SUMMARY
Recent findings have brought optimism that development of a successful human immunodeficiency virus type-1 (HIV-1) vaccine lies within reach. Studies of early events in HIV-1 infection have revealed when and where HIV-1 is potentially vulnerable to vaccine-targeted immune responses. With technical advances in human monoclonal antibody production, clues about how antibodies recognize the HIV-1 envelope proteins have uncovered new antigenic targets for immunogen design. A recent vaccine regimen has shown modest efficacy against HIV-1 acquisition. However, inducing long-term T and B cell memory and coping with HIV-1 diversity remain a high priority. Mediators of innate immunity may play pivotal roles in blocking infection and shaping immunity; vaccine strategies to capture these activities are under intense investigation. Key challenges remain in integrating basic, preclinical and clinical research to improve predictions of types of immunity associated with vaccine efficacy, to apply these insights to immunogen design, and to accelerate evaluation of vaccine efficacy in persons at-risk for acquiring infection.
Human immunodeficiency virus type-1 (HIV-1): the epidemic and the need for a vaccine
Since 1981, more than 25 million people have died of Acquired Immune Deficiency Syndrome (AIDS). As of 2009, UNAIDS estimates that 33.4 million now live with HIV-1 infection, and 2 million become newly diagnosed with HIV-1 each year. Sub-Saharan Africa continues to bear the major burden with 22 million HIV-infected persons. Anti-retroviral therapy (ART) can suppress viral replication, increasing life expectancy among those infected, but cannot cure infection; with rare exceptions, HIV-1 infection left untreated leads to death. Sustaining affordable ART coverage in resource-poor, HIV-1 endemic regions is a daunting global health problem. A safe, efficacious vaccine affords the best long-term solution to ending the HIV-1 epidemic.
Several modalities can reduce HIV-1 infection rates in persons at risk for exposure, including screening of donor blood products, risk reduction counseling, behavioral modifications, condom usage and male circumcision. Pre-exposure or post-exposure ART prophylaxis may reduce susceptibility, with one recent trial demonstrating 39% efficacy in lowering HIV-1 incidence rates among South African women using a tenofovir vaginal gel before and after sexual activities (Karim et al., 2010). Treatment of infected persons can markedly reduce transmission risk from mother to child, in exposed persons living in high-seroprevalence communities, and between heterosexual discordant couples. Together, these interventions can slow the epidemic and complement partially effective vaccine regimens. However, a highly efficacious preventive vaccine is key to generating long-term immunological memory to sustain protection against HIV-1 infection.
A fundamental barrier to HIV-1 vaccine development lies with the unique properties of the virus: its entry is predominantly through mucosal surfaces, its preferred target is human CD4+ T cells, and it rapidly establishes a persistent reservoir of latently infected cells. Properties of transmitted (founder) viruses from mucosal transmission indicate that in 70-80% of cases, a single virus or virus-infected cell establishes productive clinical infection (Keele et al., 2008). Such viruses typically exhibit C-C chemokine receptor type 5 (CCR5)-dependence, mask functional envelope trimers needed to trigger antibody neutralization, and undergo rapid mutation as productive infection ensues (Goonetilleke et al., 2009; Keele et al., 2008). Taken together, these viral properties have direct implications in defining specific host innate and adaptive immune pathways that can efficiently defend against HIV-1 entry and productive infection, and in optimizing ways to elicit these responses at the site of exposure.
As a result of genetic sequence variability created by its error-prone reverse transcriptase as well as mutations selected by host immune pressure, HIV-1 has evolved into multiple subtypes or clades together with circulating recombinant forms (collected at http://www.hiv.lanl.gov). Because of this global diversity (up to 35% in envelope gp120) it may impossible to design a single vaccine candidate that can induce potent effector immunity to multiple key antigenic determinants among worldwide circulating, infecting HIV-1 strains.
State of the HIV-1 vaccine field
Following the identification of HIV-1 as the etiologic agent of AIDS, non-human primate models were established to examine vaccine effects following experimental retroviral challenge; the utility and limitations of these models in predicting vaccine efficacy have been well-described (Sodora et al., 2009). Since 1987, more than 30 candidate HIV-1 vaccines whose prototypes have elicited varying degrees of protective responses in non-human primate models have advanced to human clinical trials, alone or in combinations (Mascola and Montefiori, 2010; Ross et al., 2010). These include replication-competent or incompetent viral vectors (pox, adenovirus, alphavirus, adeno-associated virus) containing HIV-1 gene inserts; HIV-1 viral-like particles; HIV-1 DNA plasmids; and soluble HIV-1 proteins and peptides, with or without adjuvant formulations (Table 1). Prime-boost heterologous regimens have been employed to enhance the potency and breadth of antibody and T cell responses.
Table 1.
Overview of candidate HIV-1 vaccine regimen prototypes evaluated in clinical trials and summary of findings*
| Vaccine | Immunogenicity | Extended Evaluation |
|---|---|---|
| Recombinant Env gp120 proteins with adjuvants | ||
|
| ||
|
|
|
|
| ||
| HIV-1 DNA plasmid | ||
|
| ||
|
|
|
|
| ||
| Viral vectors | ||
|
| ||
|
|
|
|
| ||
| Prime-boost regimens | ||
|
| ||
|
|
|
See comprehensive summary in Ross et al, 2010. Phase IIb and III trials are highlighted in bold.
Those candidate regimens extended to large-scale international phase IIb or III studies (highlighted in Table 1) include the recombinant bivalent HIV-1 envelope gp120 vaccines, AIDSVAX B/E with alum (Vax003 trial) (Pitisuttithum et al., 2006) and AIDSVAX B/B with alum (Vax004 trial) (Flynn et al., 2005); the replication-incompetent adenovirus serotype 5 (Ad5) vectored HIV-1 trivalent vaccine (Step and Phambili trials) (Buchbinder et al., 2008; Gray, 2010); and the canarypox ALVAC-HIV vCP1521 plus AIDSVAX gp120 clades B and E (RV144 trial) (Rerks-Ngarm et al., 2009). Only the RV144 trial, evaluating a recombinant canarypox-HIV vector prime and recombinant HIV-1 envelope gp120 subunit protein plus alum boost in Thailand, showed low-level efficacy (31%) in reducing HIV-1 infection rates (Rerks-Ngarm et al., 2009). No regimen has induced vaccine responses associated with set-point viral load reduction following acute infection or other sustained clinical parameters related to improved outcomes.
Apart from safety, the overriding principles guiding HIV vaccine development have been the predicted ability of the immunogen to induce either HIV-1 neutralizing antibodies, HIV-1-specific CD8+ T cells, or both. Although there have been strong proponents of either antibodies or T cells alone as the most effective strategy, taking into account the correlates of immune protection against other viral pathogens as well as understanding the immune components that control HIV-1 in vivo, the consensus view now is that a highly effective vaccine will need to elicit coordinated B cell, CD4+ and CD8+ T cell responses. Moreover, it is anticipated that specific adjuvants and vectors can trigger innate immune signaling pathways that can improve the potency and quality of adaptive immunity. Recent studies in acute infection have emphasized the rapidly destructive nature of HIV-1, and the need for protective immune responses to be present concurrently with, if not before, HIV-1 transmission (Haase, 2010; McMichael et al., 2010).
