Abstract
Background
Activation of renal D3 receptor induces natriuresis and diuresis in Wistar-Kyoto (WKY) rats; in the presence of ETB receptor antagonist, the natriuretic effect of D3 receptor in WKY rats is reduced. We hypothesize that ETB receptor activation may regulate D3 receptor expression in renal proximal tubule (RPT) cells from WKY rats, which is impaired in RPT cells from spontaneously hypertensive rats (SHRs).
Methods
D3 receptor expression was determined by immunoblotting; the D3/ETB receptor linkage was checked by coimmunoprecipitation; Na+-K+-ATPase activity was determined as the rate of inorganic phosphate released in the presence or absence of ouabain.
Results
In RPT cells from WKY rats, the ETB receptor agonist BQ3020 increased D3 receptor protein. In contrast, in RPT cells from SHRs, BQ3020 did not increase D3 receptor. There was coimmunoprecipitation between D3 and ETB receptors in RPT cells from WKY and SHRs. Activation of ETB receptor increased D3/ETB coimmunoprecipitation in RPT cells from WKY rats, but not from SHRs. The basal levels of D3/ETB receptor coimmunoprecipitation were greater in RPT cells from WKY rats than in those from SHRs. Stimulation of D3 receptor inhibited Na+-K+-ATPase activity, which was augmented by the pretreatment with the ETB receptor agonist BQ3020 in WKY RPT cells, but not in SHR RPT cells.
Conclusion
ETB receptors regulate and physically interact with D3 receptors differently in WKY rats and SHRs. The impaired natriuretic effect in SHRs may be, in part, related to impaired ETB and D3 receptor interactions.
Key Words: Essential hypertension, Dopamine receptor, Endothelin receptor
Introduction
Dopamine has been recognized as an important modulator of central as well as peripheral physiologic functions in both humans and animals, which produces its biologic effects through five genetically distinct dopamine receptor subtypes: D1, D2, D3, D4, and D5. These receptors are categorized into two groups known as D1-like (D1 and D5, whose rat homologs are D1A and D1B) and D2-like (D2, D3, and D4) dopamine receptors based on their ability to stimulate and inhibit adenylyl cyclase, respectively [1,2,3]. Dopamine receptors have been identified in a number of organs and tissues, which include kidney [4,5,6]. Within the kidney, dopamine receptors are present along the nephron, with highest density on renal proximal tubule (RPT) cells, where activation of dopamine receptor, mainly via D1 and D3 receptor, decreases sodium transport [1,2,3]. Under euvolemic conditions and magnified during moderate volume expansion, dopamine acts to increase sodium excretion and decrease blood pressure [1,2,3].
Endothelin, like dopamine, is produced by renal tubules where it can regulate sodium transport in an autocrine or paracrine manner [7]. Endothelin regulates epithelial sodium transport via two receptor subtypes (ETA and ETB). The effect of endothelin on sodium transport is complicated; there are both stimulatory and inhibitory effects on sodium reabsorption. Endothelin increases sodium reabsorption [8,9]. Whereas there are also reports that endothelin-1 inhibits Na+-K+-ATPase activity in this nephron segment [10], our previous study showed that activation of ETB receptor inhibits Na+-K+-ATPase activity in RPT cells from Wistar-Kyoto (WKY) rats [11]. ETB receptors can lower blood pressure by decreasing endothelin-1 levels, and promoting renal loss of sodium and water [12,13,14]. Indeed, in uninephrectomized rats given deoxycorticosterone acetate and a high salt diet, spontaneously hypertensive rats (SHRs), and humans with essential hypertension ETB receptor activation may be a counter-regulatory mechanism to the increase in blood pressure [12,14]. Naturally occurring or induced deletion of the ETB receptor gene and chronic pharmacological blockade of ETB receptors result in salt-sensitive hypertension in rats [15].
Disruption of the D3 receptor gene in mice produces hypertension that is associated with a decreased ability to excrete a sodium load [16]. Our previous studies found that activation of renal D3 receptor induces natriuresis and diuresis in WKY rats; in the presence of ETB receptor antagonist, the natriuretic effect of D3 receptor is reduced [17]. The mechanisms underlying the D3 and ETB receptor interaction are not known. We hypothesize that ETB receptor activation may regulate D3 receptor expression in RPT cells from WKY rats, which is impaired in RPT cells from SHRs. The interaction between D3 and ETB receptors was studied further in RPT cells from WKY rats and SHRs, which have characteristics similar to freshly obtained RPT brush border membranes and RPTs, at least with regard to D1 receptors and responses to G protein stimulation [18,19,20,21]. We now report that activation of ETB receptor increased D3 receptor expression in RPT cells from WKY rats. In contrast, BQ3020 no longer affected D3 receptor expression in SHR cells. D3/ETB receptors coimmunoprecipitated in both WKY and SHR cells. BQ3020 treatment increased D3/ETB receptor coimmunoprecipitation in WKY cells but had no effect in SHR cells, and basal D3/ETB receptor coimmunoprecipitation was higher in WKY cells than in SHR cells. Stimulation of D3 receptor inhibited Na+-K+-ATPase activity, which was augmented by the pretreatment with ETB receptor agonist, BQ3020, in WKY RPT cells, but not in SHR RPT cells.
