Abstract
While the popularity of and expenditures for herbal therapies (aka “ethnomedicines”) have increased globally in recent years, their efficacy, safety, mechanisms of action, potential as novel therapeutic agents, cost-effectiveness, or lack thereof, remain poorly defined and controversial. Moreover, published clinical trials evaluating the efficacy of herbal therapies have rightfully been criticized, post hoc, for their lack of quality assurance and reproducibility of study materials, as well as a lack of demonstration of plausible mechanisms and dosing effects. In short, clinical botanical investigations have suffered from the lack of a cohesive research strategy which draws on the expertise of all relevant specialties.
With this as background, US and Chinese co-investigators with expertise in Traditional Chinese Medicine (TCM), botany, chemistry and drug discovery, have jointly established a prototype library consisting of 202 authenticated medicinal plant and fungal species that collectively represent the therapeutic content of the majority of all commonly prescribed TCM herbal prescriptions. Currently housed at Harvard University, the library consists of duplicate or triplicate kilogram quantities of each authenticated and processed species, as well as “detanninized” extracts and sub-fractions of each mother extract. Each species has been collected at 2–3 sites, each separated geographically by hundreds of miles, with precise GPS documentation, and authenticated visually and chemically prior to testing for heavy metals and/or pesticides contamination. An explicit decision process has been developed whereby samples with the least contamination were selected to undergo ethanol extraction and HPLC sub-fractionation in preparation for high throughput screening across a broad array of biological targets including cancer biology targets. As envisioned, the subfractions in this artisan collection of authenticated medicinal plants will be tested for biological activity individually and in combinations (i.e., “complex mixtures”) consistent with traditional ethnomedical practice.
This manuscript summarizes the rationale, methods and preliminary “proof of principle” for the establishment of this prototype, authenticated medicinal plant library. It is hoped that these methods will foster scientific discoveries with therapeutic potential and enhance efforts to systematically evaluate commonly used herbal therapies worldwide.
Keywords: Herbal medicine, Library, Traditional Chinese, Ethnomedicine
1. Introduction
The topic of whether and how plant based medicines (aka herbal remedies) predictably alter the natural course of human disease has been an essential and complex aspect of medicine for thousands of years. By contrast, efforts to systematically apply modern scientific strategies to prove or disprove the therapeutic value of specific medicinal plants, individually or in complex mixtures, and to optimize their rightful place in modern health care, represent a more recent trans-disciplinary challenge.
Focusing on Traditional Chinese Medicine (TCM), there was a singular moment in recent history when practitioners of TCM and advocates of modern western medicine were explicitly called upon to jointly learn from one another, teach one another and, in the process, attempt to generate new knowledge for the common good of the next generation. The time was August 1950. The setting was the first National Health Congress of the newly established People’s Republic of China. Chairman Mao Ze Dong spoke on the occasion of the proposed establishment of the first five accredited schools of TCM and the need for collaboration across disparate expert groups. “We should unite all the young and experienced medical professionals from both Traditional Chinese Medicine and Modern Western Medicine to form a firmly united front to jointly strive for a great enhancement of the people’s health [1]!” Mao’s intention was clear and practical. He sought to proactively engage medical experts from both eastern and western traditions to jointly explore what he called “The Treasurehouse of Traditional Chinese Medicine,” including its rich pharmacopeia.
2. Relevance of herbal and TCM products
Roughly half of all approved prescription drugs are natural products, mostly from plants and microbial sources, their semi-synthetic derivatives or fully synthetic analogs [2]. Close to 70% of all cancer drugs originated from natural products [3,4]. Therefore, the application of state-of-the-art technologies to the systematic evaluation of traditionally used plant based medicines (aka ethnobotanicals) remains a highly relevant yet scientifically challenging line of inquiry.
3. Epidemiology and market relevance of herbal and TCM products
Herbal medicine use by the American public has increased dramatically over the past two decades. The percentage of US adults reporting the use of herbal (non-vitamin, non-mineral) products to treat or prevent disease increased from 2.5% in 1990 [5] to 12% in 1997 [6] to 14% in 2000 [7] to 19% in 2002 [8] and 18% in 2007 [9]. The estimated out-of-pocket expenditures for herbal therapies by the US adult population in 2007 was $14.8B. This is equivalent to approximately one-third of the total out-of-pocket spending on all prescription drugs ($47.6B) that same year [10].
A marketing analysis suggested that sales of TCM herbal products from China increased at an annual rate of 24% between 2004 and 2008 [11]. In 2008, TCM herbal product sales accounted for an estimated 22% of China’s overall healthcare product revenue and were estimated at a value of $26 billion US dollars [11].
4. Rationale to build a prototype library based on challenges and lessons learned
4.1. Lessons learned from selected clinical trials
In 2003, the NIH’s National Center for Complementary and Alternative Medicine (NCCAM) warned that a lack of reproducibility, quality control and dosage schedules involving natural products might lead to methodologically questionable and/or negative clinical studies, thereby diminishing opportunities for further investigations of ethnobotanicals [12].
