Abstract
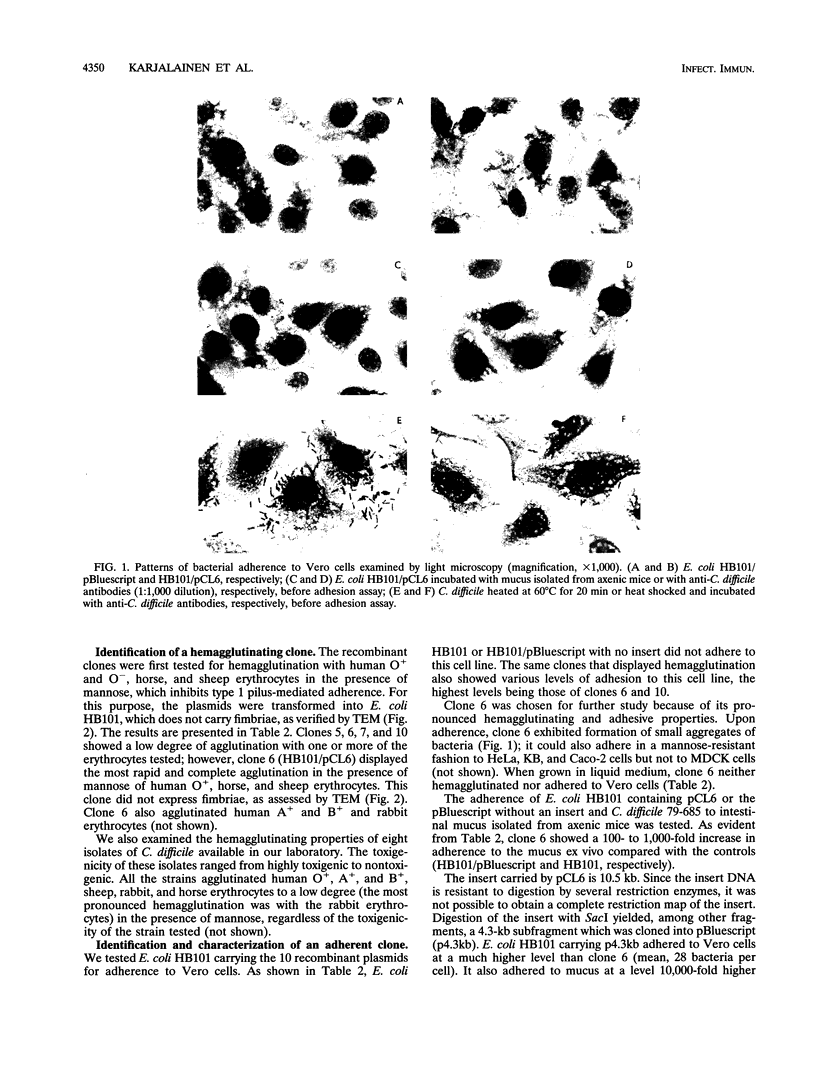
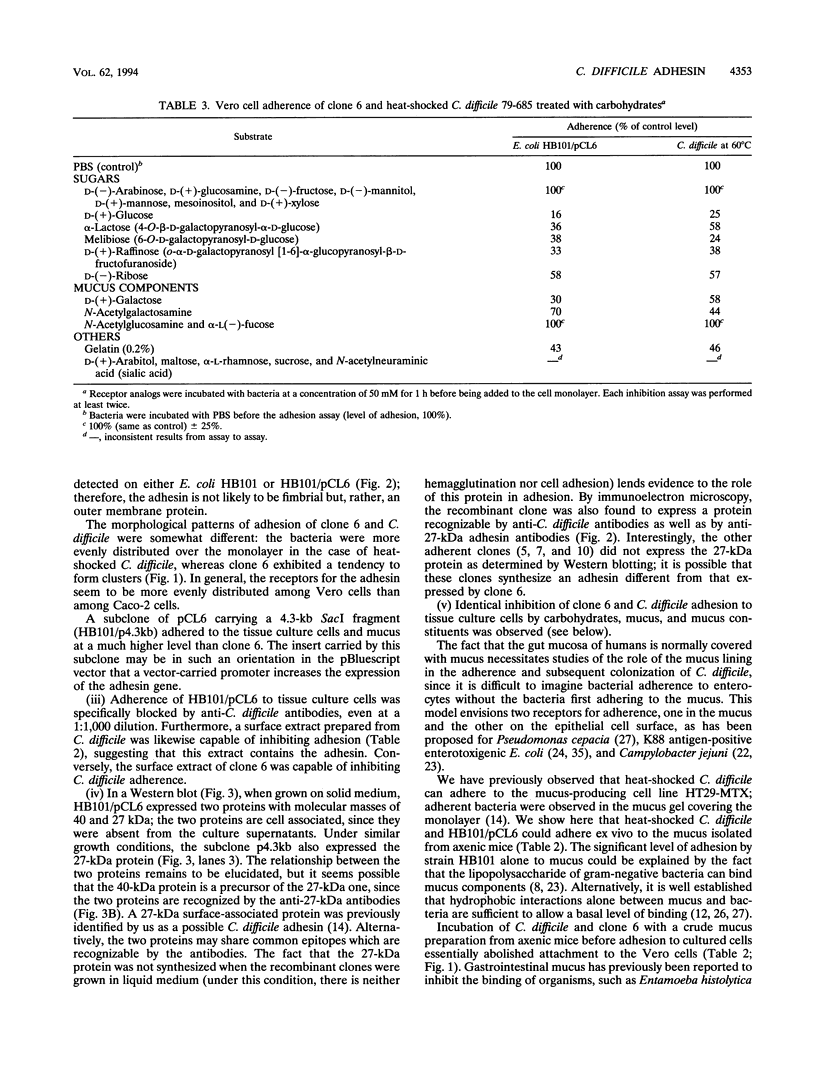
Our laboratory has previously shown that Clostridium difficile adherence to Caco-2 cells is greatly enhanced after heat shock at 60 degrees C and that it is mediated by a proteinaceous surface component. The experiments described here show that C. difficile could adhere to several types of tissue culture cells (Vero, HeLa, and KB) after heat shock. The type of culture medium (liquid or solid, with or without blood) had little effect on adhesion. To clone the adhesin gene, polyclonal antibodies against C. difficile heated at 60 degrees C were used to screen a genomic library of C. difficile constructed in lambda ZapII. Ten positive clones were identified in the library, one of which (pCL6) agglutinated several types of erythrocytes in the presence of mannose. In Western blots (immunoblots), this clone expressed in Escherichia coli a 40- and a 27-kDa protein; a 27-kDa protein has been previously identified in the surface extracts of heat-shocked C. difficile as a possible adhesin. The clone adhered to Vero, Caco-2, KB, and HeLa cells; the adherence was blocked by anti-C. difficile antibodies, by a surface extract of C. difficile, and by mucus isolated from axenic mice. Furthermore, the clone could attach ex vivo to intestinal mucus isolated from axenic mice. Preliminary studies on the receptor moieties implicated in C. difficile adhesion revealed that glucose and galactose could partially block adhesion to tissue culture cells, as did di- or trisaccharides containing these sugars, suggesting that the adhesin is a lectin. In addition, N-acetylgalactosamine, a component of mucus, and gelatin partially impeded cell attachment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Borriello S. P., Welch A. R., Barclay F. E., Davies H. A. Mucosal association by Clostridium difficile in the hamster gastrointestinal tract. J Med Microbiol. 1988 Mar;25(3):191–196. doi: 10.1099/00222615-25-3-191. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. A., Borriello S. P. Detection of capsule in strains of Clostridium difficile of varying virulence and toxigenicity. Microb Pathog. 1990 Aug;9(2):141–146. doi: 10.1016/0882-4010(90)90088-8. [DOI] [PubMed] [Google Scholar]
- Delmée M., Mackey T., Hamitou A. Evaluation of a new commercial Clostridium difficile toxin A enzyme immunoassay using diarrhoeal stools. Eur J Clin Microbiol Infect Dis. 1992 Mar;11(3):246–249. doi: 10.1007/BF02098089. [DOI] [PubMed] [Google Scholar]