Innate mechanisms altering HIV-1 vaccine immunity and protection
Although innate immune mechanisms contribute to HIV-1 control (Alter et al., 2007), it remains unclear if recapitulating these responses with a vaccine will enhance protection against HIV-1 acquisition. Understanding how innate cells capture and present HIV-1 antigens through vector delivery and adjuvant formulations is receiving growing interest, with investigations ongoing to identify chemoattractants that recruit inflammatory cells at the vaccination site, promote dendritic cell (DC) activation and maturation, optimize antigen intracellular presentation or cross-presentation, and delineate innate cell populations and signaling pathways for selectively inducing long-term B versus T cell responses.
Natural killer (NK) cells provide the first wave of attack against viral infection, mediating antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI) and secreting antiviral cytokines and chemokines. HIV-1-infected subjects expressing NK receptors KIR3DS1 and KIR3DL1 in conjunction with HLA-B Bw480I have slower HIV-1 disease progression, and expansions of these NK cell populations have been observed in acute infection (Alter et al., 2009). NK cells can also contribute to recall responses and thus may serve as memory cells in some viral infections (Sun et al., 2010). Efforts are underway to investigate the contribution of NK cells to the low-level protective effect observed in the RV144 trial; conceivably, NK cells bound to HIV-1 envelope (Env) antibodies may have mediated ADCC.
A variety of host genetic determinants impacting HIV-1 co-receptor interactions, replication and immune responsiveness can contribute to altered HIV-1 susceptibility or disease progression that may occur independent of, or contribute to, vaccine effects (Fellay et al., 2007; O’Brien and Nelson, 2004). To date, two genetic studies have shown relevance to HIV vaccine efficacy— polymorphisms at gene loci encoding FcγRIIa and FcγRIIIa influenced the degree to which ADCVI responses to gp120 predicted rate of infection in the Vax004 trial (Forthal et al., 2007), and the presence of certain protective or non-protective HLA-B alleles was associated with the magnitude of Gag-specific T cell responses in male vaccinees in the Step trial (Fellay et al., 2009). The extent to which these factors alter immune pathways and HIV-1 susceptibility should be taken into consideration in the evaluation of future HIV-1 vaccine candidates.
Role of Envelope Antibodies in HIV-1 Prevention
Neutralizing antibodies (nAbs) are correlates of protective immunity of all FDA approved vaccines against infectious agents. While both infection and HIV-1 envelope immunization can induce nAbs, those antibodies that broadly neutralize a wide range of HIV-1 strains are neither common nor of long duration. However, passive administration of rare human anti-Env monoclonal broadly neutralizing antibodies (bnAbs) with titers that can be achieved by immunization can protect against simian-human immunodeficiency chimeric virus (SHIV, containing some HIV-1 genes, including env, tat and rev, on a simian immunodeficiency virus [SIV] backbone) challenge in rhesus macaques (Hessell et al., 2007; Hessell et al., 2009a; Hessell et al., 2009b; Mascola, 2002; Mascola and Montefiori, 2010; Montefiori and Mascola, 2009). Thus, a major goal of HIV-1 vaccine development is to design immunogens capable of inducing antibodies that can broadly neutralize HIV-1 (Mascola and Montefiori, 2010; Stamatatos et al., 2009).
One major obstacle in HIV-1 vaccine development is constructing Env immunogens with immunogenic broadly neutralizing epitopes. First, conserved Env epitopes targeted by bnAbs are poorly immunogenic because they either are masked by carbohydrates (Binley et al., 2010; Wei et al., 2003), appear transiently (Frey et al., 2008), are sterically hindered (Labrijn et al., 2003; Schief et al., 2009) or must overcome entropy for antibody binding (Kwong et al., 2002). Second, Env glycans are antigenically similar to host carbohydrates (Astronomo et al., 2008). Third, some Env protein epitopes have homologies with self proteins, and when immunogenic, induce polyreactive antibodies that may be subjected to tolerance mechanisms (Haynes et al., 2005; Verkoczy et al., 2010). By contrast, easily induced HIV-1 Env antibodies typically recognize type-specific neutralizing epitopes located on variable loops (Davis et al., 2009; Moore et al., 2009) or recognize dominant non-neutralizing, conserved epitopes in gp120 (Palker et al., 1987) or gp41 (Gnann et al., 1987). Many of these non-neutralizing dominant epitopes are present only on structurally non-native Env. Finally, even when antibodies can neutralize the infecting strain, their effect is transient because escape mutations are selected (Richman et al., 2003; Wei et al., 2003). Thus, immunization of non-human primates and humans with HIV-1 Env monomers or trimers has failed to induce antibodies recognizing broadly neutralizing envelope epitopes. Rather, induced antibodies neutralize the strain represented in the immunogen sequence or easily neutralized strains (termed Tier 1 strains); only sporadic and weak neutralization is observed for difficult to neutralize (Tier 2 and Tier 3 strains) primary HIV-1 isolates that are representative of clinically relevant field isolates (reviewed in (Gilbert et al., 2010; Mascola and Montefiori, 2010) and references therein).
Specificities of Broadly Neutralizing Antibodies
Approximately 20% of chronically HIV-1 infected subjects have of nAbs that neutralize multiple HIV-1 strains, and about 2-4% of chronically infected subjects have serum antibodies that broadly neutralize most HIV-1 strains tested (Simek et al., 2009). Even though these subjects can make bnAbs, they are not made until months to years after infection. Thus, bnAbs that develop in the context of chronic HIV-1 infection do not protect from disease progression (Euler et al., 2010).
Broad reactivities can be explained either by many different specificities that contribute to breadth (Scheid et al., 2009), or by only one or a few antibody specificities that are responsible for breadth (Walker et al., 2010; Wu et al., 2010). An important development of the past year has been the application of recombinant human monoclonal antibody (mAb) isolation and cloning to HIV-1 vaccine development (Hicar et al., 2010; Liao et al., 2009; Scheid et al., 2009; Walker et al., 2009; Wu et al., 2010). Table 2 summarizes the specificities of representative types of bnAbs and their targets, and Figure 1 shows a structural model of HIV-1 Env trimer and locations of conserved nAb epitopes. The specificities of broad neutralizing antibodies can be grouped into four categories: CD4 binding site; gp41 membrane proximal external region (MPER); quarternary V2, V3 loop; and carbohydrate.
Table 2.
Characteristics of human antibodies capable of broadly neutralizing HIV-1
| Antibody | Specificity | Isotype | HCDR3 length |
VH, VK/VL Family |
VH% mutated | Polyreactivity |
|---|---|---|---|---|---|---|
| 2F5 | gp41 MPER | IgG3 | 24 | 2-5, K1-13 | 12.4% | Yes |
| 4E10 | gp41 MPER | IgG3 | 20 | 1-69, K3-20 | 6.3% | Yes |
| 2G12 | Env glycans | IgG1 | 16 | 3-21, K1-5 | 19.6% | Reacts with fungal carbohydrates |
| 1b12 | CD4 binding site |
IgG1 | 20 | 1-3, K3-20 | 11.8% | Yes |
| VRC01 | CD4 binding site |
IgG1 | 14 | 1-2, K3D-15 | 31.2% | No |
| HJ16 | Core/CD4 binding site/DMR |
IgG1 | 21 | 3-3, K4-1 | 13.7% | N/A |
| PG9 | Quaternary gp120 (V2, V3) |
IgG1 | 30 | 3-33, L2-14 | 12.8% | No |
| PG16 | Quaternary gp120 (V2, V3) |
IgG1 | 30 | 3-33, L2-14 | 11.9% | No |
N/A, not available. Data on HJ16 courtesy of personal communication from Davide Corti and Antonio Lanzavecchia.
Figure 1. Unliganded Model of the HIV-1 Envelope Trimer.
