Abstract
Abnormal lung inflammation and oxidant burden are associated with a significant reduction in histone deacetylase 2 (HDAC2) abundance and steroid resistance. We hypothesized that Nrf2 regulates steroid sensitivity via HDAC2 in response to inflammation in mouse lung. Furthermore, HDAC2 deficiency leads to steroid resistance in attenuating lung inflammatory response, which may be due to oxidant/antioxidant imbalance. Loss of antioxidant transcription factor Nrf2 resulted in decreased HDAC2 in lung, and increased inflammatory lung response which was not reversed by steroid. Thus, steroid resistance or inability of steroids to control lung inflammatory response is dependent on Nrf2-HDAC2 axis. These findings have implications in steroid resistance, particularly during the conditions of oxidative stress when the lungs are more susceptible to inflammatory response, which is seen in patients with chronic obstructive pulmonary disease, asthma, rheumatoid arthritis, and inflammatory bowel disease.
Keywords: Cigarette smoke, oxidant, antioxidants, Nrf2, inflammation
Introduction
Inhaled corticosteroids remain the therapy of choice for inflammatory diseases, such as asthma and chronic obstructive pulmonary disease (COPD). However, inhaled or systemic corticosteroids fail to attenuate chronic inflammation in these patients due to increased oxidative stress [1–3]. Corticosteroids suppress inflammation by recruiting histone deacetylase 2 (HDAC2) to NF-κB-driven pro-inflammatory gene promoters thereby inhibiting the transcription of these genes [4]. Thus, the loss of corticosteroid ability to suppress inflammation in COPD and asthma may be due to the loss of HDAC2 protein [5]. However, the mechanism for steroid resistance via HDAC2 reduction in oxidative stress-prone condition in vivo is not known.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) regulates the cellular antioxidant response by upregulating genes involved in augmenting cellular antioxidant capacity and by inducing the genes that detoxify reactive oxygen species or electrophilic compounds [6,7]. The level of Nrf2 are decreased in conditions of oxidative stress [8–10], which accounts for persistent and abnormal inflammation due to imbalance of oxidants/antioxidants in the lung [10–12]. Mice lacking Nrf2 gene are susceptible to cigarette smoke (CS)-induced pulmonary inflammation and emphysema [13]. Hence, it is possible that abnormal inflammation seen in these mice is oxidant-dependent and resistance to steroids perhaps due to oxidant-mediated reduction in HDAC2 levels.
We hypothesized that Nrf2 regulates steroid sensitivity via HDAC2 in response to inflammation in mouse lung. Furthermore, HDAC2 deficiency leads to steroid resistance in attenuation of lung inflammatory response triggered by increased oxidative stress. We tested this hypothesis in mice lacking Nrf2 and HDAC2 to determine the ability of corticosteroids to inhibit lung inflammatory response induced by CS and LPS.
Materials and Methods
Materials
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma (St. Louis, MO). HDAC2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal β-actin antibody was obtained from Calbiochem (La Jolla, CA).
Mice
The Nrf2 knockout (KO) mouse strain (Nrf2−/− on C57BL/6J background) used in this study is described earlier [5] and was generously supplied by Prof. Masayuki Yamamoto, University of Tsukuba, Japan via the RIKEN BioResource Center, Tsukuba, Japan. HDAC2 mutant (HDAC2−/− on C57BL/6J background) mice were kindly provided by Dr. J.A. Epstein (University of Pennsylvania School of Medicine, Philadelphia, PA) [14]. These mice express a truncated and catalytically inactive form of HDAC2 with exons 9 – 14 replaced by a LacZ fusion gene created via a gene-trap method [14]. Wild-type (WT) C57BL/6J mice were purchased from Jackson laboratories (Bar Harbor, ME). All animal protocols for this study were approved by the University Committee on Animal Research of the University of Rochester.