Methods
Cell Culture
Immortalized RPT cells from 4- to 8-week-old SHR and WKY rats were cultured at 37°C in 95% air/5% CO2 atmosphere in DMEM/F-12 culture media as previously described [22,23]. The cells (80% confluence) were extracted in ice-cold lysis buffer (phosphate-buffered saline with 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), sonicated, placed on ice for 1 h, and centrifuged at 16,000 g for 30 min. The supernatants were stored at −70° until used for immunoblotting and/or immunoprecipitation.
Immunoblotting
The antibodies were polyclonal, purified, and antipeptides. The rat ETB receptor-immunizing peptide (298- CEMLRKKSGMQIALND-314; Alomone Labs, Jerusalem, Israel) [24,25] and the rat D3 receptor-immunizing peptide (288-QPP SPG QTH GGL KRY YSI C-306; Alpha Diagnostic International, San Antonio, Tex., USA) [26,27,28] were located on the third extracellular loop of their corresponding receptors. The specificity of these antibodies had been reported [24,25,26,27,28]. RPT cells were treated with vehicle (dH2O), BQ3020 (Sigma, Co., St. Louis, Mo., USA) [29,30], or an ETB receptor antagonist (BQ788; Sigma) [29,30] at the indicated concentrations and times. Immunoblotting was performed as reported, except that the transblots were probed with ETB (1:300) or D3 receptor antibodies (1:250) [24,25,26,27,28].
Immunoprecipitation
RPT cells were incubated with vehicle or BQ3020 (10−8M) for 30 min as described above. The cells were lysed with ice-cold lysis buffer for 1 h and centrifuged at 16,000 g for 30 min. Equal amounts of lysates, except as indicated (200 μg protein/ml supernatant for WKY RPT cells and 800 μg protein/ml supernatant for SHR RPT cells) were incubated with the anti-ETB receptor antibody (1 μg/ml) for 1 h, and protein-G agarose at 4°C for 12 h. The immunoprecipitates were pelleted and washed four times with lysis buffer. The pellets were suspended in sample buffer, boiled for 10 min, and subjected to immunoblotting with anti-D3 receptor antibody. In order to determine the specificity of the bands, normal rabbit IgG (negative control) and D3 receptor antibody (positive control) were used as the immunoprecipitants instead of the ETB receptor antibody [22,23,24].
Na+-K+-ATPase Activity Assay
Na+-K+-ATPase activity was determined as the rate of inorganic phosphate released in the presence or absence of ouabain [31,32,33]. To prepare membranes for Na+-K+-ATPase activity assay, RPT cells cultured in 21-cm2 plastic culture dishes were washed twice with 5 ml chilled phosphate-free modified Krebs buffer (118 mM NaCl, 4 mM KCl, 27.2 mM NaHCO3, 1.2 mM MgCl2·6H2O, 10 mM Hepes and 0.25 mM CaCl2·2H2O), and centrifuged at 3,000 g for 10 min. The cells were then placed on ice and lysed in 2 ml of lysis buffer (1 mM NaHCO3, 2 mM CaCl2 and 5 mM MgCl2). Cell lysates were centrifuged at 3,000 g for 2 min to remove intact cells, debris, and nuclei. The resulting supernatant was suspended in an equal volume of 1 M sodium iodide, and the mixture was centrifuged at 48,000 g for 25 min. The pellet (membrane fraction) was washed 2 times and suspended in 10 mM Tris and 1 mM EDTA (pH 7.4). Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories, Hercules, Calif., USA.) and adjusted to 1 mg/ml. The membranes were stored at −70°C until further use. To measure Na+-K+-ATPase activity, 100-μl aliquots of membrane fraction were added to a 800-μl reaction mixture (75 mM NaCl, 5 mM KCl, 5 mM MgCl2, 6 mM sodium azide, 1 mM Na4EGTA, 37.5 mM imidazole, 75 mM Tris HCl, and 30 mM histidine; pH 7.4) with or without 1 mM ouabain (final volume = 1 ml) and preincubated for 5 min in a water bath at 37°C. Reactions were initiated by adding Tris ATP (4 mM) and terminated after 15 min of incubation at 37°C by adding 50 μl of 50% trichloracetate. For determination of ouabain-insensitive ATPase activity, NaCl and KCl were omitted from the reaction mixtures containing ouabain. To quantify the amount of phosphate produced, 1 ml of coloring reagent (10% ammonium molybdate in 10 N sulfuric acid + ferrous sulfate) was added to the reaction mixture. The mixture was then combined thoroughly and centrifuged at 3,000 g for 10 min. Formation of phosphomolybdate was determined spectrophotometrically at 740 nm against a standard curve prepared from K2HPO4. Na+-K+-ATPase activity was estimated as the difference between total and ouabain-insensitive ATPase activity, and results in this experiment were expressed as μmol phosphate released per mg protein per min.
