synonymous and the remaining nine resulted in an amino acid change (Thr-4Ala, Gly-56Ala, Leu-255Met, Ser-293Phe, Pro-339Leu, Leu-362Met, Ile-425Val, Lys-605Asn and Pro-621Ser) (summarized in Table 4). Murphy and colleagues reported that two unrelated probands and their family members with Obsessive-Compulsive Disorder (OCD) contained the Ile-425Val coding variant carried on the 5-HTTLPR/1/1 background (Ozaki et al., 2003). In functional studies, this variant has been found to exhibit elevated 5-HT uptake activity without any change in SERT surface expression and is insensitive to stimulation by nitric oxide donors in transfected cells (Kilic et al., 2003). Recently, a thorough study reveals that PKG activator, 8-Br-cGMP stimulates the activity of wild type SERT, but not Ile-425Val, and the activity of wild type SERT stimulated by 8-Br-cGMP was similar to that of the Ile-425Val mutant. In addition, more prolonged treatment with inhibitors of soluble guanylyl cyclase or PKG decreased activity of the Ile-425Val mutant, but not the wild type, indicating that Ile-425Val mutant transporters could exist in high activity forms due to sustained targeting by PKG. In addition, mutation of the PKG phosphorylation site Thr-276 into alanine not only prevented activation of wild type hSERT through the PKG pathway but also blocked the inhibition of Ile-425Val- 5-HT uptake activity by inhibitors of the pathway (Zhang et al., 2007). Furthermore, the elevated 5-HT uptake activity found in Thr-276Asp is similar to the level of 5-HT uptake activity found in Ile-425V. In addition, double mutants containing both Ile-425Val and Asp at 276 did not lose activity when treated with a soluble guanylyl cyclase inhibitor (ODQ) in contrast with Ile-425Val alone, in which activity decreased over the incubation time. ODQ also had no effect on Thr-276Asp (Ramamoorthy et al., 2007; Zhang et al., 2007). Collectively, these findings suggest that mimicking PKG phosphorylation by mutating Thr-276 into Asp-276 results in an elevated 5-HT transport phenotype that parallels that of Ile-425Val and is insensitive to PKG-linked SERT modulation.
Table 4.
Effects of human SERT coding variants on SERT expression and regulation
| hSERT variants | Effect on SERT Activity (versus wild type) | ||||
|---|---|---|---|---|---|
| Basal | PKG- activation | P38 MAPK-activation | PKC-activation | Surface expression | |
| hSERT-WT | 100% | Stimulation | Stimulation | Inhibition | 100% |
| Thr4Ala1 | Higher | Insensitive | Insensitive | More-sensitive | Similar to WT |
| Gly56Ala1,3*# | Higher | Insensitive | Insensitive | More-sensitive | Similar to WT |
| Lys201Asn2 | Higher | NT | NT | NT | Similar to WT |
| Glu215Lys1 | Similar to WT | Insensitive | Insensitive | Similar to WT | Similar to WT |
| Leu255Met1 | Similar to WT | Similar to WT | Similar to WT | Similar to WT | Similar to WT |
| Ser293Phe1 | Higher | Similar to WT | Similar to WT | Similar to WT | Similar to WT |
| Pro339Leu1 | Lower | Similar to WT | Similar to WT | Similar to WT | Lower |
| Leu362Met1 | Higher | Similar to WT | Similar to WT | Similar to WT | Similar to WT |
| Ile425Val11, 4 | Higher | Insensitive | NT | Similar to WT | Similar/higher to WT |
| Ile425Leu3 | Higher | Less-sensitive | Less-sensitive | < = Less-sensitive | Higher |
| Phe465Leu3* | Higher | Less-sensitive | Less-sensitive | < = Less-sensitive | Higher |
| Leu550Val3 | Higher | Less-sensitive | Less-sensitive | < = Less-sensitive | Higher |
| Lys605Asn1 | Similar to WT | Insensitive | Insensitive | More-sensitive | Similar to WT |
| Pro621Ser1 | Similar to WT | Insensitive | Insensitive | More-sensitive | Similar to WT |
NT: Not tested; < = comparable or slightly reduced sensitivity in comparison with WT;
Less sensitive to PP2 inhibition with calyculin and fostriecin in comparison with WT;
exhibits elevated basal phosphorylation.
Other human variants such as Gly-55Ala, Ile-425Leu, Phe-465Leu and Leu-550Val are linked to autism (Prasad et al., 2005; Sutcliffe et al., 2005). Autism linked hSERT variants Ile-425Val, Gly-55Ala, Ileu-425Leu, Phe-465Leu and Leu-550Val exhibit elevated 5-HT transport (Prasad et al., 2009). Remarkably, human SERT variants, Thr-4Ala and Gly-56Ala failed to show 5-HT uptake stimulation when tested with 8-Br-cGMP and the p38 MAPK inhibitor, anisomycin, typical of wild type hSERT. Importantly, in a physiologically relevant human cell model, such as the Epstein–Barr virus (EBV)-transformed lymphocyte which natively expresses the most common of these variants (Gly-56Ala), a loss of 5-HT uptake stimulation by PKG and p38 MAPK activators was seen as was insensitivity to PP2A inhibition and enhanced sensitivity to PKC mediated regulation. Furthermore, HeLa cells transfected with the Gly-56Ala variant exhibit elevated basal phosphorylation and, unlike wild type hSERT, could not be further phosphorylated after 8-Br-cGMP treatments (Prasad et al., 2005). Other coding variants associated with autism such as Ile-425Leu, Phe-465Leu and Leu-550Val exhibit low sensitivity to PP2A inhibition, but unaltered response to PKG or p38 MAPK stimulation. These results suggest that the effect of the Gly-56Ala or Ile-425Val mutation may shift the balance of SERT toward the phosphorylated form, possibly by elevated/sustained phosphorylation of PKG-dependent-Thr-276 residue due, in part, to an altered role of PP2A.