Cigarette smoke and lipopolysacharide (LPS) exposure to mice
Eight to ten week-old adult wild-type (WT), Nrf2−/− and HDAC2−/− were exposed to diluted mainstream CS generated from 3R4F filtered research grade cigarettes as described previously [15,16] for 1 h twice daily with 1 h interval in between for 3 days using a Baumgartner-Jaeger CSM2072i cigarette smoke-generating machine (CH Technologies, Westwood, NJ). Smoke concentration and airflow were adjusted to obtain a constant particulate matter concentration of 300 mg/m3 total particulate matter (TPM) [15,16]. Mice exposed to filtered air served as controls. Mice were sacrificed at 24 h post-last CS exposure.
LPS exposure studies in mice were performed as described previously [17]. Briefly, age-matched WT, Nrf2−/− and HDAC2−/− were exposed to aerosolized Escherichia coli LPS (1 mg/ml) for 8 minutes. Aerosolized saline-exposed mice were used as controls, and animals were sacrificed at 24 h post last exposure.
Corticosteroid treatment
Budesonide in dry-powered form was dissolved in 70% ethanol and then diluted with saline prior to administration. Twenty-five microliters of budesonide solution, corresponding to 1 or 3 mg/kg body weight, was administered via an intranasal route to each mouse for 3 days followed by LPS exposure at 1 h after last budesonide treatment [18].
Bronchoalveolar lavage
Mice were anaesthetized by pentobarbital (Abbott Laboratories, Abbott Park, IL) intraperitoneal injection (100 mg/kg body weight) before sacrifice. Lungs were then removed and lavaged three times with 0.6 ml of 0.9% sodium chloride with cannula inserted into the trachea. Total lavage fluid for each mouse was combined and then centrifuged. Supernatants were frozen at −80°C until required for further analysis and cell pellets resuspended in 1 ml of saline, and total number of cells determined using a haemocytometer. Differential counts (minimum 500 per slide) were determined using Diff-Quik (Dade Behring, Newark, DE)-stained cytospin slides.
Lung tissue protein extraction
Cytoplasmic and nuclear proteins were extracted from frozen lung tissue samples as described [19]. Whole cell lysate was extracted from lung tissue after homogenization in RIPA buffer [15,16].
Immunoblotting
Protein estimation was performed by the bicinchoninic acid (BCA) method as described by the manufacturer (Pierce, Rockford, IL). Mouse lung nuclear extracts (10 μg–20 μg) were electrophoresed on 7.5% SDS-polyacrylamide gels, electro-blotted on PVDF membranes (Millipore, Burlington, MA). Membranes were incubated with primary antibody in a 1:1000 dilution in 5% BSA in TBS. After extensive washing, primary antibodies were detected with secondary antibodies linked to horse-radish peroxidase (Dako, Carpinteria, CA) and bound complex detected with enhanced chemiluminescence (PerkinElmer, Waltham, MA).
Cytokine Analysis
Monocyte chemotactic protein 1 (MCP-1) levels were measured from mouse lung soluble protein by enzyme-linked immunosorbent assay (ELISA) with the duo-antibody kit (R & D Systems). Results were normalized to protein concentration per sample.
HDAC2 activity assay
HDAC2 was immunoprecipitated from lung homogenates (500 μg protein) by incubating overnight with anti-HDAC2 antibody (2 μg). Beads were washed and incubated with Color de Lys substrate (Biomol) for 80 minutes with rocking at 37 °C. 30 μL aliquots from each sample were placed in 96-well plates and HDAC specific buffer added. Color de Lys developer was then added and incubated for a further 20 minutes with rocking at 37 °C. Color development was monitored at 405 nm. HDAC2 activity was expressed relative to standard curve generated from 0 – 500 μM Color de Lys deacetylated standard.
Statistical analysis
Data expressed as mean±SEM. Statistical significance was calculated using one-way Analysis of Variance (ANOVA) with STATVIEW software. NIH ImageJ software was used for densitometry analysis. P<0.05 as significant compared to relative controls.