To eliminate the effect of proteases on the results, we added protease inhibitor (1 mM phenylmethylsulfonyl fluoride, 10 mg/ml each leupeptin and aprotinin) in all solutions in this experiment [34].
Statistical Analysis
The data are expressed as mean ± SEM. Comparison within groups was made by repeated-measures ANOVA, and comparison among groups was made by factorial ANOVA with Duncan's test. A value of p < 0.05 was considered significant.
Results
Activation of ETB Receptor Increases D3 Receptor Expression in Rat RPT Cells from WKY Rats, but Not from SHRs
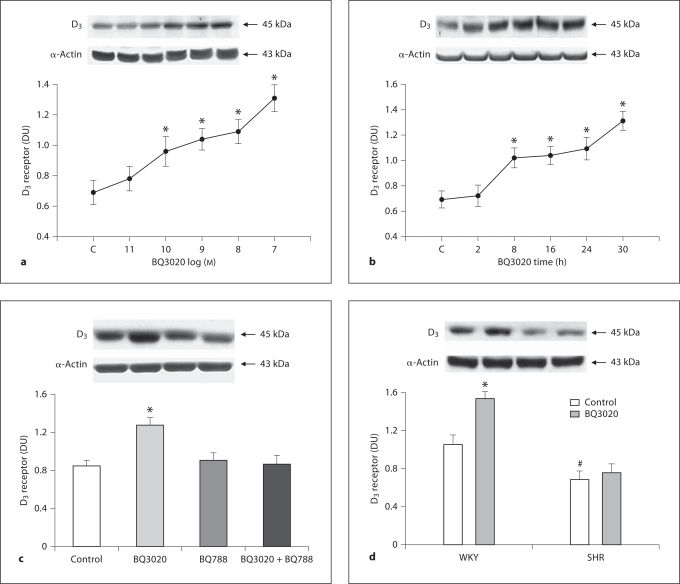
An ETB receptor agonist, BQ3020, increased D3 receptor expression in a concentration- and time-dependent manner in RPT cells from WKY rats. The stimulatory effect was evident at 10−10M (fig. 1a). The stimulatory effect of BQ3020 (10−8M) was noted as early as 8 h and maintained for at least 30 h (fig. 1b). The specificity of BQ3020 as an ETB receptor agonist was also determined by studying the effect of the ETB receptor antagonist BQ788. Consistent with the studies shown in figure 1a and b, BQ3020 (10−8M/24 h) increased D3 receptor expression. The ETB receptor antagonist BQ788 (10−8M) by itself had no effect on D3 receptor expression, but reversed the stimulatory effect of BQ3020 on D3 receptor expression (fig. 1c).
Fig. 1.
