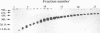Abstract
It has been shown that gamma interferon (IFN-gamma)-producing CD4+ T cells, which are generated only by immunization with viable bacteria, exert a significant role in protective immunity against mycobacteria in mice. In this study, we have tried to determine the antigen recognized by the T cells in search of a possible protective antigen. T cells from viable Mycobacterium bovis BCG-immunized mice were stimulated with several antigens, and IFN-gamma production was measured. Purified protein derivative and viable and killed BCG lysates caused significant IFN-gamma production, and almost the same level of IFN-gamma activity was detected in both groups stimulated with viable and killed BCG lysates. However, heat shock protein (HSP) 65 and HSP 70 were not a major antigen for IFN-gamma production. The antigen provoking IFN-gamma production is localized mainly in the membrane fraction of BCG cells, and the approximate molecular size was 18 kDa. On the other hand, T cells from killed BCG-immunized mice never responded to this antigen for IFN-gamma production, whereas they could mount a delayed-type hypersensitivity response. These results showed that the antigen provoking IFN-gamma production was present in killed as well as viable BCG. In addition to the antigen presentation by antigen-presenting cells, some kinds of differentiation factor (such as monokines) that are produced only by stimulation with viable cells seemed to be necessary for the development of IFN-gamma-producing T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Lu S., Abrams J. S., Wang E., Yamamura M., Modlin R. L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993 