8.2. The effect of NET coding variants on NET regulation in disease states
Nonsynonymous single nucleotide polymorphisms (SNPs) in the human NET (hNET) gene that influence transporter function can contribute to disease, such as the nonfunctional transporter, Ala-457Pro, identified in orthostatic intolerance. The hNET gene polymorphism with an Ala-457Pro mutation in the coding region has been identified in an individual with the autonomic disorder orthostatic intolerance (OI) (Shannon et al., 2000). The presence of the hNET-Ala-457Pro allele tracked with elevated heart rates and plasma NE levels in family members suggesting that genetic or acquired deficits in NE inactivation may underlie hyperadrenergic states that lead to orthostatic intolerance. hNET-Ala-457Pro lacked transport activity and had reduced surface expression compared to wild type hNET. Defects in biosynthetic progression and trafficking have been attributed to reduced functional expression of hNET-Ala-457Pro and dominant-negative effect on wild type hNET (Hahn et al., 2003). Similar to the Ala-457Pro variant, several other hNET variants (e.g., Ala-369Pro and Asn-292Thr) were found to be defective in glycosylation, retained intracellularly, lacked NE transport function and exhibited a dominant negative effect on wild type hNET. The most intriguing finding was that another variant, Phe-528Cys, had higher transport capacity and exhibited insensitivity to down-regulation by PKC. Phe-528Cys variant showed decreased potency for the tricyclic antidepressant desipramine (Hahn et al., 2005).
8.3. The effect of DAT coding variants on DAT regulation in disease states
Multiple, rare, hDAT coding variants have been described and characterized. An analysis of function and regulation of five naturally occurring coding variants, Val-55Ala, Arg-237Gln, Val-382Ala, Ala-559Val and Gln-602Gly, expressed in COS-7 and SH-SY5Y revealed that all variants, except Val-382Ala, exhibit levels of surface protein expression and DA transport activity comparable to wild type hDAT (Mazei-Robison & Blakely, 2005). Val-382Ala, divergent at the most highly conserved residue among reported hDAT variants, exhibited altered substrate selectivity for DA versus NE, diminished surface expression and decreased sensitivity to phorbol ester induced internalization (Mazei-Robinson & Blakely, 2006). Although a single variant within the hDAT gene is unlikely to play a major role in the ADHD, Mazei-Robison et al. recently identified a pedigree containing two male children diagnosed with ADHD who share a rare DAT coding variant, Ala-559Val (Mazei-Robison et al., 2008). This variant has only been isolated once previously, in a female subject with bipolar disorder. Although hDAT Ala-559Val supports normal DAT protein and cell surface expression, as well as normal DA uptake, the variant exhibited anomalous DA efflux from DA-loaded cells. The Ala599Val variant exhibited increased sensitivity to intracellular Na+, but not intracellular DA, and displayed exaggerated DA efflux at depolarized potentials. In contrast to wild type DAT, amphetamine and methylphenidate, two of the most commonly used ADHD medications blocked hDAT Ala-559Val-mediated DA efflux rather than inducing the efflux as occurs with wild type DAT. It has been hypothesized that AMPH-induced changes in intracellular signaling pathways and hDAT phosphorylation (Fog et al., 2006) may be constitutively promoted in hDAT Ala-559Val, and AMPH application may restore an efflux-incompetent conformation. In support of this idea, elevated basal hDAT A559V phosphorylation can be attenuated by AMPH (Mazei-Robison et al., 2008). Furthermore, recent studies demonstrated that hDAT A559V exhibits elevated Ser-7 and Ser-13 phosphorylation and support anomalous DA efflux in transfected HEK-293 cells (Bowton et al., 2010). Interestingly, blockade of D2R-linked CaMKII signaling attenuated anomalous DA efflux found in hDAT A559V in transfected HEK-293 cells and DA neurons. These studies provided further elucidation of the potential role of presynaptic DA autoreceptor signaling in the regulation of DAT mediated DA influx (Bolan et al., 2007; Zapata et al., 2007) and efflux (Bowton et al., 2010) for normal DA homeostasis.
Based on recent findings with naturally occurring rare MA transporter coding variants, it is tempting to speculate that altered MA transporter phosphorylation could be one of the molecular mechanisms contributing to functional deficits in transporter biosynthesis, activity, regulation and sensitivity that have been identified in human disease.
9. Conclusion
In general, accumulated evidence suggests that while activation of PKC down regulates MA transporter function, activation of MAPKs and tyrosine kinases upregulate MA transporters. As shown in Figure 1, regulation can occur at several subcellular locations of MA transporters to ensure response to external signals for proper MA transporter functional expression to regulate aminergic neurotransmission and behavior. It is noteworthy that the intrinsic transport capacity of a transporter molecule governs its own plasma membrane expression and function. There appears to be multiple mechanisms by which transporter substrates and antagonists can influence transporter function and expression. The capacity of a transporter to fine-tune its function in response to extracellular neurotransmitter would maintain a constant level of neurotransmitter at the synaptic cleft. On the other hand interactions with psychostimulants would interfere and compromise the normal activity-dependent regulation of MA transporters and may cause or initiate neuroplastic changes associated with drug addiction. SSRIs and other agents may have therapeutic utility in disease states, not only by preventing amine uptake but also by shifting the cellular distribution of MA transporters.