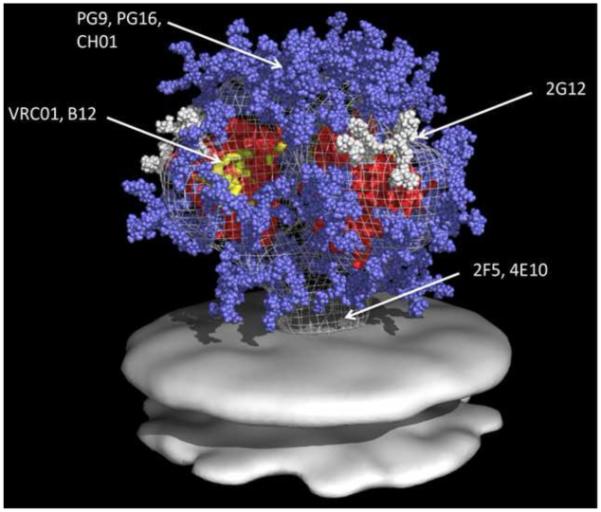
All glycans on gp160 are shown. Glycans are light blue and white, core gp120 is red, 2G12 epitopes are white (arrow), B12 and VRC01 epitopes are yellow (arrow), and the location of the quarternary epitope involving V2 and V3 loops of mAbs PG9 and PG16 is indicated. Image courtesy of Dr. William Schief, University of Washington, Seattle, WA, adapted with permission from (Schief et al., 2009).
The prototype gp120 mAb, 1b12, binds to gp120 at its CD4 binding site, surrounded and protected by N-linked glycans (reviewed in (Schief et al., 2009)). A recent antibody specificity, designated CD4bs/DMR/core, binds near the CD4 binding site, depends on gp120 amino acids D474, M475 and R476 for reactivity, and can have considerable breadth (Pietzsch et al., 2010). Wu and colleagues designed a resurfaced stabilized gp120 core to mimic the CD4 binding site where bnAbs but not weak or non-neutralizing antibodies bind. Using this resurfaced core as a fluorescent-labeled probe to capture antigen-specific memory B cells, they isolated a CD4 binding site mAb, VRC01, that neutralized 91% of tested primary isolate Env pseudoviruses, exhibiting the broadest neutralization activities of any antibody isolated to date (Wu et al., 2010). Structural analyses of the CD4 binding site bound with mAbs 1b12 (neutralizing), F105 (non-neutralizing), and VRC01 (very broadly neutralizing) revealed the narrow site to which these antibodies must bind (reviewed in (Schief et al., 2009; Zhou et al., 2010)).
Two prototype membrane-proximal external region (MPER) gp41 nAbs are 2F5 and 4E10. 2F5 binds to a core target at ELDKWA in the heptad repeat-2 region of gp41, while 4E10 binds to the NWFDIT sequence at the insertion of the gp41 stalk into the virion lipid membrane (Cardoso et al., 2005; Ofek et al., 2004). These antibodies bind to both lipid-MPER peptide complexes and to HIV virions (Alam et al., 2009) in a two-step conformation change model wherein they bind first to virion lipids, then surf the viral membrane while awaiting transient exposure of their neutralizing epitopes during fusion with a target cell.
Antibodies directed to a dominant linear epitope at the tip of the gp120 V3 loop are commonly made. While potent against Tier 1 HIV-1 strains and T-cell line adapted (easy to neutralize) HIV-1, most V3 loop antibodies cannot neutralize either Tier 2 and 3 primary strains or transmitted founder viruses (Davis et al., 2009). Zolla-Pazner isolated V3-specific human mAbs with a degree of breadth from chronically infected HIV-1 subjects and identified an antibody, 2909, that recognizes a strain-specific quaternary epitope involving gp120 V2 and V3 loops in the context of Env trimer (reviewed in (Zolla-Pazner and Cardozo, 2010)). Two new bnAbs, PG9 and PG16, also recognize quaternary gp120 V2 and V3 region epitopes, is dependent on V2 N-linked glycosylation sites and neutralize ~80% of HIV-1 primary isolates (Pancera et al., 2010; Walker et al., 2009).
The 2G12 Env carbohydrate mAb is unusual in two aspects. First, it is the only purely glycan-targeted bnAb isolated thus far. Second, it has a unique domain-swap structure such that its Fab region is comprised of a heavy and light chain from two members of a 2G12 dimer (Calarese et al., 2003; Scanlan et al., 2002). The glycans on gp120 are the result of host cell post-translational modifying glycosidases, and therefore resemble host carbohydrates (Scanlan et al., 2002), with oligomannose residues comprising most glycans on CD4+ T cell-derived virions (Doores et al., 2010). The glycans recognized by 2G12 comprise a unique conformational epitope of oligomannose glycans that is non-immunogenic (Astronomo et al., 2008). Thus, the HIV-1 envelope has at least four conserved regions, each with overlapping epitopes that can be targets for broad neutralizing antibodies. Many recombinant envelopes are antigenic and bind broad neutralizing antibodies, but for the reasons outlined below, recombinant envelopes are not immunogenic for these types of antibodies.
Characteristics of Broad Neutralizing Antibodies
HIV-1 bnAbs have unusual characteristics that include either long heavy chain complementarity determining region 3 (HCDR3), polyreactivity, or high levels of mutations (Table 2). Compelling evidence exists for immunoregulatory mechanisms that control production of B cells with immunoglobulin genes containing long HCDR3 regions (Meffre et al., 2001; Shiokawa et al., 1999). B cells with long hydrophobic HCDR3s appear to be eliminated at the naïve B cell stage (Meffre et al., 2001; Shiokawa et al., 1999).
Polyreactivity is a normal component of the antibody repertoire; in the pre-selection bone marrow repertoire, up to 40% of antibodies are polyreactive, while in the post-selection (post-tolerance) repertoire, only 15-20% of antibodies are polyreactive (Wardemann et al., 2003). In transgenic mice, polyreactive double-stranded DNA autoantibodies are either deleted in bone marrow or modified in the periphery by tolerance mechanisms (Chen et al., 1995). By contrast, the immune regulatory mechanisms for somatic hypermutation levels are unclear. Nonetheless, the mean number of mutations in a secondary immune response such as against influenza is approximately 5%, in contrast to the generally higher number of mutations in HIV-1 broad neutralizing antibodies (Table 2).
Many rare bnAbs are polyreactive and react with a number of host and other non-Env molecules, including host and virion lipids (Haynes et al., 2005). Approximately 70% of recombinant memory anti-Env B cells derived from rare subjects with broad neutralizing antibodies are polyreactive and react with host and other non-HIV antigens (M. Nussenzweig, personal communication).
Polyreactivity of HIV-1 antibodies can be functionally important. Lipid polyreactivity of gp41 nAbs is required for neutralization (Alam et al., 2009). An antibody binding to the CD4-inducible CCR5 binding site on gp120 has been found that also uses adjacent bound CD4 as a part of the CD4i epitope (Diskin et al., 2010). Because of the low number of Env trimer spikes on the HIV-1 virion, the likelihood of bivalent antibody-virion binding in the absence of antibody polyreactivity is small (Klein and Bjorkman, 2010). Nussenzweig and Mouquet have demonstrated that induction of polyreactive antibodies by HIV-1 facilitates Env antibody bivalent binding (avidity) on virions by allowing antibody cross-linking via Env and virion-host molecules (M. Nussenzweig, personal communication). Thus, the hypothesis is that the host needs to induce polyreactive antibodies, in effect, to overcome HIV-1 escape from antibody avidity (Klein and Bjorkman, 2010). In the majority of subjects, tolerance mechanisms would then limit expression of high affinity HIV-1 antibodies that could broadly neutralize (Haynes et al., 2005). In this regard, mice that only express the 2F5 variable heavy (VH) immunoglobulin chain regulate 2F5 VH expression by both central and peripheral tolerance mechanisms (Verkoczy et al. 2009).