Results
HDAC2−/− mice are more susceptible to CS-induced lung inflammation
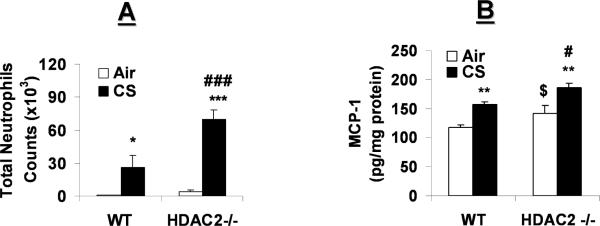
CS exposure induced neutrophil influx in bronchoalveolar lavage fluid (BALF) of WT mice, which was significantly increased in HDAC2−/− mice (Fig. 1A). Consistent with this finding, air- and CS-exposed HDAC2−/− mice showed significant increase in MCP-1, a monocyte chemoattractant (Fig. 1B); and KC, a neutrophil chemoattractant (data not shown), release in the lungs as compared to corresponding air- and CS- exposed WT mice, suggesting the susceptibility of HDAC2 deficient mice to CS-induced lung inflammation.
Fig. 1. HDAC2−/− mice are more susceptible to CS-induced lung inflammation.
WT and HDAC2−/− mice were exposed to filtered air or acute CS for 3 days. Mice were sacrificed at 24 h post-last exposure, and lungs were lavaged. Bronchoalveolar lavage cells were prepared on cytospin slides and stained with Diff-Quik. (A) Total number of neutrophils in BALF. (B) MCP-1 levels were measured in mouse lung tissue by ELISA. Data are shown as mean±SEM (n=4 to 6). *P<0.05, **P<0.01, ***P<0.001 significant compared with corresponding air groups. #P<0.05, and ###P<0.001 compared with corresponding WT mice exposed to CS. $P<0.05, compared with air-exposed WT mice.
HDAC2−/− mice are less responsive to budesonide in inhibiting LPS-induced lung inflammation
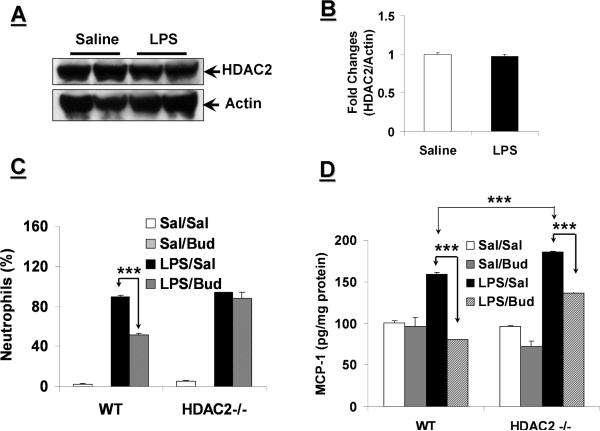
LPS was used as a different lung inflammation-triggering agent rather than CS since HDAC2 is inactivated and degraded in response to CS exposure both in mouse lungs in vivo, and in epithelial cells and macrophages in vitro [19–22], whereas LPS aerosolization in WT mice had no effect on HDAC2 protein levels in mouse lung (Figs. 2A and B). Not surprisingly, neutrophil counts in BALF were significantly reduced in budesonide-pretreated LPS-exposed WT mice as compared to saline-pretreated LPS-exposed WT mice (Fig. 2C).
Fig. 2. HDAC2−/− mice are less responsive to budesonide in response to LPS-induced lung inflammation.
(A) WT mice were exposed to saline or aerosolized LPS (1 mg/ml). Nuclear extracts from mouse lungs were separated on SDS-PAGE gel, and HDAC2 protein levels were determined by immunoblotting. (B) Band intensity of HDAC2 levels normalized to actin. (C and D) WT and HDAC2−/− mice were pretreated with budesonide (3 mg/kg body weight) for 3 days at 1 h prior to aerosolization of LPS. (C) Neutrophils in BALF were determined following differential staining on cytospin slides. (D) MCP-1 levels were measured from lung homogenates of mouse lung tissue by ELISA. Data are shown as mean±SEM (n=4 to 6). ***P<0.001, significant compared with corresponding controls as denoted in figure. Sal: Saline; Bud: Budesonide.