Figure 1. Hypothetical representation of functional regulation of MA transporters.
Based on published reports, MA-transporters are subject to regulation at the levels of transcription, translation and functional delivery. Presynaptic homo- and hetero- receptors can trigger specific signaling cascades and hence MA transporter regulation to maintain normal amine neurotransmission. At the gene level, transporter gene transcription can be regulated by many cellular signals and transcription factors. At the protein level, MA transporters may be organized as a complex in the plasma membrane motifs (lipid rafts versus non-lipid rafts) that can be regulated by several cellular mechanisms, such as phosphorylation, protein-protein interactions, and ubiquitination. Transporter activity can be regulated through trafficking dependent and/or independent mechanisms. Kinase/phosphatase mediated post translational modifications such as phosphorylation regulate transporter catalytic functions (amine influx/efflux), surface residency or both. At the plasma membrane level, lipid raft microdomains, in which MA transporters along with other signalosomes are located, may serve as morphological “conveyers” of signal transduction in the milieu of various incoming signals. Phosphorylated transporters may be subjected to trafficking dependent or independent regulatory mechanisms. Transporters may enter into a regulated endocytic pathway and, thus, internalized transporters may be subjected to degradation or recycling depending on the signals and cell system. Internalized transporters could be dephosphorylated by associated protein phosphatases allowing the return of nonphosphorylated transporters to the cell surface. MA transporters such as endogenous amines and psychostimulants (substrates or blockers) in concert with other regulatory cascades can modulate the retention time of MA transporters at the plasma membrane via activity dependent-transporter phosphorylation. Thus, disease linked human coding variants can influence activity dependent regulation, thereby, producing an abnormal phenotype. (T: Transporters; TAP: Transporter Associated Proteins; PP2A: Protein Phosphatase 2A; P; phosphorylation; ER: Endoplasmic Reticulum).
The past few years have been an active period of research with regard to coding variants of MA transporters and their impact on normal transporter regulation. These investigations strongly support the idea that naturally occurring human disease linked coding variants exhibit altered amine- transport phenotypes as a consequence of altered transporter phosphorylation. Much progress has been made in identifying the contribution of kinase cascades, transporter interacting proteins and phosphorylation to MA transporter regulation in a variety of systems using receptor/kinase-activators or inhibitors. Fundamental questions, however, remain as to whether such regulation is important for MA signaling and MA-dependent behaviors. Translating information obtained from in vitro and ex vivo studies to in vivo settingsis a challenging question and must be addressed in the next decade. Developing genetically modified mice bearing deficits in specific regulatory elements and/or introducing naturally identified mutants should facilitate systems level analysis of transporter response in the face of neuronal excitability and receptor-second-messenger activation and aid our understanding of the contribution of that particular regulatory cascade in amine signaling, behavior and psychiatric illnesses.
In summary, investigations from several laboratories provide clear evidence that MA transporters are principle players in regulating normal and abnormal amine signaling in the CNS and periphery and, hence, are key players in the control of complex behavioral and physiological functions. Future studies are needed to delineate cis/trans acting elements and signals for acute and chronic amine transporter regulation and their physiological relevance. Many of the tools needed to address these issues are already in place and should allow for rapid progress in understanding the role of cellular regulation of amine transporters in amine signaling and behavior. The results from this work will aid in the development of novel targets for manipulating MA transporter regulation and more effective therapies for the treatment of depression, addiction and other psychiatric disorders.
Acknowledgments
The support from the National Institutes of Health (MH062612, MH083928, MH091633 to SR) (GM081054 to LDJ) and (NIDA IRP to TSS) is greatly acknowledged.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 8-Br-cGMP
8-bromoguanosine 3′:5′-cyclic monophosphate
- Akt
protein kinase B
- AMPH
D-amphetamine
- Asp
aspartic acid
- CaMK
calcium/calmodulin dependent protein kinase
- CPu
caudate-putamen
- DA
dopamine
- DAT
dopamine transporter
- ERK1/2
extracellular signal-regulated kinase
- HEK-293 cells
human embryonic kidney-293 cells
- MA
monoamines
- MDMA
3,4-methylenedioxymethamphetamine
- METH
methamphetamine
- NAcc
nucleus accumbens
- NE
norepinephrine
- NET
norepinephrine transporter
- p38 MAPK
p38 mitogen-activated protein kinase
- PI-3 kinase
phosphoinositide 3 kinase
- PKC
protein kinase C
- PKG
protein kinase G
- PP2Ac
protein phosphatase 2A catalytic subunit
- Ser
serine
- SERT
serotonin transporter
- β-PMA
phorbol 12-myristate 13 acetate
- SSRI
selective serotonin reuptake inhibitor
- Thr
threonines
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, et al. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- Adriani W, Boyer F, Gioiosa L, Macri S, Dreyer JL, Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- Ahern TH, Javors MA, Eagles DA, Martillotti J, Mitchell HA, Liles LC, et al. The effects of chronic norepinephrine transporter inactivation on seizure susceptibility in mice. Neuropsychopharmacol. 2006;31:730–738. doi: 10.1038/sj.npp.1300847. [DOI] [PubMed] [Google Scholar]
- Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, et al. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117:133–142. doi: 10.1016/s0165-5728(01)00317-4. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Horne WC. Activators of protein kinase C decrease serotonin transport in human platelets. Biochim Biophys Acta. 1992;1137:331–337. doi: 10.1016/0167-4889(92)90154-4. [DOI] [PubMed] [Google Scholar]