The construction of inferred reverted unmutated ancestor antibodies (RUAs) that are candidates for being the germline antibodies of naïve B cell precursors of bnAbs has demonstrated that in some cases the RUAs of bnAbs do not react with the same epitopes as the highly mutated observed descendant antibodies (Pancera et al., 2010; Xiao et al., 2009; Zhou et al., 2010). Thus, induction of bnAbs may be complicated by the need for antigens other than targets of the somatically mutated bnAb to stimulate affinity maturation of the appropriate naïve B cell clone to recognize conserved Env regions (Alam et al., 2010; Bonsignori et al., 2010; Dimitrov, 2010; Xiao et al., 2009; Zhou et al., 2010).
The search for correlates of protection in the RV144 trial
Preliminary analyses of the canarypox ALVAC-HIV vCP1521 plus AIDSVAX gp120 B/E (RV144) trial demonstrate that binding antibodies to clade B and E gp120s were present in 99% of vaccinated subjects, but titers waned approximately 90% over 20 weeks (J. Kim and M. De Souza, personal communication). ADCC with HIV-1 clade B and E gp120-coated targets were detected in ~75% of vaccinees for clade B and ~25% for clade E; as with binding antibodies, titers were not stable and waned over 20 weeks (M. De Souza and J. Kim, personal communication). Neutralizing antibodies targeted a subset of Tier 1 and 2 viruses and were less potent than the failed Vax003 and Vax004 trials testing gp120 without the ALVAC-HIV prime (D. Montefiori, personal communication). Interestingly, in Vax004 (clade B gp120MN and gp120GNE8), the vaccine induced robust gp120 binding and neutralization antibody levels to the Tier 1 strain HIV-1 MN, but neutralization breadth for Tier 2 HIV-1 isolates was weak and sporadic, consistent with the lack of observed protection (Gilbert et al., 2005; Graham and Mascola, 2005).
Thus, a correlate of protection in RV144 may be a short-lived antibody response that can neutralize or block an early phase of the HIV-1 mucosal transmission event (Figure 2). Other considerations for immune correlates are CD8+ and CD4+ T-cell responses as well as induction of innate immune factors such as NK cells or anti-HIV-1 chemokines (DeVico and Gallo, 2004). Because the Vax003 trial of clades B and E gp120 alone in Thai intravenous drug users failed (Pitisuttithum et al., 2006), the protective effect in RV144 could be due to a short-lived immune response induced by ALVAC alone, or due to synergy of ALVAC with clades B and E gp120.
Figure 2. Steps in mucosal HIV-1 transmission potentially amenable to intervention by vaccination.
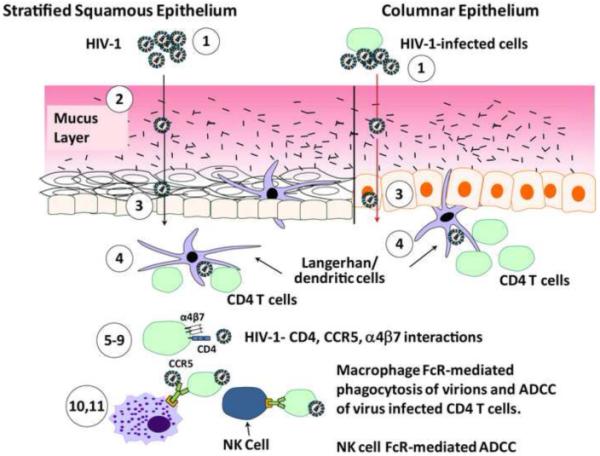
Steps are keyed to assays listed in Table S1. Antibodies that score positive in in vitro assays and protect against SHIV acquisition in in vivo passive protection trials in non-human primates are candidates for correlates of protective immunity in the RV144 vaccine trial. Free virions or virions from infected cells can be aggregated by antibodies (1) and their movement through the mucus layer inhibited (2). Possible sites of transmission are female vagina and cervix (squamous epithelium), endocervix and rectum (columnar epithelium) as well as male rectum (columnar epithelium and forskin (squamous epithelium). Transcytosis through either squamous or columnar epithelium may be blocked by antibodies (3), as can the transfer of DC-bound virions to CD4+ T cells (4). Various assays measure the ability of antibody to block CD4+ T cell and monocyte-macrophage (not shown) infection such as inhibition of gp120 binding to α4β7 (5), pseudovirus infection inhibition (not shown) (6), PBMC infection inhibition (not shown) (7), syncytium inhibition (not shown) (8), and cell-to-cell infection inhibition (4 and implied in 9). ADCVI and ADCC, as well as phagocytosis assays of opsonized virions, can measure Fc receptor mediated anti-HIV activities. Antibody and/or immune complexes bound to IgG FcR-bearing immune cells such as NK cells and/or monocyte macrophages can also release anti-HIV-1 chemokines and other factors.
A number of in vitro assays are available to study naturally occurring and vaccine-induced antibody effector functions, described in Table S1 and highlighted in Figure 2, to show where in HIV-1 transmission these functions may be useful. It is unclear which assays will identify protective antibodies against HIV-1 infection in humans. A critical step to defining correlates of protection in RV144 is to identify serum HIV-1 antibody activity, use fluorescent-labeled gp120 Env as a probe to sort memory B cells making such antibodies, and then test these antibodies alone or in combination for efficacy in rhesus macaque passive protection trials with CCR5-utilizing (R5) SHIVs (Mascola, 2002). Additional R5 SHIVs are critically needed for the non-human primate validation studies. Thus, the search is on to unravel the immune responses induced by the RV144 vaccine and to deduce the nature of the immune responses responsible for the modest protection seen, in order to begin to rationally improve on immunogen design.
What antibody titers are needed to protect?
In SHIV models, high titers of nAbs are needed to protect against high-dose intravenous challenges (Mascola, 2002). However, Hessell et al. demonstrated that a serum titer of 1:1 in macaques after infusion of the broad neutralizing anti-carbohydrate antibody 2G12 was able to protect from intravaginal SHIV challenge (Hessell et al., 2009b). Whereas protection from high-dose intravaginal SHIV challenge required relatively high serum titers of the CD4 binding site antibody 1b12, far lower serum titers of 1b12 can protect against low-dose intravaginal SHIV challenge (Hessell et al., 2009a). At low serum dilutions, 90% neutralization is a benchmark to be attained by new vaccine candidates; the Vax003 and Vax004 gp120 trials did not achieve this benchmark. Therefore, if protective antibodies can be induced, the necessary plasma titers will be sufficiently low so as to be attainable by vaccination.
Easily Induced HIV-1 Envelope Antibodies
Unlike the initial CD8+ T cell response in acute HIV-1 infection that rapidly selects for escape mutants (Cao et al., 2003; Goonetilleke et al., 2009), the initial antibody response to Env, although virion-binding, does not have neutralizing activity and does not drive escape mutation. The first autologous neutralizing antibody response does not develop until approximately three months after transmission (McMichael et al., 2010; Richman et al., 2003; Wei et al., 2003). This autologous response occurs in virtually all infected subjects, is usually specific for the transmitted (founder) virus, and is frequently targeted to variable Env regions (Moore et al., 2009). A major goal of HIV-1 vaccine development is to determine whether autologous nAb targets fall into groups or patterns such that a polyvalent immunogen might be made to target a sufficient number of founder viruses.