Budesonide pretreatment had no effect on neutrophil counts in BALF of HDAC2−/− mice (Fig. 2C). However, pretreatment of budesonide significantly lowered the MCP-1 (Fig. 2D) and KC (data not shown) release in WT mouse lung in response to LPS exposure. The levels of MCP-1 in lungs of HDAC2−/− mice were reduced by budesonide in response to LPS exposure (Fig. 2D). However, the efficacy of budesonide in decreasing MCP-1 release in HDAC2−/− mice (26.4%) was lower than that in WT mice (49.4%, P<0.01). Budesonide, however, had no appreciable effect on KC release in response to LPS exposure in HDAC2−/− mice (data not shown). Consistent with the MCP-1 and KC data, matrix metalloproteinase 9 (MMP9) activity was significantly elevated in HDAC2−/− mouse lungs compared to WT lungs with only a minimal effect of budesonide treatment observed (data not shown). Overall, these data suggest that HDAC2 deficiency leads to steroid insensitivity in terms of attenuating lung inflammatory response.
Enhanced lung inflammation is associated with HDAC2 reduction in Nrf2−/− mice exposed to CS
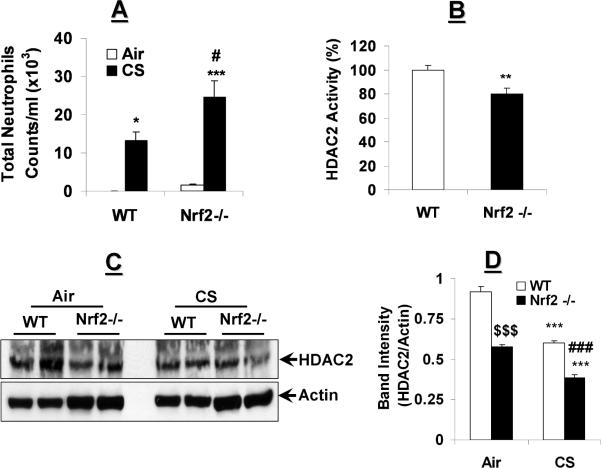
We determined whether mice deficient in the antioxidant transcription factor, Nrf2, would be more susceptible to CS-mediated oxidative stress to cause lung inflammation. Neutrophil influx, as a marker for increased vascular permeability and pulmonary inflammation, was increased in lungs of Nrf2−/− mice as compared to WT mice in response to 3 days CS exposure (Fig. 3A).
Fig. 3. Increased lung inflammation is associated with HDAC2 reduction in Nrf2−/− mice exposed to CS.
(A) WT and Nrf2−/− mice were exposed to CS for 3 days. Differential counts from BALF were determined by Diff-Quik staining of cytospin slides. (B) Immunoprecipitated HDAC2 from lungs of naïve WT and Nrf2−/− mice were analyzed for deacetylase activity using specific HDAC deacetylase activity kit. (C) WT and Nrf2−/− mice were exposed to filtered air or whole body CS for 3 days. Lung tissue nuclear extracts were analyzed for HDAC2 relative expression. (D) HDAC2 levels normalized to actin expression. Data are shown as mean±SEM (n=4 to 9). *P<0.05, **P<0.01, and ***P<0.001, significant compared with corresponding air-exposed controls. #P<0.05 and ###P<0.001, significant compared with CS-exposed WT controls. $$$P<0.001, compared with air-exposed WT mice.
HDAC2 protein level and deacetylase activity are significantly decreased in response to CS-mediated oxidative stress in mouse lungs [19], therefore, we speculated that HDAC2 protein levels in lung may be altered in Nrf2−/− mice, which are susceptible to oxidative stress. Naïve Nrf2−/− mouse lung showed decreased HDAC2 activity compared to WT mouse lungs (Fig. 3B). To determine if this difference was due to accelerated loss of HDAC2 protein in lungs of Nrf2−/− mice, we compared HDAC2 protein abundance in nuclear extracts of Nrf2−/− and WT mice. Consistent with reduction in HDAC2 activity, the HDAC2 protein level was also significantly decreased in lung nuclear extracts of Nrf2−/− mice as compared to WT mice in the absence of CS exposure (Figs. 3C and D). Three days of CS exposure further decreased the levels of HDAC2 protein in Nrf2−/− mice compared to WT mice (Figs. 3C and D). These results suggest that the loss of HDAC2 in Nrf2 deficient mice is a crucial component of increased susceptibility to oxidative stress-induced inflammatory response in the lungs.