- Annamalai B, Mannangatti P, Arapulisamy O, Ramamoorthy S, Jayanthi LD. Involvement of Threonine 258 and Serine 259 Motif in Amphetamine-Induced Norepinephrine Transporter Endocytosis. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06898.x. “Accepted Article”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansah TA, Ramamoorthy S, Montanez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. PKC-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998a;287:733–743. [PubMed] [Google Scholar]
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998b;287:733–743. [PubMed] [Google Scholar]
- Apparsundaram S, Schroeter S, Blakely RD. Acute regulation of norepinephrine transport. II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Bannon MJ, Granneman JG, GK . The Dopamine Transporter. Potential involvement in neuropsychiatric disorders. In: Bloom FE, KD, editors. Psychopharmacology: the Fourth Generation of Progress. New York: Raven Press; 1995. pp. 179–187. [Google Scholar]
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters: Molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 321–333. [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymetamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, et al. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, et al. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda F, Lute BJ, Dipace C, Blakely RD, Galli A. The N-terminus of the norepinephrine transporter regulates the magnitude and selectivity of the transporter-associated leak current. Neuropharmacol. 2006;50:354–361. doi: 10.1016/j.neuropharm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, et al. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blier P. Norepinephrine and selective norepinephrine reuptake inhibitors in depression and mood disorders: their pivotal roles. J Psychiatry Neurosci. 2001;26(Suppl):S1–2. [PMC free article] [PubMed] [Google Scholar]
- Bohm M, La Rosse K, Schwinger RHG, Erdmann E. Evidence for reduction norepinephrine uptake sites in the failing human heart. J Amer Col Cardiol. 1995;25:146–153. doi: 10.1016/0735-1097(94)00353-r. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Bonisch H, Bruss M. The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol. 2006:485–524. doi: 10.1007/3-540-29784-7_20. [DOI] [PubMed] [Google Scholar]
- Bönisch H, Hammermann R, Brüss M. Role of protein kinase C and second messengers in regulation of the norepinephrine transporter. Adv Pharmacol. 1998;42:183–187. doi: 10.1016/s1054-3589(08)60723-1. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008;39:211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30:6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd FT, Clarke DW, Muther TF, Raizada MK. Insulin receptors and insulin modulation of norepinephrine uptake in neuronal cultures from rat brain. J Biol Chem. 1985;260:15880–15884. [PubMed] [Google Scholar]
- Boyd FT, Clarke DW, Raizada MK. Insulin inhibits specific norepinephrine uptake in neuronal cultures from rat brain. Brain Research. 1986;398:1–5. doi: 10.1016/0006-8993(86)91242-4. [DOI] [PubMed] [Google Scholar]
- Brüss M, Kunz J, Lingen B, Bönisch H. Chromosomal mapping of the human gene for the tricyclic antidepressant-sensitive noradrenaline transporter. Hum Genet. 1993;91:278–280. doi: 10.1007/BF00218272. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, et al. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci U S A. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Blakely RD. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J Biol Chem. 2006;281:24769–24780. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MG, Gainetdinov RR. Role of dopamine transporters in neuronal homeostasis. In: Iversen LL, Iversen SD, Dunnett SB, BjÖrklund A, editors. Dopamine Handbook. New York: Oxford; 2010. pp. 88–99. [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A. 2008;105:14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, et al. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002a;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, et al. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002b;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Gould GW. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem. 2002;277:49750–49754. doi: 10.1074/jbc.M206936200. [DOI] [PubMed] [Google Scholar]
- Chang AS, Starnes DM, Chang SM. Possible existence of quaternary structure in the high-affinity serotonin transport complex. Biochem Biophys Res Commun. 1998;249:416–421. doi: 10.1006/bbrc.1998.9158. [DOI] [PubMed] [Google Scholar]
- Chang MY, Lee SH, Kim JH, Lee KH, Kim YS, Son H, et al. Protein kinase C-mediated functional regulation of dopamine transporter is not achieved by direct phosphorylation of the dopamine transporter protein. J Neurochem. 2001;77:754–761. doi: 10.1046/j.1471-4159.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacol. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Chen JG, Liu-Chen S, Rudnick G. Determination of external loop topology in the serotonin transporter by site-directed chemical labeling. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- Chen N, Appell M, Berfield JL, Reith ME. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur J Pharmacol. 2003;478:89–95. doi: 10.1016/j.ejphar.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RWt, Leitges M, et al. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Cirillo P, Golino P, Ragni M, Battaglia C, Pacifico F, Formisano S, et al. Activated platelets and leucocytes cooperatively stimulate smooth muscle cell proliferation and proto-oncogene expression via release of soluble growth factors. Cardiovasc Res. 1999;43:210–218. doi: 10.1016/s0008-6363(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Central serotonin and impulsive aggression. Br J Psychiatry. 1989;155:52–62. [PubMed] [Google Scholar]
- Compagnon P, Ernouf D, Narcisse G, Daoust M. Serotonin in animal models of alcoholism. Alcohol Suppl. 1993;2:215–219. [PubMed] [Google Scholar]