IgM and IgA multimeric antibodies can aggregate virions and inhibit movement through mucous and epithelial layers (Hladik and Hope, 2009). Others bind to Fc receptors on monocytes, NK cells and other hematopoietic cells to either mediate killing of infected targets via ADCC (Florese et al., 2009), inhibit HIV-1 via ADCVI (Forthal et al., 2005; Hladik and Hope, 2009), or bind to FcRs and induce anti-HIV-1 chemokines or other antiviral factors (DeVico and Gallo, 2004). Using the CD4 binding site antibody 1b12 in passive protection studies in non-human primates, Fc receptor binding but not complement binding activity was necessary for protection against vaginal SHIV challenge (Hessell et al., 2007). Thus for both neutralizing and non-neutralizing anti-HIV-1 activity, antibody binding to Fc receptors is important for optimal anti-HIV-1 activity.
Fc piece glycosylation is required for an antibody to bind to Fc receptors and for mediation of effector functions (Jefferis et al., 1995). Antibodies from HIV-1 infected subjects have glycan profiles similar to those associated with chronic inflammation (e.g., autoimmune diseases), characterized by agalactosylated antibodies that are less effective at binding FcR (Moore et al., 2005). HIV-1 elite controllers have elevated ADCC-mediating antibody titers compared to chronic or acutely infected subjects (Lambotte et al., 2009). ADCC and ADCVI activity of serum antibodies, as well as transcytosis-blocking activity in mucosal secretions, correlated with control of viral load following vaccination with replicating recombinant adenovirus followed by boosting with recombinant gp140 Env (Xiao et al., 2010). Whether non-neutralizing ADCC- or ADCVI-mediating antibodies can protect against HIV-1 acquisition remains to be determined.
Antibodies present in mucosa but not in blood may block HIV-1 transit across epithelial layers (Horton et al., 2009; Tudor et al., 2009; Xiao et al., 2010). Recent studies have found subjects with HIV-1 co-receptor CCR5 autoantibodies at mucosal sites that are capable of down-regulating CCR5 and blocking HIV-1 infection in vitro; a subset of these subjects have mucosal (cervical fluid or seminal plasma) anti-Env IgA that is not present in the plasma (Tomaras et al., 2010). These observations imply that multiple HIV-1 exposures can induce host immunity and that the mucosal immune system can respond with local antibodies that are independent of systemic antibody responses.
Thus, it is critical to determine if any of the above-mentioned easy-to-induce antibody types, if present before transmission, can protect. If not, then the effort must be focused entirely on induction of broadly neutralizing antibodies.
Impact of T cell immunity on natural HIV infection: considerations for HIV-1 vaccines
A central theme of HIV-1 vaccine design has been to elicit antiviral CD8+ cytotoxic T lymphocytes (CTL) to control HIV-1 replication and CD4+ T cells that can help induce and maintain CD8+ and B cell responses. Extensive experimental evidence in macaque SIV vaccine challenge models and correlative data in human HIV-1 infection indicate that CD8+ T cells play a pivotal role in controlling viremia during acute infection, that they exert immune selective pressure early in infection, and that the resulting escape variants frequently demonstrate reduced viral fitness (reviewed in (Goulder and Watkins, 2008)). Further, the association of HIV-1 disease progression with distinct class I MHC molecules is linked to CD8+ CTL recognition of specific HIV-1 epitopes (Dinges et al., 2010). T-cell based vaccines targeting specific Gag epitopes have been a common approach, as responses to these have been associated with lower viral loads in population-based studies (Kiepiela et al., 2007) and conserved regions in Gag are critical for viral fitness (Troyer et al., 2009).
The mere presence or magnitude of anti-viral CD8+ T cells is not sufficient to control acute or chronic HIV-1 infection. Moreover, CD8+ T cell responses were induced in vaccine recipients in the Step trial, but the response rate and magnitude at the peak immunogenicity time point after vaccination were not substantially different in vaccinated individuals who became HIV-infected versus matched vaccinated controls (McElrath et al., 2008). A threshold level of T cell responses, recognizing one or more epitopes, may be necessary to provide an efficient recall response at the initial site of infection, and conceivably this level was not attained in the Step trial vaccine recipients because the vaccine regimen might have been poorly immunogenic. Thus, more extensive anti-viral functional analyses of effector and central memory CD8+ T cells have now assumed greater importance (Table S2). Attributes ascribed to a protective phenotype are polyfunctional cytokine production (e.g., IFN-γ, TNF-α, IL-2, and/or MIP-1β), high proliferative capacity, cytolytic potential, avidity, suppression of HIV-1 replication from infected cells, recognition of specific epitopes restricted by protective HLA-B alleles, long-lived central memory cells, and effector memory cells acting rapidly at sites of mucosal exposure (Almeida et al., 2007; Horton et al., 2006; Migueles et al., 2008; Saez-Cirion et al., 2007). Additionally, molecular signatures consistent with T cell exhaustion and antiviral control will be important to assess in vaccine studies to ensure that vaccine designs induce memory T cells with favorable qualities (Day et al., 2006; Trautmann et al., 2006). The assays to measure these activities are in place, some validated and all standardized, and outlined in Table S2.
Although HIV-specific CD4+ T cells are induced during acute HIV-1 infection, their helper function is compromised (Malhotra et al., 2001). Their protective role is suggested in the less virulent HIV-2 infection, since maintenance of HIV-2-specific CD4+ T cells correlates with lower HIV-2 viremia (Zheng et al., 2004). HIV-1 preferentially infects HIV-specific T cells, and thus a major concern is that CD4+ T cells induced by vaccination can serve as immediate HIV-1 targets, particularly since most if not all immunogenic candidate HIV vaccines evaluated induce HIV-specific CD4+ T cells. However, to date no evidence exists that CD4+ T cell activation or vaccine-induced CD4+ T cells result in heightened HIV-1 acquisition or viremia post-infection (McElrath et al., 2008; Rerks-Ngarm et al., 2009). In SIV-challenged rhesus macaques, data suggest that CD8+ T cell function has a greater impact on viremia than the activation status of CD4+ target cells (Okoye et al., 2009). However, although postulated from in vitro studies (Benlahrech et al., 2009), whether vaccine vectors such as Ad5 heighten activation of memory CD4+ T cells that preferentially home to mucosal sites of HIV-1 exposure in vivo remains unanswered. Certainly, CD4+ T cell help is critical for generating effective, long-lived CD8+ and B cell memory responses, for efficiently directing T cell responses to the mucosa, and for exerting direct antiviral activities.
Of note, vaccine-induced CD4+ lymphoproliferation was the most substantial detectable T cell response elicited by the prime-boost regimen in the RV144 trial. Investigations are in progress to comprehensively define the phenotypic and functional properties of these T helper responses, as outlined in Table S2. Moreover, because CD4+ T cell responses were also elicited by gp120 plus alum vaccine regimens similar to those in the Vax003 and Vax004 studies (McElrath et al., 2000), understanding the role of the canarypox vector prime in shaping the CD4+ response may lend further insight into understanding RV144 correlates of immune protection.