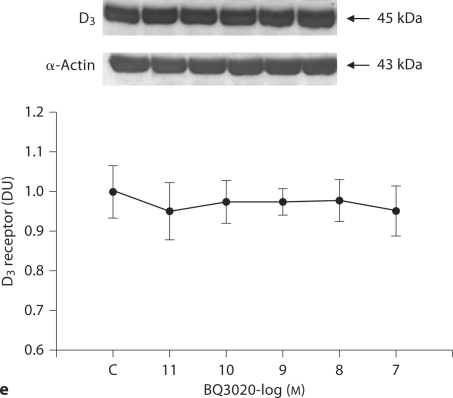
Effect of BQ3020 on D 3 receptor expression in RPT cells from SHRs and WKY rats. a Concentration-response of D3 receptor expression in RPT cells from WKY rats treated with BQ3020. Immunoreactive D3 receptor expression was determined after 24-hour incubation with the indicated concentrations of BQ3020. Results are expressed as the ratio of D3 receptor to α-actin densities [n = 6, ∗ p<0.05 vs. control (C), ANOVA, Duncan's test]. DU = Density units. b Time course of D3 receptor expression in RPT cells from WKY rats treated with BQ3020. The cells were incubated for the indicated times with 10−8 M BQ3020. Results are expressed as the ratio of D3 receptor to α-actin densities [n = 7, ∗ p < 0.05 vs. control (0 time), ANOVA, Duncan's test]. c Effect of BQ3020 and an ETB antagonist (BQ788) on D3 receptor expression in RPT cells from WKY rats. The cells were incubated with the indicated reagents (BQ3020, 10−8 M; BQ788, 10−8 M) for 24 h. Results are expressed as the ratio of D3 receptor to α-actin densities (n = 6, ∗ p < 0.05 vs. others, ANOVA, Duncan's test). d Differential effects of BQ3020 (10−8 M/24 h) on D3 receptor expression in RPT cells from both WKY rats and SHRs. Results are expressed as the ratio of D3 receptor to α-actin densities (n = 10, ∗ p < 0.05 vs. control, # p < 0.05 vs. WKY control, ANOVA, Duncan's test). Effect of BQ3020 on D3 receptor expression in RPT cells from SHRs and WKY rats. e Concentration-response of D3 receptor expression in SHR RPT cells treated with BQ3020. Immunoreactive D3 receptor expression was determined after 24-hour incubation with the indicated concentrations of BQ3020. Results are expressed as the ratio of D3 receptor to α-actin densities (n = 5, p = NS vs. control, ANOVA, Duncan's test).
In contrast, in RPT cells from SHRs, BQ3020 (10−8M) had no effect on D3 receptor expression (fig. 1d). To determine whether higher concentrations of BQ3020 could have any effect on D3 receptor expression, SHR cells were treated with varying concentrations of BQ3020 for 24 h. Consistent with the results from figure 1d, 10−7–10−11M BQ3020 had no effect on D3 receptor protein expression (fig. 1e).
ETB Receptor Coimmunoprecipitates with the D3 Receptor in Rat RPT Cells
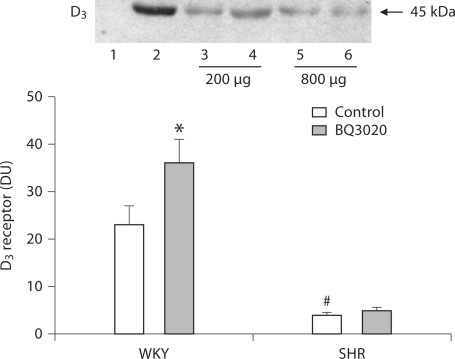
To determine whether there is a physical interaction between the ETB and the D3 receptor, additional experiments were performed. ETB receptors were first immunoprecipitated with anti-ETB receptor antibodies and then probed with anti-D3 receptor antibodies. As shown in figure 2, the 45-kDa band representing the coimmunoprecipitated ETB and D3 receptors, was increased by treatment with BQ3020 (10−8M) in RPT cells from WKY rats for 30 min; however, BQ3020 had no effect on ETB/D3 receptor coimmunoprecipitation in SHR cells, the basal level of D3/ETB receptor coimmunoprecipitation was higher in WKY cells than in SHR cells.
Fig. 2.
Effect of BQ3020 on coimmunoprecipitation of D3 and ETB receptors in rat RPT cells. The cells were incubated with BQ3020 (10−8 M) for 30 min. Thereafter, the samples were immunoprecipitated with anti-ETB antibodies and immunoblotted with anti-D3 antibodies (∗ p < 0.05 vs. control, # p < 0.05 vs. WKY rats, n = 8, ANOVA, Duncan's test). One immunoblot (45 kDa) is depicted in the inset (lane 1 = negative control, lane 2 = positive control, lane 3 = vehicle-treated RPT cell of WKY rats, lane 4 = BQ3020- treated RPT cell of WKY rats, lane 5 = vehicle-treated RPT cell of SHRs, lane 6 = BQ3020-treated RPT cell of SHRs).