- Copeland BJ, Vogelsberg V, Neff NH, Hadjiconstantinou M. Protein kinase C activators decrease dopamine uptake into striatal synaptosomes. J Pharmacol Exp Ther. 1996;277:1527–1532. [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin- mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gerhardt GA, Frazer A. 5-HT1B antagonists modulate clearance of extracellular serotonin in rat hippocampus. Neurosci Lett. 1999;266:165–168. doi: 10.1016/s0304-3940(99)00277-3. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, et al. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Dipace C, Sung U, Binda F, Blakely RD, Galli A. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol Pharmacol. 2007;71:230–239. doi: 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- Doolen S, Zahniser NR. Protein tyrosine kinase inhibitors alter human dopamine transporter activity in Xenopus oocytes. J Pharmacol Exp Ther. 2001;296:931–938. [PubMed] [Google Scholar]
- Doolen S, Zahniser NR. Conventional protein kinase C isoforms regulate human dopamine transporter activity in Xenopus oocytes. FEBS Lett. 2002;516:187–190. doi: 10.1016/s0014-5793(02)02554-1. [DOI] [PubMed] [Google Scholar]
- Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, et al. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J Neurosci. 2005;25:10198–10206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Rasmussen SG, Rasmussen TN, Vaegter CB, Cha JH, Zou MF, et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci. 2009;29:6794–6808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Alvarenga M, Pier C, Richards J, El-Osta A, Barton D, et al. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol. 2006;20:60–66. doi: 10.1177/1359786806066055. [DOI] [PubMed] [Google Scholar]
- Esler M, Jackman G, Bobik A, Leonard P, Kelleher D, Skews H, et al. Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension. 1981;3:149–156. doi: 10.1161/01.hyp.3.2.149. [DOI] [PubMed] [Google Scholar]
- Faraj BA, Olkowski ZL, Jackson RT. Expression of a high-affinity serotonin transporter in human lymphocytes. Int J Immunopharmacol. 1994;16:561–567. doi: 10.1016/0192-0561(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bentson K, Ocrant I. The effect of insulin on norepinephrine uptake by PC12 cells. Brain Research Bulletin. 1993;32:425–431. doi: 10.1016/0361-9230(93)90210-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Research. 1993;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Fontana AC, Sonders MS, Pereira-Junior OS, Knight M, Javitch JA, Rodrigues V, et al. Two allelic isoforms of the serotonin transporter from Schistosoma mansoni display electrogenic transport and high selectivity for serotonin. Eur J Pharmacol. 2009;616:48–57. doi: 10.1016/j.ejphar.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiological Reviews. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Cervinski MA, Holden HE, Vaughan RA. Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Res Mol Brain Res. 2003;110:100–108. doi: 10.1016/s0169-328x(02)00645-9. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: Evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kruger S, Pakstis AJ, Pacholczyk T, Sparkes RS, Kidd KK, et al. Assignment of the norepinephrine transporter protein (NET1) locus to chromosome 16. Genomics. 1993;18:690–692. doi: 10.1016/s0888-7543(05)80375-1. [DOI] [PubMed] [Google Scholar]
- Ghisi V, Ramsey AJ, Masri B, Gainetdinov RR, Caron MG, Salahpour A. Reduced D2-mediated signaling activity and trans-synaptic upregulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal. 2009;21:87–94. doi: 10.1016/j.cellsig.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, El Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, et al. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M. Serotonin receptor subtypes. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd; 1995. pp. 415–429. [Google Scholar]
- Gobbi M, Mennini T, Garratini S. Mechanism of neurotransmitter release induced by amphetamine derivatives: pharmacological and toxicological aspects. Current Topics in Pharmacol. 1997;3:217–227. [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, Barnes NM. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. 2003;24:438–443. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacol. 2005;49:759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur J Pharmacol. 2003;479:139–152. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, et al. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol. 2009;75:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Linsel K, Bruss M, Gilsbach R, Propping P, Nothen MM, et al. Association of major depression with rare functional variants in norepinephrine transporter and serotonin(1A) receptor genes. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30912. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blackford JU, Haman K, Mazei-Robison M, English BA, Prasad HC, et al. Multivariate permutation analysis associates multiple polymorphisms with subphenotypes of major depression. Genes Brain Behav. 2008;7:487–495. doi: 10.1111/j.1601-183X.2007.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Mazei-Robison MS, Blakely RD. Single nucleotide polymorphisms in the human norepinephrine transporter gene affect expression, trafficking, antidepressant interaction, and protein kinase C regulation. Mol Pharmacol. 2005;68:457–466. doi: 10.1124/mol.105.011270. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Robertson D, Blakely RD. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild-type transporters. J Neurosci. 2003;23:4470–4478. doi: 10.1523/JNEUROSCI.23-11-04470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, et al. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. Behavioral responses to social stress in noradrenaline transporter knockout mice: effects on social behavior and depression. Brain Res Bull. 2002;58:279–284. doi: 10.1016/s0361-9230(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Helmeste DM, Tang SW. Tyrosine kinase inhibitors regulate serotonin uptake in platelets. Eur J Pharmacol. 1995;280:R5–7. doi: 10.1016/0014-2999(95)00323-d. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, et al. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacol. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem. 1997;68:225–232. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- Imamura M, Lander HM, Levi R. Activation of histamine H3-receptors inhibits carrier-mediated norepinephrine release during protracted myocardial ischemia. Comparison with adenosine A1-receptors and alpha2-adrenoceptors. Circ Res. 1996;78:475–481. doi: 10.1161/01.res.78.3.475. [DOI] [PubMed] [Google Scholar]