Analysis of T cell responses elicited by candidate HIV vaccines and their contribution to protection
The development of validated assays to detect vaccine-induced HIV-specific T cell responses has afforded the opportunity to reliably quantify and identify epitope specificities of multiple cytokine-secreting CD4+ and CD8+ T cells from cryopreserved PBMC obtained from study participants worldwide. These procedures also have permitted immunogenicity comparisons between vaccine regimens evaluated in separate trials, and more importantly, have provided the opportunity to conduct case-cohort control immune correlates analyses in Phase IIb-III trials. Two validated assays, IFN-γ ELISpot and intracellular cytokine staining by flow cytometry, were applied in the evaluation of vaccine-induced T cells in the Step trial (McElrath et al., 2008). Importantly, the remarkably high response rates (90% in male vaccinees after three immunizations), the observation that two-thirds of responding T cells expressed two or more antiviral cytokines, and the persistence of the CD8+ T cell responses after one year indicates that these measurements, although useful as screening assays for T cell immunogenicity, did not predict vaccine efficacy in the Step trial (McElrath et al., 2008).
Another potential explanation for why protection against HIV-1 acquisition did not occur in the Step trial is that the number and specificity of epitopes recognized by vaccine-induced T cells was not adequate to mount immune pressure on the infecting viral strains. CD8+ T cells from Step vaccinees recognized a median of 1-2 epitopes, in contrast to rhesus macaques who recognize a mean of 12 epitopes from Gag, Pol and Nef when given similar vaccine regimens that are protective (Wilson et al., 2009). Recent viral sequence analysis of early founder viruses in cases receiving vaccine in contrast to those receiving placebo prior to infection indicates that the Step vaccine did exert substantial immune pressure (Rolland et al., 2009). Ongoing longitudinal sequence analysis will lend insight into whether the vaccine effect was lost soon after acute infection and will determine the impact of protective HLA-B and KIR alleles on post-infection viremia. Taken together, the lack of efficacy in the Step trial may be attributed to insufficient potency, antiviral activities, and breadth of epitope recognition. As a consequence of the findings in Step and RV144, in future trials it will be important to evaluate more comprehensively the functional properties and the epitope specificities of the T cells induced by vaccination (Table S2).
Improved vaccine adjuvants and delivery
Recent advances in unraveling the molecular interactions in the innate immune signaling pathway hold promise for new adjuvant formulations to enhance immunogenicity of HIV-1 immunogens. Alum and MF59 adjuvants have been most widely used with envelope gp120 immunogens in phase II or III studies (Table 1). Other adjuvants formulated with proteins or peptides evaluated in phase I studies include incomplete Freund’s adjuvant, muramyl dipeptide (MDP), MTP-PE, monophosphoryl lipid A (MPL), QS21, AS02a, liposomes with lipid-A, and lipopeptides. While some adjuvants generated 2-3-fold increased antibody titers and CD4+ T cell responses in contrast to alum or MF59, these effects waned with repeated dosing, and increased local and systemic reactogenicity was observed with some preparations (Evans et al., 2001; McElrath, 1995). Of note, these studies were carried out when less sophisticated immunological assays were available to define phenotypic and functional differences in B and T cell responses. Adjuvants (CRL1005) and cytokines (IL-12, IL-15) have also been employed with HIV-1 DNA plasmids (Table 1), but HIV-1-specific IFN-γ-secreting T cells were not substantially enhanced by these studies (Asmuth et al., 2010). However, preliminary findings hold promise that delivery of DNA by electroporation may improve immunogenicity (Vasan et al., 2009).
Increasing the pace of adjuvant development for HIV-1 vaccines is challenging. Optimizing the relative adjuvant-antigen dose in humans is rarely feasible because of financial and time constraints, and must rely on predictions from preclinical studies or empiric selection. Comparative studies are usually restricted to products held by one sponsor. Formulation of newer adjuvants with HIV-1 envelope immunogens to retain its antigenic structure, particularly conformational neutralizing epitopes, is an important requirement. Nevertheless, Phase I studies are in development to evaluate poly IC-LC (TLR3-MDA5 signaling) and synthetic gamma-linolenic acid (TLR4 ligand) as protein adjuvants, as well as targeting Gag protein to DCs through the DEC-205 receptor (Carter, 2010; Longhi et al., 2009). These approaches to varying degrees may enhance antibody avidity, improve CD4+ T cell help, and prime CD8+ T cells through cross-presentation. Incorporating systems biology assessments of the earliest innate immune responses within hours to the first week following immunization will also accelerate understanding the key properties that lead to durable, protective B and T cell responses (Pulendran, 2009).
Improving vaccine breadth with mosaic and conserved vaccine inserts
Due to high diversity of circulating HIV-1 strains, vaccines using sequences from reference strains might be insufficient for protection, given that T cell responses to only a few epitopes are observed. A mosaic vaccine strategy using in silico algorithms to recombine vaccine sequences may improve coverage of sequences found in circulating HIV-1 strains (Fischer et al., 2007). The first experimental studies in rhesus macaques look encouraging and suggest that the mosaic approach may elicit broad T cell responses in the context of many vaccine regimens (Barouch et al., 2010; Santra et al., 2010). Other groups have pursued designs featuring conserved HIV-1 regions representing multiple clades (Letourneau et al., 2007) and HIV-1 M group strains (Rolland et al., 2007) to circumvent induction of responses to variable regions that can readily escape CTL pressure. Taken together, these approaches will have the greatest utility if they can efficiently broaden CD4+ T cell, CD8+ T cell as well as B cell responses in clinical trials.
Developing alternative vaccine vectors
Adenovirus-based vaccine vectors have many desirable properties, but since the Step trial results were announced there has been concern that inclusion of subjects with Ad5 neutralizing antibodies prior to vaccination with an Ad5 vector could have a negative impact on immunogenicity as well as increase potential risk for infection, particularly among uncircumcised males (Buchbinder et al., 2008). If these concerns are borne out, there is a need for alternate adenovirus vectors based on serotypes with low prevalence in target populations. Efforts thus far have primarily focused on human adenoviruses Ad26 and Ad35 and several chimpanzee adenoviruses (reviewed in (Barouch, 2010)).
Poxvirus vectors have a long successful history in the veterinary field and feature many of the same potential benefits as adenovirus-based vectors in carrying gene inserts and in inducing T cell responses. Concentrated efforts are underway to develop and improve several poxvirus vectors, including ALVAC as deployed in the Thai trial, MVA, NYVAC and fowlpox (Pantaleo et al., 2010).
While both adenovirus- and poxvirus-based vectors are effective at stimulating CD4+ and CD8+ T cell responses, those responses tend to peak rapidly and attenuate after vaccination. An intriguing alternate strategy to maintain differentiated effector memory T cells uses a cytomegalovirus (CMV)-based vector. A rhesus CMV vaccine containing SIV gene inserts was able to persistently infect rhesus macaques and maintain robust SIV-specific CD4+ and CD8+ T cell responses. Although local mucosal infection occurred in most vaccinated animals following low-dose mucosal SIV challenge, protection from systemic infection was observed for extended periods in the majority of animals (Hansen et al., 2009). Additional studies are needed to further assess the mechanisms of SIV protection with this or other persistent vectors that could more rapidly arm the mucosa with effector T cells.
Perspectives and Conclusions
The obstacles of designing an HIV-1 vaccine that can induce protective immunity are complex and daunting. Despite nearly three decades of searching for a vaccine, we do not know the specific immune components sufficient to protect against HIV-1 and we do not know how best to elicit responses that we predict will be protective. Clues from findings in non-human primate SIV vaccine models and from persons who exhibit unusual control of HIV-1 replication have not translated into vaccine efficacy in humans. The Step trial elicited the desired HIV-1-specific CD8+ T cells, yet the trial failed. In contrast, the RV144 trial, which did not elicit these responses, seemed to modestly protect. Moreover, it has been generally assumed that vaccine-induced protection against HIV-1 acquisition would require potent bnAbs, but the RV144 vaccine regimen does not appear to have induced bnAbs despite showing a low-level reduction in infection rates.