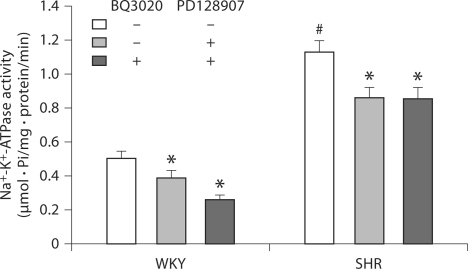
Pretreatment with BQ3020 Increases the Inhibitory Effect of D3 Receptor on Na+-K+-ATPase Activity in WKY Cells, but Not in SHR Cells
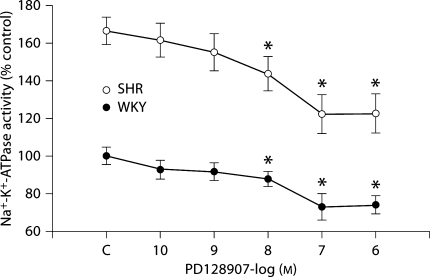
To investigate the physiological significance of the ETB/D3 receptor interaction, the effects of D3 or/and ETB receptor stimulation on Na+-K+-ATPase activity were determined in WKY and SHR cells. Stimulation of D3 receptors by PD128907 (10−10–10−5M) for 15 min decreased Na+-K+-ATPase activities in a concentration-dependent manner in WKY and SHR cells (fig. 3). However, the basal levels of Na+-K+-ATPase activity were higher in SHR cells than in WKY cells. Pretreatment with BQ3020 (10−8M) for 24 h augmented the inhibitory effect of D3 (10−7M/15 min) on Na+-K+-ATPase activity in WKY cells, but not in SHR cells (fig. 4). The cells pretreated with BQ3020 for 24 h were washed three times (15 min/wash) with serum-free culture medium to remove all the added BQ3020, kept in serum-free culture medium for 2 h, and then treated with vehicle or PD128907 for 15 min.
Fig. 3.
Effect of the D3 receptor on Na+-K+-ATPase activity in RPT cells from WKY rats and SHRs (n = 6). The RPT cells were treated with the indicated concentrations of the D3 receptor agonist PD128907 (10−10-10−6 M) for 15 min. Results in this experiment are expressed as percent change from control [∗ p < 0.01 vs. control (C), ANOVA, Duncan's test].
Fig. 4.
Effect of pretreatment with the ETB receptor agonist BQ3020 on inhibitory effect of D3 receptor on Na+-K+-ATPase activity in WKY and SHR RPT cells. The cells were pretreated with BQ3020 (10−8 M) or vehicle (dH2O) for 24 h. After washing for 15 min, the cells were treated with the D3 receptor agonist PD128907 (10−7 M) for 15 min. Results in this experiment are expressed as μmol phosphate released per mg protein per min (∗ p < 0.05 vs. control, # p < 0.05 vs. WKY rats, n = 12, ANOVA, Duncan's test).
Discussion
There are several novel observations in our study. First, we showed that an ETB receptor agonist, BQ3020, increased D3 receptor protein expression in rat WKY RPT cells, but not in SHR cells. This effect was clearly exerted at the ETB receptor because an ETB receptor antagonist, BQ788, blocked the effect of the ETB receptor agonist. Second, we demonstrated that ETB receptors coimmunoprecipitated with D3 receptors in rat RPT cells. ETB receptor stimulation with BQ3020 increased ETB/D3 receptor coimmunoprecipitation in WKY cells, but not in SHR cells, and the basal levels of ETB/D3 receptor coimmunoprecipitation was lower in SHR cells than in WKY cells. Third, stimulation of D3 receptor inhibited Na+-K+-ATPase activity, which was augmented by the pretreatment with ETB receptor agonist BQ3020 in WKY RPT cells, but not in SHR RPT cells.
ETB receptor has been found to have a natriuretic effect in renal medullary collecting ducts and medullary thick ascending limb of Henle [35,36,37]. However, both inhibitory and stimulatory effects have been reported in the proximal tubules [10,36,37,38,39]. There is evidence that endothelin-1 inhibits Na+-K+-ATPase activity in this nephron segment [10]; our previous study showed that activation of ETB receptor inhibits Na+-K+-ATPase activity in WKY RPT cells [11]. Short-term stimulation of ETB receptors in opossum kidney cells, a proximal tubular cell line, activates the sodium hydrogen exchanger 3 (NHE3) [39]. However, chronic treatment of the same opossum kidney cells by endothelin has an opposite effect on NHE3 activity. Thus, a 6-hour exposure of opossum kidney cells to endothelin-1 inhibits NHE3 expression and activity [36]. It is of interest that the ability of the ETB receptor agonist to increase D3 receptor expression also occurs within the same time frame, as the enhanced ability of ETB to inhibit NHE3 expression and activity.