Nrf2−/− mice are not responsive to budesonide in inhibiting LPS-induced lung inflammation
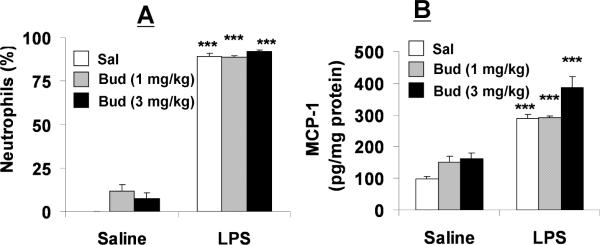
Decreased HDAC2 protein levels and deacetylase activity in naïve Nrf2−/− mice suggested that these mice may also exhibit resistance to steroid-mediated attenuation of lung inflammation. As shown in Figure 2C, budesonide (3 mg/kg) pretreatment attenuated lung neutrophil influx following LPS exposure by ~50% in WT mice. However, the neutrophils influx in BALF was not altered by pretreatment of budesonide (1 and 3 mg/kg) in Nrf2−/− mice in response to LPS exposure (Fig. 4A). Similarly, pretreatment of budesonide did not reduce LPS-induced release of MCP-1 (Fig. 4B) and KC (data not shown) release in Nrf2−/− mouse lung. These results suggest that the inability of steroids to inhibit lung inflammation possibly be due to HDAC2 reduction in lungs of Nrf2 deficient mice.
Fig. 4. Nrf2−/− mice are unresponsive to budesonide following LPS exposure.
Nrf2−/− mice were treated with budesonide (1 or 3 mg/kg body weight) by intranasal administration followed by LPS exposure. (A) Percentage of neutrophils in BALF was determined following differential staining on cytospin slides. (B) MCP-1 levels were measured in lung tissue homogenates by ELISA. Data are shown as mean±SEM (n=4 to 6). ***P<0.001 significant compared with corresponding controls. Sal: Saline; Bud: Budesonide.
Discussion
Although HDAC2 has been implicated in increased susceptibility to inflammation and steroid resistance in vitro [21,23], no such studies are available to determine the role of HDAC2 in vivo in lung in response to inhaled toxicants. In this study, we used mice expressing mutant HDAC2 [14,24,25] to study the steroid resistance in response to CS exposure. HDAC2−/− mice showed increased neutrophil recruitment in the lung in response to CS as compared to WT mice exposed to CS. Neutrophil recruitment was correlated strongly with the release of KC and MCP-1 in lung tissue suggesting the susceptibility of these mice to augmentation of CS-induced inflammation.
Since CS induces degradation of other HDACs (HDAC1 and HDAC3) that may be crucial for inflammation [21], it was important to determine a specific role for HDAC2 in steroid resistance utilizing an exposure model with no effect on HDAC levels or activity. Hence, we hypothesized that HDAC2−/− mice would exhibit a poor response to budesonide in response to LPS exposure. As expected, budesonide had no significant effect in reducing KC release in HDAC2−/− mice lungs in response to LPS. Furthermore, budesonide partially blocked MCP-1 release in the lungs of HDAC2−/− mice versus complete inhibition of MCP-1 in WT mice. Budesonide was also ineffective in reducing MMP9 activity in the mutant mice. Thus, these data suggest that basal HDAC2 deficiency leads to increased inflammatory response in the lung which is further augmented by LPS exposure. Furthermore, steroids have poor efficacy in HDAC2-ablated mice in response to pro-inflammatory challenge in the lung.