Where does the field go from here? For induction of protective antibodies the main research directions are to define ways to induce bnAbs systemically and mucosally, to determine if any easily induced antibodies can interrupt transmission, and to understand why HIV-1 Env antibodies are short-lived in comparison to other viral envelope antibodies. The path to induction of bnAbs will come from understanding the B cell regulatory and tolerance pathways of anti-Env antibody production, and from design of optimal immunogens targeted at naïve and memory B cell populations that can respond to HIV-1 envelope. The path to determining correlates of protection mediated by easily induced antibodies that act by Fc receptor-mediated anti-HIV-1 activity or by blocking the earliest events in transmission will come from isolating human mAbs with various anti-HIV-1 activities (Table 2) and testing their ability to prevent HIV-1 acquisition in passive transfer non-human primate studies. The path to induction of long-lived systemic mucosal antibodies lies in the understanding of, and optimizing, the B cell germinal center anti-Env antibody response.
For induction of protective T cells, the critical research areas will be directed toward in-depth understanding of the functional profiles that CD8+ T cells require to restrict early infection and how to target these effectors to the mucosa, defining new strategies to increase breadth for CD4+ and CD8+ T cell epitope recognition, and identifying the properties of CD4+ T cells that can simultaneously stimulate the quality and persistence of protective CD8+ T cell and B cell responses.
Finally, over the next five years, the field will strive to replicate and improve on the RV144 trial results by: 1) conducting a detailed immune correlates analysis of the RV144 trial, 2) completing the HVTN 505 trial evaluating the effect of a DNA-prime Ad5-boost regimen, including multiclade env inserts, on reducing post-infection viral set point, 3) evaluating the utility of mosaic vaccine designed to elicit increased T and B cell breadth, 4) defining effects of new adjuvant formulations on innate, T and B cell responses, 5) conducting immunogenicity comparative studies on the effectiveness of alternate serotype adenovirus and novel pox vectors for eliciting improved quality and breadth of T cell and antibody responses, and 6) conducting detailed study into the biology of vaccine induction of protective responses targeting mucosal anti-HIV-1 immune responses. Coordination between non-human primate vaccine challenge studies and human vaccine clinical trials will be essential to advance new vaccine concepts into the product development pipeline, and to accelerate the pace of initiating future HIV-1 vaccine efficacy trials.
Highlights.
Efficacy trial results suggest need for new approaches to define immune correlates
Recently isolated broad neutralizing antibodies point to new targets for vaccines
Tolerance controls blocking broad neutralizing antibody expansion have been found
Strategies to improve T-cell breadth and quality are moving to clinical trials
Supplementary Material
Acknowledgments
The authors thank the following colleagues for discussions, preliminary data or review of the manuscript: S. Voght, N. Frahm, L. Corey, S. Self, S. De Rosa, P. Gilbert, J. Hural, A. McMichael, M. Cohen, D. Goldstein, N. Letvin, J. Sodroski, G. Shaw, A. Lanzavecchia, D. Corti, G. Alter, G. Tomaras, G. Kelsoe, D. Dimitrov, L. Verkoczy, A. Moody, H.-X. Liao, M. Alam, M. Sekaran, and J. Arthos. We thank W. Schief for Figure 1. We apologize to colleagues whose publications we were unable to cite due to space limitations, and are grateful for all of their exceptional work done in the effort to develop an HIV-1 vaccine. This work was supported by the HIV Vaccine Trials Network Laboratory Program and the Center for HIV/AIDS Vaccine Immunology (CHAVI), funded by the NIH, NIAID, Division of AIDS; and by Collaboration for AIDS Vaccine Discovery (CAVD) grants to BFH and MJM, funded by the Bill and Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Tomaras GD, Liao HX, Parks R, Meyerhoff R, Jaeger F, Foulger A, Donathan M, Lucas JT, Kelsoe G, et al. Reverted unmutated ancestor VH2-5 alleles of Mab 2F5 differentially bind gp41 Env and intestinal bacteria: a potential genetic basis for B cell responses to gp41 neutralizing determinants. AIDS Vaccine 2010, abstract. 2010 [Google Scholar]
- Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Rihn S, Walter K, Nolting A, Martin M, Rosenberg ES, Miller JS, Carrington M, Altfeld M. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmuth DM, Brown EL, DiNubile MJ, Sun X, del Rio C, Harro C, Keefer MC, Kublin JG, Dubey SA, Kierstead LS, et al. Comparative cell-mediated immunogenicity of DNA/DNA, DNA/adenovirus type 5 (Ad5), or Ad5/Ad5 HIV-1 clade B gag vaccine prime-boost regimens. J Infect Dis. 2010;201:132–141. doi: 10.1086/648591. [DOI] [PubMed] [Google Scholar]
- Astronomo RD, Lee HK, Scanlan CN, Pantophlet R, Huang CY, Wilson IA, Blixt O, Dwek RA, Wong CH, Burton DR. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–6368. doi: 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–390. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlahrech A, Harris J, Meiser A, Papagatsias T, Hornig J, Hayes P, Lieber A, Athanasopoulos T, Bachy V, Csomor E, et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci U S A. 2009;106:19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol. 2010;84:5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Hwang KK, Chen X, Tsao C-Y, Bilska M, Fang W, Marshall DJ, Whitesides J, Crump JA, Noel S, et al. Immunoregulation of HIV-1 Broadly neutralizing antibody responses: deciphering maturation paths for antibody induction. AIDS Vaccine 2010, abstract. 2010 [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Cao J, McNevin J, Malhotra U, McElrath MJ. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J Immunol. 2003;171:3837–3846. doi: 10.4049/jimmunol.171.7.3837. [DOI] [PubMed] [Google Scholar]
- Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Carter D, Reed SG. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010;5:409–413. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, Nason MC, Martin JE, Rucker S, Andrews CA, Gomez PL, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B-cell deletion. Nature. 1995;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- Cooney EL, McElrath MJ, Corey L, Hu SL, Collier AC, Arditti D, Hoffman M, Coombs RW, Smith GE, Greenberg PD. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, Graham BS, Keefer MC, Pinter A, Morris L, et al. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009;387:414–426. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. MAbs. 2010;2:347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges WL, Richardt J, Friedrich D, Jalbert E, Liu Y, Stevens CE, Maenza J, Collier AC, Geraghty DE, Smith J, et al. Virus-specific CD8+ T-cell responses better define HIV disease progression than HLA genotype. J Virol. 2010;84:4461–4468. doi: 10.1128/JVI.02438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin R, Marcovecchio PM, Bjorkman PJ. Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity. Nat Struct Mol Biol. 2010;17:608–613. doi: 10.1038/nsmb.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci U S A. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis. 2010;201:1045–1053. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, Keefer MC, Kallas EG, Corey L, Gorse GJ, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19:2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Cirulli ET, McElrath MJ, Casimiro DR, Geraghty DE, Frahm N, Goldstein DB. A genome-wide association study of host genetic determinants of T cell responses to the MRKAd5 HIV-1 gag/pol/nef vaccine in the STEP trial. Retrovirology. 2009;6:P398. doi: 10.1093/infdis/jiq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Phan TB, Becerra J. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J Virol. 2005;79:2042–2049. doi: 10.1128/JVI.79.4.2042-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, D’Souza P, Rodriguez-Chavez IR, Decamp A, Giganti M, et al. Magnitude and Breadth of a Nonprotective Neutralizing Antibody Response in an Efficacy Trial of a Candidate HIV-1 gp120 Vaccine. J Infect Dis. 2010;202:595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- Gnann JW, Jr., Schwimmbeck PL, Nelson JA, Truax AB, Oldstone MB. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987;156:261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Mascola JR. Lessons from failure--preparing for future HIV-1 vaccine efficacy trials. J Infect Dis. 2005;191:647–649. doi: 10.1086/428406. [DOI] [PubMed] [Google Scholar]
- Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009a;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009b;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicar MD, Chen X, Briney B, Hammonds J, Wang JJ, Kalams S, Spearman PW, Crowe JE., Jr. Pseudovirion particles bearing native HIV envelope trimers facilitate a novel method for generating human neutralizing monoclonal antibodies against HIV. J Acquir Immune Defic Syndr. 2010;54:223–235. doi: 10.1097/QAI.0b013e3181dc98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- Horton H, Frank I, Baydo R, Jalbert E, Penn J, Wilson S, McNevin JP, McSweyn MD, Lee D, Huang Y, et al. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J Immunol. 2006;177:7406–7415. doi: 10.4049/jimmunol.177.10.7406. [DOI] [PubMed] [Google Scholar]
- Horton RE, Ball TB, Wachichi C, Jaoko W, Rutherford WJ, McKinnon L, Kaul R, Rebbapragada A, Kimani J, Plummer FA. Cervical HIV-specific IgA in a population of commercial sex workers correlates with repeated exposure but not resistance to HIV. AIDS Res Hum Retroviruses. 2009;25:83–92. doi: 10.1089/aid.2008.0207. [DOI] [PubMed] [Google Scholar]
- Jefferis R, Lund J, Goodall M. Recognition sites on human IgG for Fc gamma receptors: the role of glycosylation. Immunol Lett. 1995;44:111–117. doi: 10.1016/0165-2478(94)00201-2. [DOI] [PubMed] [Google Scholar]
- Karim QA, Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010 doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, et al. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172) J Infect Dis. 2010;201:600–607. doi: 10.1086/650299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Klein JS, Bjorkman PJ. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 2010;6:e1000908. doi: 10.1371/journal.ppat.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. Aids. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, Hanke T. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007;2:e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, et al. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods. 2009;158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra U, Holte S, Dutta S, Berrey MM, Delpit E, Koelle DM, Sette A, Corey L, McElrath MJ. Role for HLA class II molecules in HIV-1 suppression and cellular immunity following antiretroviral treatment. J Clin Invest. 2001;107:505–517. doi: 10.1172/JCI11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine. 2002;20:1922–1925. doi: 10.1016/s0264-410x(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- McElrath MJ. Selection of potent immunological adjuvants for vaccine construction. Semin Cancer Biol. 1995;6:375–385. doi: 10.1016/1044-579x(95)90007-1. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, Corey L, Montefiori D, Wolff M, Schwartz D, Keefer M, Belshe R, Graham BS, Matthews T, Wright P, et al. AIDS Vaccine Evaluation Group A phase II study of two HIV type 1 envelope vaccines, comparing their immunogenicity in populations at risk for acquiring HIV type 1 infection. AIDS Res Hum Retroviruses. 2000;16:907–919. doi: 10.1089/08892220050042846. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig MC, Schiff C. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J Clin Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC, Mascola JR. Neutralizing antibodies against HIV-1: can we elicit them with vaccines and how much do we need? Curr Opin HIV AIDS. 2009;4:347–351. doi: 10.1097/COH.0b013e32832f4a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. Aids. 2005;19:381–389. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- Moore PL, Gray ES, Morris L. Specificity of the autologous neutralizing antibody response. Curr Opin HIV AIDS. 2009;4:358–363. doi: 10.1097/COH.0b013e32832ea7e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet. 2004;36:565–574. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, Jr., et al. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker TJ, Matthews TJ, Clark ME, Cianciolo GJ, Randall RR, Langlois AJ, White GC, Safai B, Snyderman R, Bolognesi DP, et al. A conserved region at the COOH terminus of human immunodeficiency virus gp120 envelope protein contains an immunodominant epitope. Proc Natl Acad Sci U S A. 1987;84:2479–2483. doi: 10.1073/pnas.84.8.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, McLellan JS, Wu X, Zhu J, Changela A, Schmidt SD, Yang Y, Zhou T, Phogat S, Mascola JR, Kwong PD. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84:8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS. 2010;5:391–396. doi: 10.1097/COH.0b013e32833d1e87. [DOI] [PubMed] [Google Scholar]
- Pietzsch J, Scheid JF, Mouquet H, Klein F, Seaman MS, Jankovic M, Corti D, Lanzavecchia A, Nussenzweig MC. Human anti-HIV-neutralizing antibodies frequently target a conserved epitope essential for viral fitness. J Exp Med. 2010 doi: 10.1084/jem.20101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9:741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3:e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Tovanabutra S, Gilbert PB, anders-Buell E, Heath L, deCamp AC, Magaret CC, Bose M, Bradfield A, O’Sullivan A, et al. Evidence of vaccine-induced changes in breakthrough HIV-1 strains from the Step trial. Retrovirology. 2009;6:O42. [Google Scholar]
- Ross AL, Brave A, Scarlatti G, Manrique A, Buonaguro L. Progress towards development of an HIV vaccine: report of the AIDS Vaccine 2009 Conference. Lancet Infect Dis. 2010;10:305–316. doi: 10.1016/S1473-3099(10)70069-4. [DOI] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Schief WR, Ban YE, Stamatatos L. Challenges for structure-based HIV vaccine design. Curr Opin HIV AIDS. 2009;4:431–440. doi: 10.1097/COH.0b013e32832e6184. [DOI] [PubMed] [Google Scholar]
- Shiokawa S, Mortari F, Lima JO, Nunez C, Bertrand FE, 3rd, Kirkham PM, Zhu S, Dasanayake AP, Schroeder HW., Jr. IgM heavy chain complementarity-determining region 3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J Immunol. 1999;162:6060–6070. [PubMed] [Google Scholar]
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Lucas JT, Yates NL, Shen X, Ashley VC, Moody MA, Liao HX, Vandergrift N, Cohen M, Montefiori DC, et al. Mucosal HIV specific IgA immune responses in CHAVI002 exposed and uninfected subject. 2010 [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Troyer RM, McNevin J, Liu Y, Zhang SC, Krizan RW, Abraha A, Tebit DM, Zhao H, Avila S, Lobritz MA, et al. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 2009;5:e1000365. doi: 10.1371/journal.ppat.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera MM, Vittorino RM, Caskey M, Andersen J, et al. In vivo electroporation enhances the immunogenicity of ADVAX, a DNA-based HIV-1 vaccine candidate, in healthy volunteers. Retrovirology. 2009;6:O31. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, Liang X, Wang F, Thoryk E, Heidecker GJ, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83:6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational Design of Envelope Identifies Broadly Neutralizing Human Monoclonal Antibodies to HIV-1. Science. 2010 doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, Zhang MY, Longo NS, Dimitrov DS. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng NN, Kiviat NB, Sow PS, Hawes SE, Wilson A, Diallo-Agne H, Critchlow CW, Gottlieb GS, Musey L, McElrath MJ. Comparison of human immunodeficiency virus (HIV)-specific T-cell responses in HIV-1- and HIV-2-infected individuals in Senegal. J Virol. 2004;78:13934–13942. doi: 10.1128/JVI.78.24.13934-13942.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid J, Shi W, Xu L, et al. Structural Basis for Broad and Potent Neutralization of HIV-1 by Antibody VRC01. Science. 2010 doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cardozo T. Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat Rev Immunol. 2010;10:527–535. doi: 10.1038/nri2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