As a major subtype of D2-like receptor, the natriuretic effect of D3 receptor is reported [16]. Our previous study found that D1-like and D2-like receptors synergistically increase sodium excretion in WKY rats [28,40]; and this synergistic effect occurs between D1 and D3 receptors [23]. Homozygous D3 receptor null mice develop hypertension with an impaired ability to excrete a sodium load, indicating a natriuretic effect of D3 receptor in the kidney [16]. This effect is confirmed in salt-sensitive Dahl rats, WKY rats and SHRs [41,42], which may contribute the inhibitory effect of D3 receptor on Na+-K+-ATPase activity in RPTs [43,44]. Our present study found that stimulation of D3 receptor inhibits Na+-K+-ATPase activity in RPT cells.
An increasing body of evidence shows dopamine and endothelin systems can interact with each other. Dopamine and ETB receptors have been found in brain and spinal regions known to control cardiovascular function [45]. In the rat striatum, a decrease in dopamine production decreases endothelin receptor, while endothelin via ETB receptors induces the release of dopamine [46]. In the peripheral system, an ETB receptor blocker for both ETB1 and ETB2 receptors decreases, whereas a selective ETB1 blocker increases blood pressure in D2 dopamine receptor knockout mice, but not in D2 receptor wild-type control mice. ETB receptor expression is greater in D2 receptor knockout mice than in D2 receptor wild-type mice [47]. This study found activation of ETB receptor increases D3 receptor expression and D3/ETB receptor coimmunoprecipitation, and those effects are lost in SHR cells, supporting the interaction between dopamine and endothelin system in the kidney. It indicates that impaired natriuretic effect in SHRs may be, in part, related to impaired D3 and ETB receptor interactions. Our present study found that pretreatment with BQ3020 for 24 h augments the inhibitory effect of D3 receptor on Na+-K+-ATPase activity in WKY cells, but not in SHR cells. The aberrant interaction between D3 and ETB receptor might be involved in the pathogenesis of hypertension.
The mechanism of the increase in ETB receptors caused by D3 receptors was not studied. The D2 receptor has been reported to regulate expression of other receptors [48]; it is possible that D3 receptors regulate ETB receptor expression by the similar mechanism(s). We also found that D3 and ETB receptors can physically interact, as determined by their coimmunoprecipitation. Moreover, ETB receptor stimulation results in an increase in the interaction between D3 and ETB receptors in WKY rats. The increase of D3/ETB receptor coimmunoprecipitation in RPT cells following ETB receptor agonist stimulation could have been caused by the physical interaction between the D3 and ETB receptors because the stimulation period is only 30 min, which is too short to change the protein expressions. In the basal state, there was less D3/ETB receptor coimmunoprecipitation in SHRs compared to WKY rats. This could be due to decreased D3 expression in SHRs relative to WKY rats, but could not be caused by differences in ETB expression, in agreement with a previous study.
In summary, we have demonstrated that ETB receptors positively regulate the expression of D3 receptors in rat RPT cells. Furthermore, D3 and ETB receptors coimmunoprecipitate in RPT cells. Activation of ETB receptor increases D3/ETB receptor coimmunoprecipitation in WKY cells, but has no effect in SHRs. Stimulation of D3 receptor inhibited Na+-K+-ATPase activity, which was augmented by the pretreatment with ETB receptor agonist BQ3020 in WKY RPT cells, but not in SHR RPT cells.
We conclude that ETB receptors regulate D3 receptors by direct physical receptor interaction and receptor expression. The impaired natriuretic effect in SHRs may be, in part, related to impaired ETB receptor regulation of D3 receptors.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health, HL23081, DK39308, HL68686, HL074940, HL092196, the National Natural Science Foundation of China 30470728, 30672199, the National Basic Research Program of China (973 Program, 2008CB517308), Natural Science Foundation Project of CQ CSTC (CSTC,2009BA5044) and the Grants for Distinguished Young Scholars of China from the National Natural Science Foundation of China (30925018).