Aug;61(8):3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci S., Beschin A., Tuosto L., Ameglio F., Gandolfo G. M., Cocito C., Fiorucci F., Saltini C., Piccolella E. Mycobacterial antigen complex A60-specific T-cell repertoire during the course of pulmonary tuberculosis. Infect Immun. 1993 Feb;61(2):439–447. doi: 10.1128/iai.61.2.439-447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Hinman A. R., Dooley S. W., Fischl M. A., Sepkowitz K. A., Goldberger M. J., Shinnick T. M., Iseman M. D., Jacobs W. R., Jr Tuberculosis symposium: emerging problems and promise. J Infect Dis. 1993 Sep;168(3):537–551. doi: 10.1093/infdis/168.3.537. [DOI] [PubMed] [Google Scholar]
- Fifis T., Costopoulos C., Radford A. J., Bacic A., Wood P. R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991 Mar;59(3):800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Mitsuyama M., Muramori K., Tsukada H., Nomoto K. Interleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenes. Infect Immun. 1990 Dec;58(12):3973–3979. doi: 10.1128/iai.58.12.3973-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Mathur N. K., Job C. K., Nath I., Cohn Z. A. Effect of multiple interferon gamma injections on the disposal of Mycobacterium leprae. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8073–8077. doi: 10.1073/pnas.86.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
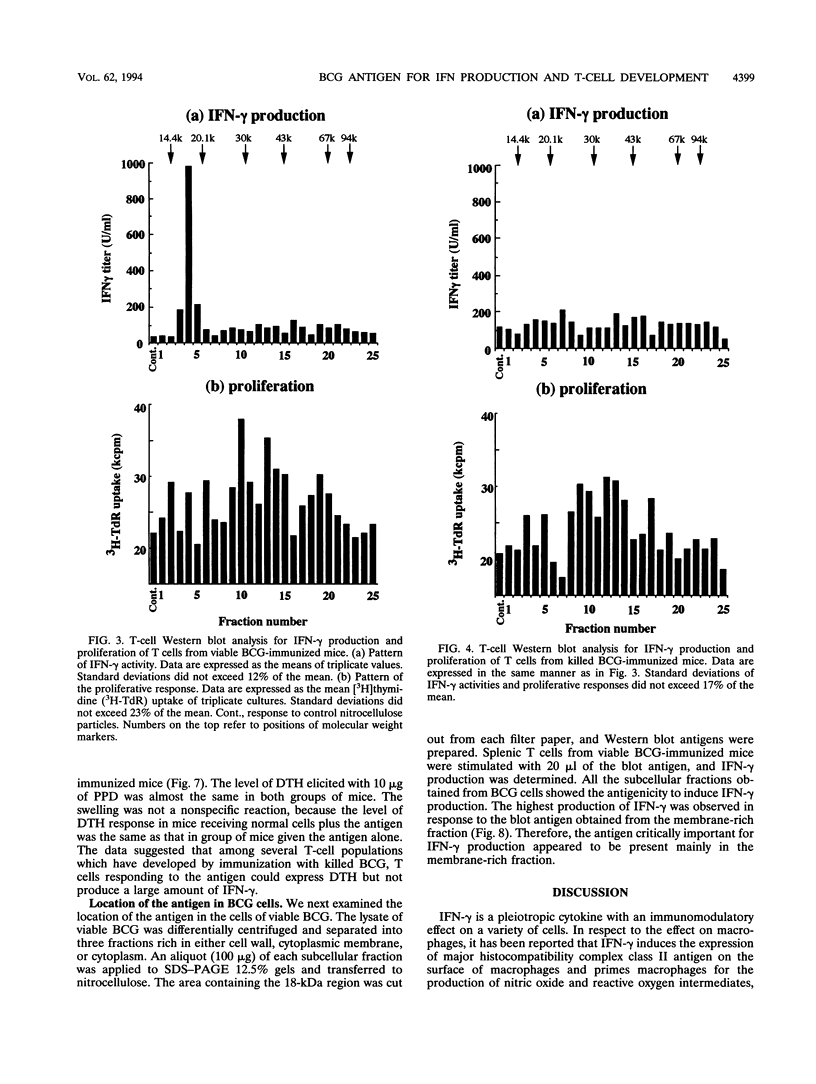
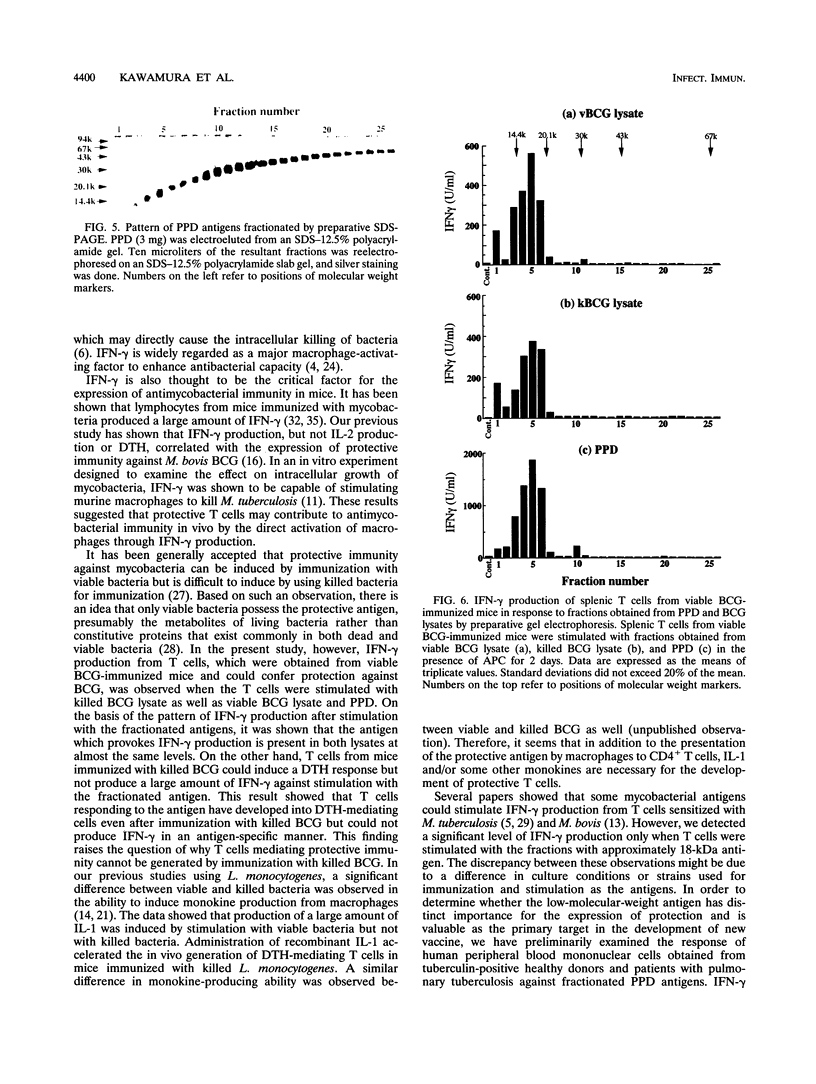
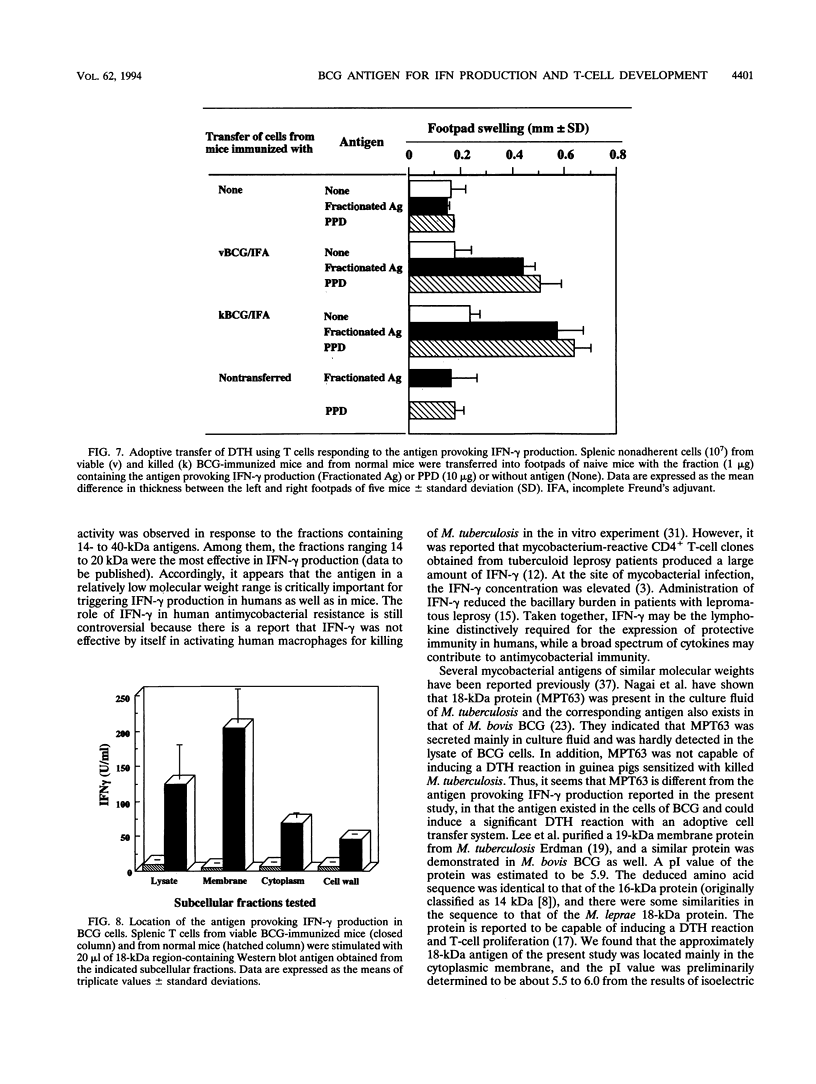
- Kawamura I., Tsukada H., Yoshikawa H., Fujita M., Nomoto K., Mitsuyama M. IFN-gamma-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992 May 1;148(9):2887–2893. [PubMed] [Google Scholar]
- Kingston A. E., Salgame P. R., Mitchison N. A., Colston M. J. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987 Dec;55(12):3149–3154. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Lee B. Y., Hefta S. A., Brennan P. J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992 May;60(5):2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvik M., Closs O. Repeated delayed-type hypersensitivity reactions against Mycobacterium lepraemurium antigens at the infection site do not affect bacillary multiplication in C3H mice. Infect Immun. 1982 May;36(2):768–774. doi: 10.1128/iai.36.2.768-774.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Igarashi K., Kawamura I., Ohmori T., Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990 May;58(5):1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Andersen P., Boom W. H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993 Jun;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- Singh I. G., Mukherjee R., Talwar G. P., Kaufmann S. H. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun. 1992 Jan;60(1):257–263. doi: 10.1128/iai.60.1.257-263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr The tuberculin skin test. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):108–118. doi: 10.1164/arrd.1982.125.3P2.108. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Kawamura I., Arakawa M., Nomoto K., Mitsuyama M. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect Immun. 1991 Oct;59(10):3589–3595. doi: 10.1128/iai.59.10.3589-3595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K. B., Butler R., Colston M. J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1992 Mar 15;148(6):1885–1889. [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]