Increased susceptibility to oxidative stress (or deficiency of antioxidants) may exhibit phenotypes similar to HDAC2−/− mice in terms of steroid resistance. We used Nrf2−/− mice, which are susceptible to oxidative stress/cigarette smoke, to study the steroid resistance in controlling the lung inflammatory response. Disruption of Nrf2 enhanced susceptibility to acute CS-induced neutrophilic inflammation which is consistent with an earlier report [13]. Surprisingly, in air-exposed Nrf2−/− mice, neutrophil infiltration and RelA/p65 phosphorylation on ser276 and ser536 (unpublished data) were markedly enhanced in the lungs. It is possible that loss of Nrf2 leads to activation of NF-κB by increasing the pool of CREB-binding protein (CBP) available to interact with RelA/p65 [26,27], and thus causing induction of NF-κB-dependent pro-inflammatory genes. Our data suggest that Nrf2 deficiency led to increased lung neutrophil influx and basal activation of NF-κB, which are augmented by CS exposure.
The finding that loss of Nrf2 induces an oxidative-stress phenotype in the lungs led us to hypothesize that increased oxidative stress status of the lung in Nrf2−/− mice would induce a reduction in HDAC2 activity and level. Lungs from naïve Nrf2−/− mice showed marked reduction in HDAC2 abundance and subsequently reduced HDAC2 deacetylase activity compared to naïve WT mice. Consistent with these data, exposure of Nrf2−/− mice to CS rapidly accelerated the rate of HDAC2 degradation suggesting the oxidant burden in lungs of Nrf2−/− is a key factor in loss of HDAC2 with or without exposure to environmental toxicants. The reduction in levels of HDAC2 in Nrf2−/− mice alternatively suggests that ARE-mediated Nrf2 activation may be required for HDAC2 transcription. However, the molecular regulation of HDAC2 expression or transcription factor(s) binding site is not clearly known [25]. It may also be possible that nuclear Nrf2 stabilizes the HDAC2 co-repressor complex on pro-inflammatory genes, whereas deficiency of Nrf2 leads to degradation of HDAC2 and activation of NF-κB-CBP binding on pro-inflammatory promoters [26,27]. Our preliminary data support this contention showing activation of CBP in lungs of Nrf2 null mice (unpublished data).
We next determined whether the increased inflammatory response and decreased HDAC2 protein levels in Nrf2−/− mice render them resistant to steroids in inhibiting the inflammatory response. WT and Nrf2−/− mice were exposed to aerosolized LPS following intranasal budesonide treatment. LPS induced severe neutrophilic inflammation in mouse lungs (unlike macrophage influx by CS) with no subsequent reduction in HDAC2 protein expression. Pre-treatment of WT mice with a high dose of budesonide significantly blocked LPS-induced lung neutrophilic influx which is consistent with budesonide-mediated inhibition of lung tissue KC levels. However, LPS-exposed Nrf2−/− mice failed to respond to both low and high dose budesonide treatments in blocking neutrophil influx, attenuating KC and MCP-1 release, and inhibiting RelA/p65 nuclear accumulation (unpublished data) in the lung. The observation of steroid resistance in Nrf2−/− and HDAC2−/− mice with the finding that HDAC2 protein levels are diminished in Nrf2−/− mice suggests a strong in vivo linkage between HDAC2 abundance and steroid sensitivity via oxidative stress.
In conclusion, loss of HDAC2 is a critical factor in inhaled toxicant-mediated lung inflammation especially in regulating the anti-inflammatory effects of glucocorticoids in mouse lungs. Oxidative stress-susceptible Nrf2−/− mice showed reduced HDAC2 levels and deacetylase activity in lungs, and are susceptible to CS- and LPS-induced inflammation. Interestingly, the loss of Nrf2 potentially leads to steroid resistance due to HDAC2 reduction. We speculate that the high oxidant status in the lung of Nrf2−/− mice leads to inactivation of endogenous HDAC2 through post-translational modifications, such as nitrosylation of tyrosine residues and carbonylation of cysteine, histidine and lysine residues. This may then block the ability of ligand-bound glucocorticoid receptors to recruit active HDAC2 to promoters of pro-inflammatory genes. Nevertheless, the rapid loss of HDAC2 and NF-κB activation in CS-exposed Nrf2−/− mice suggest steroid resistance is clearly a multifactorial cascade of events that perhaps start with oxidant/antioxidant imbalance and then precipitates at a much more rapid decline in HDAC2. This may have implications for devising better therapies for patients who are refractory/insensitive to steroid treatments, such as patients with COPD, asthma, rheumatoid arthritis, and inflammatory bowel disease [5].