References
- 1.Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther. 1998;80:149–182. doi: 10.1016/s0163-7258(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Shin Y, Kumar U, Patel Y, Patel SC, Sidhu A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens. 2003;21:199–207. doi: 10.1097/00004872-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Ricci A, Marchal-Victorion S, Bronzetti E, Parini A, Amenta F, Tayebati SK. Dopamine D4 receptor expression in rat kidney: evidence for pre- and postjunctional localization. J Histochem Cytochem. 2002;50:1091–1096. doi: 10.1177/002215540205000811. [DOI] [PubMed] [Google Scholar]
- 6.Muhlbauer B, Kuster E, Luippold G. Dopamine D3 receptors in the rat kidney: role in physiology and pathophysiology. Acta Physiol Scand. 2000;168:219–223. doi: 10.1046/j.1365-201x.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- 7.Haug C, Grill C, Schmid-Kotsas A, Gruenert A, Jehle PM. Endothelin release by rabbit proximal tubule cells: modulatory effects of cyclosporine A, tacrolimus, HGF and EGF. Kidney Int. 1998;54:1626–1636. doi: 10.1046/j.1523-1755.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- 8.Walter R, Helmle-Kolb C, Forgo J, Binswanger U, Murer H. Stimulation of Na+/H+ exchange activity by endothelin in opossum kidney cells. Pflugers Arch. 1995;430:137–144. doi: 10.1007/BF00373849. [DOI] [PubMed] [Google Scholar]
- 9.Garcia NH, Garvin JL. Endothelin's biphasic effect on fluid absorption in the proximal straight tubule and its inhibitory cascade. J Clin Invest. 1994;93:2572–2577. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvin J, Sanders K. Endothelin inhibits fluid and bicarbonate transport in part by reducing Na+/K+ATPase activity in the rat proximal straight tubule. J Am Soc Nephrol. 1991;2:976–982. doi: 10.1681/ASN.V25976. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na+-K+ ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res. 2009;32:846–852. doi: 10.1038/hr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohuchi T, Yanagisawa M, Gariepy CE. Renal tubular effects of endothelin-B receptor signaling: its role in cardiovascular homeostasis and extracellular volume regulation. Curr Opin Nephrol Hypertens. 2000;9:435–439. doi: 10.1097/00041552-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Miki S, Takeda K, Kiyama M, Hatta T, Morimoto S, Kawa T, Itoh H, Nakata T, Sasaki S, Nakagawa M. Augmented response of endothelin-A and endothelin-B receptor stimulation in coronary arteries of hypertensive hearts. J Cardiovasc Pharmacol. 1998;31(suppl 1):S94–S98. doi: 10.1097/00005344-199800001-00029. [DOI] [PubMed] [Google Scholar]
- 14.Pollock DM, Allcock GH, Krishnan A, Dayton BD, Pollock JS. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2000;278:F279–F286. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- 15.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–929. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int. 1996;50:125–134. doi: 10.1038/ki.1996.295. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. D1 receptor, Gsα, and Na+/H+ exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. doi: 10.1161/01.hyp.36.3.395. [DOI] [PubMed] [Google Scholar]
- 20.Yu P, Asico LD, Eisner GM, Hopfer U, Felder RA, Jose PA. Renal protein phosphatase 2A activity and spontaneous hypertension in rats. Hypertension. 2000;36:1053–1058. doi: 10.1161/01.hyp.36.6.1053. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1064–R1073. doi: 10.1152/ajpregu.2000.278.4.R1064. [DOI] [PubMed] [Google Scholar]
- 22.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases AT1 angiotensin receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Wang Z, Yu P, Zheng S, Wu L, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension. 2006;47:573–579. doi: 10.1161/01.HYP.0000199983.24674.83. [DOI] [PubMed] [Google Scholar]
- 24.Zeng C, Wang Z, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Aberrant ETB receptor regulation of AT1 receptors in renal proximal tubule cells of spontaneously hypertensive rats. Kidney Int. 2005;68:623–631. doi: 10.1111/j.1523-1755.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 25.Moridaira K, Morrissey J, Fitzgerald M, Guo G, McCracken R, Tolley T, Klahr S. ACE inhibition increases expression of the ETB receptor in kidneys of mice with unilateral obstruction. Am J Physiol Renal Physiol. 2003;284:F209–F217. doi: 10.1152/ajprenal.00352.2001. [DOI] [PubMed] [Google Scholar]
- 26.Zeng C, Asico LD, Wang X, Hopfer U, Eisner GM, Felder RA, Jose PA. Angiotensin II regulation of AT1 and D3 dopamine receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2003;41:724–729. doi: 10.1161/01.HYP.0000047880.78462.0E. [DOI] [PubMed] [Google Scholar]