- Iversen LL. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL. Uptake processes for biogenic amines. In: Iversen I, editor. Handbook of Psychopharmacology. 3. New York: Prenum Press; 1978. pp. 381–442. [Google Scholar]
- Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Mannangatti P, Arapulisamy O, Ramamoorthy S. Cocaine upregulation of the norepinephrine transporter requires threonine 30 phosphorylation by p38 mitogen-activated protein kinase. Paper presented at the Society for Neuroscience; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi LD, Ramamoorthy S. Regulation of monoamine transporters: influence of psychostimulants and therapeutic antidepressants. Aaps J. 2005;7:E728–738. doi: 10.1208/aapsj070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi LD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Calmodulin-dependent regulation of the catalytic function of the human serotonin transporter in placental choriocarcinoma cells. J Biol Chem. 1994;269:14424–14429. [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Vargas G, DeFelice LJ. Characterization of cocaine and antidepressant-sensitive norepinephrine transporters in rat placental trophoblasts. Br J Pharmacol. 2002;135:1927–1934. doi: 10.1038/sj.bjp.0704658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jess U, Betz H, Schloss P. The membrane-bound rat serotonin transporter, SERT1, is an oligomeric protein. Fed Experiment Biol Sci. 1996;394:44–46. doi: 10.1016/0014-5793(96)00916-7. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacol. 2005;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, et al. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95(7):4029. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JB, Jr, Danek KS, Kable JW, Barker EL, Blakely RD. Voltammetric approaches to kinetics and mechanism of the norepinephrine transporter. Adv Pharmacol. 1998;42:191–194. doi: 10.1016/s1054-3589(08)60725-5. [DOI] [PubMed] [Google Scholar]
- Keller NR, Diedrich A, Appalsamy M, Miller LC, Caron MG, McDonald MP, et al. Norepinephrine transporter-deficient mice respond to anxiety producing and fearful environments with bradycardia and hypotension. Neuroscience. 2006;139:931–946. doi: 10.1016/j.neuroscience.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Keller NR, Diedrich A, Appalsamy M, Tuntrakool S, Lonce S, Finney C, et al. Norepinephrine transporter-deficient mice exhibit excessive tachycardia and elevated blood pressure with wakefulness and activity. Circulation. 2004;110:1191–1196. doi: 10.1161/01.CIR.0000141804.90845.E6. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS biology. 2004;2:16. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kilic F, Rudnick G. Oligomerization of serotonin transporter and its functional consequences. Proc Natl Acad Sci U S A. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilty JE, Lorang D, Amara SG. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254:578–580. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kim CH, Hahn MK, Joung Y, Anderson SL, Steele AH, Mazei-Robinson MS, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci U S A. 2006;103:19164–19169. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Morita K, Dohi T. Functional characterization of the splicing variants of human norepinephrine transporter. Neurosci Lett. 2001;312:108–112. doi: 10.1016/s0304-3940(01)02138-3. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drug abuse and alcoholism. Overview. Adv Pharmacol. 1998;42:969–977. doi: 10.1016/s1054-3589(08)60909-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Launay J, Bondoux D, Oset-Gasque M, Emami S, Mutel V, Haimart M, et al. Increase of human platelet serotonin uptake by atypical histamine receptors. Am J Physiol. 1994;266:526–536. doi: 10.1152/ajpregu.1994.266.2.R526. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. Embo J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. The role of noradrenaline in depression: a review. J Psychopharmacol. 1997;11:S39–47. [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Estler HC, Murphy DL, Riederer P. Isolation of a cDNA encoding the human brain serotonin transporter. J Neural Transm. 1993;91:67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Murphy DL, Riederer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Ma L, Innis RB, Seneca N, Ichise M, Huang H, et al. Pharmacological and genetic characterization of two selective serotonin transporter ligands: 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine (AFM) and 3-amino-4-[2-(dimethylaminomethyl-phenylthio)]benzonitrile (DASB) J Pharmacol Exp Ther. 2004;308:481–486. doi: 10.1124/jpet.103.058636. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Liang C, Fan THM, Sullebarger JT, Sakamoto S. Decreased adrenergic neuronal uptake activity in experimental right heart failure. J Clin Invest. 1989;84:1267–1275. doi: 10.1172/JCI114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Uhl GR. Human dopamine transporter gene variation: effects of protein coding variants V55A and V382A on expression and uptake activities. Pharmacogenomics J. 2003;3:159–168. doi: 10.1038/sj.tpj.6500169. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, et al. PI3, PKC and MEK1/2 kinase regulation of dopamine transporters (DAT) requires N-terminal DAT phosphoacceptor sites. J Biol Chem. 2003;26:26. doi: 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- Logan J, Wang GJ, Telang F, Fowler JS, Alexoff D, Zabroski J, et al. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nucl Med Biol. 2007;34:667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Lu D, Yu K, Paddy MR, Rowland NE, Raizada MK. Regulation of norepinephrine transport system by angiotensin II in neuronal cultures of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996;137:763–772. doi: 10.1210/endo.137.2.8593828. [DOI] [PubMed] [Google Scholar]