Research highlights.
HDAC2 is a critical factor in inhaled toxicant-mediated lung inflammation especially in regulating the anti-inflammatory effects of glucocorticoids.
Loss of Nrf2 resulted in decreased HDAC2 in lung, and increased inflammatory lung response which was not reversed by steroid.
Steroid resistance or inability of steroids to control lung inflammatory response is dependent on Nrf2-HDAC2 axis.
Steroid resistance via Nrf2-HDAC2 axis leads to abnormal inflammatory response which is seen in patients with chronic inflammatory diseases.
Understanding the redox regulation of Nrf2-HDAC2 may lead to better therapies for patients who are refractory/insensitive to steroid treatments.
Acknowledgements
This study was supported by the NIH 1R01HL085613, 1R01HL097751, 1R01HL092842, Institute for Science and Health, NIEHS Toxicology training grant ES-07026 and NIEHS Environmental Health Sciences Center Grant ES-01247. The Nrf2 knockout mouse strain (C57BL/6J background) was generously supplied by Prof. Masayuki Yamamoto, University of Tsukuba, Japan via the RIKEN BioResource Center, Tsukuba, Japan. We are thankful to Drs. J. A. Epstein and C. Trivedi (University of Pennsylvania School of Medicine, Philadelphia, PA) for providing the HDAC2 KO mice for this study. We thank Dr. G. Arunachalam and Suzy Bellanca for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155:542–548. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- [2].Hew M, Bhavsar P, Torrego A, Meah S, Khorasani N, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006;174:134–141. doi: 10.1164/rccm.200512-1930OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- [4].Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- [6].Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- [7].Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- [8].Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- [9].Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in Nrf2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [10].Garbin U, Fratta Pasini A, Stranieri C, Cominacini M, Pasini A, Manfro S, Lugoboni F, Mozzini C, Guidi G, Faccini G, Cominacini L. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS One. 2009;4:e8225–e8236. doi: 10.1371/journal.pone.0008225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harju T, Kaarteenaho-Wiik R, Soini Y, Sormunen R, Kinnula VL. Diminished immunoreactivity of gamma-glutamylcysteine synthetase in the airways of smokers' lung. Am J Respir Crit Care Med. 2002;166:754–759. doi: 10.1164/rccm.2112014. [DOI] [PubMed] [Google Scholar]
- [12].Tomaki M, Sugiura H, Koarai A, Komaki Y, Akita T, Matsumoto T, Nakanishi A, Ogawa H, Hattori T, Ichinose M. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm Pharmacol Ther. 2007;20:596–605. doi: 10.1016/j.pupt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [13].Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- [15].Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol. 2008;38:689–698. doi: 10.1165/rcmb.2007-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- [17].Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O'Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol. 2008;39:7–18. doi: 10.1165/rcmb.2007-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Southam DS, Ellis R, Wattie J, Young S, Inman MD. Budesonide prevents but does not reverse sustained airway hyperresponsiveness in mice. Eur Respir J. 2008;32:970–978. doi: 10.1183/09031936.00125307. [DOI] [PubMed] [Google Scholar]
- [19].Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009;40:464–473. doi: 10.1165/rcmb.2008-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, MacNee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- [21].Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- [22].Adenuga D, Rahman I. Protein kinase CK2-mediated phosphorylation of HDAC2 regulates co-repressor formation, deacetylase activity and acetylation of HDAC2 by cigarette smoke and aldehydes. Arch Biochem Biophys. 2010;498:62–73. doi: 10.1016/j.abb.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15:1110–1112. [PubMed] [Google Scholar]
- [24].Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, Floss T, Wurst W, Minucci S, Gottlicher M. Reduced body size and decreased intestinal tumor rates in HDAC2-mutant mice. Cancer Res. 2007;67:9047–9054. doi: 10.1158/0008-5472.CAN-07-0312. [DOI] [PubMed] [Google Scholar]
- [25].Kramer OH. HDAC2: a critical factor in health and disease. Trends Pharmacol Sci. 2009;30:647–655. doi: 10.1016/j.tips.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [26].Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [27].Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]