- 27.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Felder RA, Jose PA. D1 dopamine receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:654–660. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- 28.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;28:R1071–R1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 29.Drimal J, Drimal J, Jr, Orlicky J, Janecek A, Kettmann V, Drimal D, Huzavova M. Effects of human peptide endothelin-1 and two of its sterically unrestrained C-terminal fragments on coronary vascular smooth muscle. Gen Physiol Biophys. 2002;21:3–14. [PubMed] [Google Scholar]
- 30.Peter MG, Davenport AP. Characterization of the endothelin receptor selective agonist, BQ3020 and antagonists BQ123, FR139317, BQ788, 50235, Ro462005 and bosentan in the heart. Br J Pharmacol. 1996;117:455–462. doi: 10.1111/j.1476-5381.1996.tb15212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotlo K, Shukla S, Tawar U, Skidgel RA, Danziger RS. Aminopeptidase N reduces basolateral Na+/K+ ATPase in proximal tubule cells. Am J Physiol Renal Physiol. 2007;293:F1047–F1053. doi: 10.1152/ajprenal.00074.2007. [DOI] [PubMed] [Google Scholar]
- 32.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- 33.Silva E, Gomes P, Soares-da-Silva P. Overexpression of Na+/K+-ATPase parallels the increase in sodium transport and potassium recycling in an in vitro model of proximal tubule cellular ageing. J Membr Biol. 2006;212:163–175. doi: 10.1007/s00232-005-7017-5. [DOI] [PubMed] [Google Scholar]
- 34.Sorbel JD, Brooks DM, Lurie DI. SHP-1 expression in avian mixed neural/glial cultures. J Neurosci Res. 2002;68:703–715. doi: 10.1002/jnr.10262. [DOI] [PubMed] [Google Scholar]
- 35.Kohzuki M, Johnston CI, Chai SY, Casley DJ, Mendelsohn FA. Localization of endothelin receptors in rat kidney. Eur J Pharmacol. 1989;160:193–194. doi: 10.1016/0014-2999(89)90673-0. [DOI] [PubMed] [Google Scholar]
- 36.Chu TS, Tsuganezawa H, Peng Y, Cano A, Yanagisawa M, Alpern RJ. Role of tyrosine kinase pathways in ETB receptor activation of NHE3. Am J Physiol. 1996;271:C763–C771. doi: 10.1152/ajpcell.1996.271.3.C763. [DOI] [PubMed] [Google Scholar]
- 37.Knotek M, Jaksic O, Selmani R, Skorić B, Banfić H. Different endothelin receptor subtypes are involved in phospholipid signalling in the proximal tubule of rat kidney. Pflugers Arch. 1996;432:165–173. doi: 10.1007/s004240050120. [DOI] [PubMed] [Google Scholar]
- 38.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact renal tubular epithelial cells. Am J Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y, Moe OW, Chu T, Preisig PA, Yanagisawa M, Alpern RJ. ETB receptor activation leads to activation and phosphorylation of NHE3. Am J Physiol. 1999;276:C938–C945. doi: 10.1152/ajpcell.1999.276.4.C938. [DOI] [PubMed] [Google Scholar]
- 40.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol. 1998;275:R986–R994. doi: 10.1152/ajpregu.1998.275.4.R986. [DOI] [PubMed] [Google Scholar]
- 41.Luippold G, Zimmermann C, Mai M, Kloor D, Starck D, Gross G, Muhlbauer B. Dopamine D3 receptors and salt-dependent hypertension. J Am Soc Nephrol. 2001;12:2272–2279. doi: 10.1681/ASN.V12112272. [DOI] [PubMed] [Google Scholar]
- 42.Luippold G, Piesch C, Osswald H, Muhlbauer B. Dopamine D3 receptor mRNA and renal response to D3 receptor activation in spontaneously hypertensive rats. Hypertens Res. 2003;26:855–861. doi: 10.1291/hypres.26.855. [DOI] [PubMed] [Google Scholar]
- 43.Gomes P, Soares-da-Silva P. D2-like receptor-mediated inhibition of Na+-K+-ATPase activity is dependent on the opening of K+ channels. Am J Physiol Renal Physiol. 2002;283:F114–F123. doi: 10.1152/ajprenal.00244.2001. [DOI] [PubMed] [Google Scholar]
- 44.Pedrosa R, Gomes P, Zeng C, Hopfer U, Jose PA, Soares-da-Silva P. Dopamine D3 receptor-mediated inhibition of the Na+/H+ exchanger in spontaneously hypertensive rat proximal tubular epithelial cells. Br J Pharmacol. 2004;142:1343–1353. doi: 10.1038/sj.bjp.0705893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Buuse M, Webber KM. Endothelin and dopamine release. Prog Neurobiol. 2000;60:385–405. doi: 10.1016/s0301-0082(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 46.Webber KM, Pennefather JN, Head GA, van den Buuse M. Endothelin induces dopamine release from rat striatum via endothelin-B receptors. Neuroscience. 1998;86:1173–1180. doi: 10.1016/s0306-4522(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 47.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 48.Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]