- Lute BJ, Khoshbouei H, Saunders C, Sen N, Lin RZ, Javitch JA, et al. PI3K signaling supports amphetamine-induced dopamine efflux. Biochem Biophys Res Commun. 2008;372:656–661. doi: 10.1016/j.bbrc.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochem. 2006;97:310–333. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Matheus N, Mendoza C, Iceta R, Mesonero JE, Alcalde AI. Regulation of serotonin transporter activity by adenosine in intestinal epithelial cells. Biochem Pharmacol. 2009;78:1198–1204. doi: 10.1016/j.bcp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Matthies HJ, Han Q, Shields A, Wright J, Moore JL, Winder DG, et al. Subcellular localization of the antidepressant-sensitive norepinephrine transporter. BMC Neurosci. 2009;10:65. doi: 10.1186/1471-2202-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies HJ, Moore JL, Saunders C, Matthies DS, Lapierre LA, Goldenring JR, et al. Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking. J Neurosci. 2010;30:7863–7877. doi: 10.1523/JNEUROSCI.4574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol. 2001a;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol. 2001b;59:113–121. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- Mazei-Robinson MS, Blakely RD. ADHD and the dopamine transporter: are there reasons to pay attention? Handb Exp Pharmacol. 2006:373–415. doi: 10.1007/3-540-29784-7_17. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Blakely RD. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacol. 2005;49:737–749. doi: 10.1016/j.neuropharm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem. 1993;61:764–767. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Arora RC, Baber R, Tricou BJ. Serotonin uptake in blood platelets of psychiatric patients. Arch Gen Psychiatry. 1981;38:1322–1326. doi: 10.1001/archpsyc.1981.01780370024002. [DOI] [PubMed] [Google Scholar]
- Merlet P, Dubois-Rande JL, Adnot S, Bourguignon MH, Benvenuti C, Loisance D, et al. Myocardial -adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J Cardiovascular Pharmacol. 1992;19:10–16. doi: 10.1097/00005344-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hoffman BJ. Adenosine A3 receptors regulate serotonin transoprt via nitric oxide and cGMP. J Biol Chem. 1994;269(44):27351–27356. [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatry. 2006;60:1046–1052. doi: 10.1016/j.biopsych.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Larsen MB, Prasad BM, Amara SG. Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Mol Biol Cell. 2008;19:2818–2829. doi: 10.1091/mbc.E07-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Marzec U, Davidoff M, Manatunga AK, Gao F, Reemsnyder A, et al. Platelet activation and secretion in patients with major depression, thoracic aortic atherosclerosis, or renal dialysis treatment. Depress Anxiety. 2002;15:91–101. doi: 10.1002/da.10020. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clinical Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Oz M, Jaligam V, Galadari S, Petroianu G, Shuba YM, Shippenberg TS. The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor-independent mechanism. J Neurochem. 2009;114:1019–1929. doi: 10.1111/j.1471-4159.2009.06557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Libby T, Kivell B, Jaligam V, Ramamoorthy S, Shippenberg TS. Real-time, spatially resolved analysis of serotonin transporter activity and regulation using the fluorescent substrate, ASP+ J Neurochem. 2010;114:1019–1029. doi: 10.1111/j.1471-4159.2010.06828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry 8. 2003;895:933–896. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Paczkowski NJ, Vuocolo HE, Bryan-Lluka LJ. Conclusive evidence for distinct transporters for 5-hydroxytryptamine and noradrenaline in pulmonary endothelial cells of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:423–430. doi: 10.1007/BF00261439. [DOI] [PubMed] [Google Scholar]
- Page G, Peeters M, Najimi M, Maloteaux JM, Hermans E. Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem. 2001;76:1282–1290. doi: 10.1046/j.1471-4159.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC, et al. CB1-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem. 2007;101:389–396. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Price DA, Sorkin A, Zahniser NR. Cyclin-dependent kinase 5 inhibitors: inhibition of dopamine transporter activity. Mol Pharmacol. 2009;76:812–823. doi: 10.1124/mol.109.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, et al. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S. Regulation of monoamine transporters: Regulated phosphorylation, dephhosphorylation, and trafficking. 2. Book Chapter 1. Totowa, NJ: Humana Press Inc; 2002. [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Leibach FH, Mahesh VB, Ganapathy V. Partial purification and characterization of the human placental serotonin transporter. Placenta. 1993;14:449–461. doi: 10.1016/s0143-4004(05)80465-5. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- Rasmussen TN, Plenge P, Bay T, Egebjerg J, Gether U. A single nucleotide polymorphism in the human serotonin transporter introduces a new site for N-linked glycosylation. Neuropharmacol. 2009;57:287–294. doi: 10.1016/j.neuropharm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Xu C, Chen NH. Pharmacology and regulation of the neuronal dopamine transporter. Eur J Pharmacol. 1997;324:1–10. doi: 10.1016/s0014-2999(97)00065-4. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, et al. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nature Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Active transport of 5-hydroxytryptamine by plasma membrane vesicles isolated from human blood platelets. J Biol Chem. 1977;252:2170–2174. [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxymethamphetamine (MDMA)]: Serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumantir MS, Kaye DM, Jennings GL, Vaz M, Hastings JA, Esler MD. Phenotypic evidence of faulty neuronal norepinephrine reuptake in essential hypertension. Hypertension. 2000;36:824–829. doi: 10.1161/01.hyp.36.5.824. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, et al. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko V, Sung U, Blakely RD. Cell surface trafficking of the antidepressant-sensitive norepinephrine transporter revealed with an ectodomain antibody. Mol Cell Neurosci. 2003;24:1131–1150. doi: 10.1016/s1044-7431(03)00235-5. [DOI] [PubMed] [Google Scholar]
- Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- Schafers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O, et al. Myocardial presynaptic and postsynaptic autonomic dysfunction in hypertrophic cardiomyopathy. Circ Res. 1998;82:57–62. doi: 10.1161/01.res.82.1.57. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Just H, Sitte HH. Impact of oligomerization on the function of the human serotonin transporter. Biochem Soc Trans. 2001;29:732–736. doi: 10.1042/0300-5127:0290732. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J Biol Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Schroeter S, Levey AI, Blakely RD. Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol Cell Neurosci. 1997;9:170–184. doi: 10.1006/mcne.1997.0619. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Higgins GA, Tompkins DM, Romach MK. Serotonin and alcohol drinking. NIDA Res Monogr. 1992;119:141–145. [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin C, Patel A, Nanthakumar E, Gregor P, et al. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–577. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters--why amphetamines take two to tango. J Neurochem. 112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS biology. 2010;8:e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Wright J, Matthies HJ, Prasad HC, Nicki CK, et al. cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain. 2009;2:26. doi: 10.1186/1756-6606-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucic S, Dallinger S, Zdrazil B, Weissensteiner R, Jorgensen TN, Holy M, et al. The N terminus of monoamine transporters is a lever required for the action of amphetamines. J Biol Chem. 285:10924–10938. doi: 10.1074/jbc.M109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumners C, Raizada MK. Angiotensin II stimulates norepinephrine uptake in hypothalamus-brain stem neuronal cultures. Am J Physiol. 1986;250:C236–C244. doi: 10.1152/ajpcell.1986.250.2.C236. [DOI] [PubMed] [Google Scholar]
- Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, et al. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, et al. Allelic Heterogeneity at the Serotonin Transporter Locus (SLC6A4) Confers Susceptibility to Autism and Rigid-Compulsive Behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U. The TiPs lecture: functional aspects of the neuronal uptake of noradrenaline. Trends Pharmacol Sci. 1991;32:334–337. doi: 10.1016/0165-6147(91)90592-g. [DOI] [PubMed] [Google Scholar]
- Uchida J, Kiuchi Y, Ohno M, Yura A, Oguchi K. Possible involvement of calmodulin-dependent kinases in Ca2+-dependent enhancement of [3H]noradrenaline uptake in PC12 cells. J Neurosci. 1997:1–27. doi: 10.1016/0006-8993(96)00762-7. [DOI] [PubMed] [Google Scholar]
- Uchida J, Kiuchi Y, Ohno M, Yura A, Oguchi K. Ca(2+)-dependent enhancement of [3H]noradrenaline uptake in PC12 cells through calmodulin-dependent kinases. Brain Res. 1998;809:155–164. doi: 10.1016/s0006-8993(98)00850-6. [DOI] [PubMed] [Google Scholar]
- Vaughan RA. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J Pharmacol Exp Ther. 2004;310:1–7. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Vrindavanam NS, Arnaud P, Ma JX, Altman-Hamamdzic S, Parratto NP, Sallee FR. The effects of phosphorylation on the functional regulation of an expressed recombinant human dopamine transporter. Neurosci Lett. 1996;216:133–136. doi: 10.1016/0304-3940(96)13034-2. [DOI] [PubMed] [Google Scholar]
- Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A, et al. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol Pharmacol. 2007;71:835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, Kleiner-Fisman G, et al. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med. 2005;46:227–232. [PubMed] [Google Scholar]
- Whitworth TL, Herndon LC, Quick MW. Psychostimulants differentially regulate serotonin transporter expression in thalamocortical neurons. J Neurosci. 2002;22:RC192. doi: 10.1523/JNEUROSCI.22-01-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS biology. 2007a;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS biology. 2007b;5:2369–2378. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. Platelet research in psychiatry. Experientia. 1988;44:145–152. doi: 10.1007/BF01952199. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu D, Vinson GP, Raizada MK. Involvement of MAP kinase in angiotensin II-induced phosphorylation and intracellular targeting of neuronal AT1 receptors. J Neurosci. 1997;17:1660–1669. doi: 10.1523/JNEUROSCI.17-05-01660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. J Neurosci. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl- -dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacol. 2004;47(Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- Zarpellon A, Donella-Deana A, Folda A, Turetta L, Pavanetto M, Deana R. Serotonin (5-HT) transport in human platelets is modulated by Src-catalysed Tyr-phosphorylation of the plasma membrane transporter SERT. Cell Physiol Biochem. 2008;21:87–94. doi: 10.1159/000113750. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Gesmonde J, Ramamoorthy S, Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci. 2007;27:10878–10886. doi: 10.1523/JNEUROSCI.0034-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacol. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Hewlett WA, Francis SH, Corbin JD, Blakely RD. Stimulation of serotonin transport by the cyclic GMP phosphodiesterase-5 inhibitor sildenafil. Eur J Pharmacol. 2004;504:1–6. doi: 10.1016/j.ejphar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J Pharmacol Exp Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Blakely RD, Apparsundaram S, Ordway GA. Downregulation of the human norepinephrine transporter in intact 293-hNET cells exposed to desipramine. J Neurochem. 1998;70:1547–1555. doi: 10.1046/j.1471-4159.1998.70041547.x. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Shamburger S, Li J, Ordway GA. Regulation of the human norepinephrine transporter by cocaine and amphetamine. J Pharmacol Exp Ther. 2000;295:951–959. [PubMed] [Google Scholar]
- Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]