- Dinari G., Hale T. L., Washington O., Formal S. B. Effect of guinea pig or monkey colonic mucus on Shigella aggregation and invasion of HeLa cells by Shigella flexneri 1b and 2a. Infect Immun. 1986 Mar;51(3):975–978. doi: 10.1128/iai.51.3.975-978.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Neumann A. W., Policova Z., Sherman P. M. Bacterial cell surface hydrophobicity properties in the mediation of in vitro adhesion by the rabbit enteric pathogen Escherichia coli strain RDEC-1. J Clin Invest. 1989 Nov;84(5):1588–1594. doi: 10.1172/JCI114336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveillard M., Fourel V., Barc M. C., Kernéis S., Coconnier M. H., Karjalainen T., Bourlioux P., Servin A. L. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol Microbiol. 1993 Feb;7(3):371–381. doi: 10.1111/j.1365-2958.1993.tb01129.x. [DOI] [PubMed] [Google Scholar]
- George W. L. Antimicrobial agent-associated colitis and diarrhea: historical background and clinical aspects. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S208–S213. doi: 10.1093/clinids/6.supplement_1.s208. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth I., Lange S. Toxin A of Clostridium difficile: production, purification and effect in mouse intestine. Acta Pathol Microbiol Immunol Scand B. 1983 Dec;91(6):395–400. doi: 10.1111/j.1699-0463.1983.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Husar S. D. Adhesion of Yersinia enterocolitica to purified rabbit and human intestinal mucin. Infect Immun. 1993 Jun;61(6):2340–2346. doi: 10.1128/iai.61.6.2340-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Burr D. H., Walker R. I. Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun. 1987 Jun;55(6):1431–1435. doi: 10.1128/iai.55.6.1431-1435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulston J. E., Davis C. H., Schmiel D. H., Morgan M. W., Wyrick P. B. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J Biol Chem. 1993 Nov 5;268(31):23139–23147. [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990 Apr;58(4):860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U. S., Corey M., Karmali M. A., Forstner J. F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiel D. H., Knight S. T., Raulston J. E., Choong J., Davis C. H., Wyrick P. B. Recombinant Escherichia coli clones expressing Chlamydia trachomatis gene products attach to human endometrial epithelial cells. Infect Immun. 1991 Nov;59(11):4001–4012. doi: 10.1128/iai.59.11.4001-4012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon S. V., Borriello S. P. Proteolytic activity of Clostridium difficile. J Med Microbiol. 1992 May;36(5):307–311. doi: 10.1099/00222615-36-5-307. [DOI] [PubMed] [Google Scholar]
- Seddon S. V., Hemingway I., Borriello S. P. Hydrolytic enzyme production by Clostridium difficile and its relationship to toxin production and virulence in the hamster model. J Med Microbiol. 1990 Mar;31(3):169–174. doi: 10.1099/00222615-31-3-169. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen P. T., de Graaf F. K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992 May;12(5):367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]