By way of example, in 2004 a randomized trial was conducted to test the clinical effectiveness of an eight herb Chinese formula, sold under the product name “PC-SPES,” in subjects with advanced prostate cancer [13]. As documented in the medical literature, this complex herbal mixture was found to be clinically superior to the standard, high dose, estrogen salvage protocol in terms of overall reductions in PSA levels and time to progression of disease for 90 randomized study subjects. However, random testing of the herbal mixture revealed it had been adulterated with small quantities of synthetic estrogen and Coumadin [13]. The authors concluded that the true efficacy of this, and other, herbal mixtures will remain uncertain until the quality, consistency and purity of the natural products under evaluation can be ensured [13]. A review of the medical literature in 2005 documented that most publications involving the assessment of herbal therapies in clinical trials involved no independent verification of the herbal contents under evaluation [14].
The authors of the PC-SPES study also commented on the fact that the levels of estrogen identified in the commercial PC-SPES products were far too low to have explained the apparent clinical superiority of the PC-SPES therapy as compared with the estrogen salvage protocol. As such, their comments could be interpreted to raise the possibility, albeit remote, that specific components (i.e. chemical compounds) within the PC-SPES mixture, when added to low dose estrogen, might have resulted in an additive or synergistic effect powerful enough to alter the course of disease in men with advanced prostate cancer.
A second case of note involved the evaluation of the herb Echinacea (Echinacea angustifolia DC.) in the prevention of rhinovirus infection (i.e. the common cold). In this study, 437 volunteers were proactively infected with rhinovirus [15]. Subjects were then randomized to four groups. Three of the groups received various preparations and doses of a commonly sold Echinacea extract and one group received a placebo. There were no significant differences across groups with regard to rates of infection, severity of symptoms or viral titers. As such, the trial was considered to have refuted claims of clinical effectiveness of Echinacea. Subsequently, the New England Journal of Medicine published criticisms of the study’s design [16]. These included the suggestion that a different Echinacea species might have been preferable; that the dose used in the study was far too low (by a factor of 6) and that a higher dose might have made this trial more clinically and scientifically relevant [16].
A third study involved the evaluation of a popular over-the-counter preparation of the herb saw palmetto (Serenoa repens (W. Bartram) Small) in the treatment of benign prostatic hypertrophy [17]. In this study, 225 subjects were randomized to two groups, one receiving a Saw Palmetto extract in the form of a popular over-the-counter supplement and the other group receiving a placebo. There were no significant differences observed between these two groups in terms of symptomatic improvement. An accompanying editorial [18] commented that the study authors had tested a single, commercially available preparation of saw palmetto, thereby leaving open the possibility that a different preparation might still be effective. Furthermore, these authors contended that in the absence of a plausible mechanism of action, a fair comparison of this herb (or its constituents) to a more conventional FDA approved therapeutic drug, would be problematic if not impossible.
Lessons learned from these and other ambitious (and expensive) clinical trials suggest that future human clinical trials involving herbal products must ensure the reproducibility and quality of the intervention materials; and, will require an understanding of mechanisms of action and dosing prior to the implementation of new, large scale (and expensive) Phase II or III clinical trials. The current NIH guidelines involving candidate herbal therapies reflect many of these hard learned lessons [19] as do the Consort Guidelines for publications involving randomized controlled trials involving herbal interventions [20].
In hindsight, these were methodological inadequacies uncovered by individuals skilled in the design and conduct of clinical trials. They provided part of the rationale for the study described in this manuscript. What about methodological challenges from the vantage point of other relevant experts including researchers skilled in botany, chemistry, ethnobotany and drug discovery?
4.2. Lessons learned from the vantage point of drug discovery and ethnobotany
The current place of natural products in modern drug discovery is inconsistent with their past performance and future potential. Natural products have made, and continue to make, substantial contributions both to understanding basic biological processes and treating human disease. If we focus on cancer, natural products from plants have led to frontline therapies such as paclitaxel, vinblastine, camptothecin and etoposide [4]. If we look at the immediate future, geldanamycin analogs – to pick just one example – are being pursued in clinical trials [21,22]. Thus there is a strong scientific argument for continuing to explore natural products in drug discovery —an argument that is largely unheeded as pharmaceutical companies cut back on, or eliminate, their natural product programs.
Identification of natural product-based leads for Western drug discovery has usually resulted from screening of extracts or compounds from diverse biological sources, generally without regard to preexisting knowledge of the therapeutic utility of the producing plant. A good example is the remarkable portfolio of hundreds of thousands of natural products and extracts amassed by the United States National Cancer Institute (NCI) since the inception of its natural product-based efforts in 1955 [23]. Between 1960 and 1982, the NCI screened extracts of 35,000 plant species in collaboration with the U.S. Department of Agriculture (USDA). The strategy adopted was largely one of random selection of a broad range of natural product sources as opposed to selection based on medicinal use, i.e. ethnomedicine [4]. To a large extent, the driving force for NCI’s efforts was biological and geographic diversity rather than pre-existing knowledge of therapeutic utility. Focusing on biological and geographic diversity is a typical paradigm of most natural product drug discovery successes, meaning that the use of natural products for Western drug discovery has largely been one of trial and error. This approach has been referred to as “bio-prospecting.” Paclitaxel, vinblastine and camptothecin were discovered using this approach. Interestingly, Verpoorte has pointed out that there are an estimated 250,000 flowering plant species on earth while as of 2000, fewer than 15,000 (6%) had been screened for biological activity [24].
In contrast, many cultures around the world have developed ethnomedical traditions based on therapeutic utility of selected local plants and animals. Such empirical traditions are often hundreds if not thousands of years old, as in the case of TCM for which written records exist going back over 2000 years. Unfortunately, the potential value of ethnomedicines has often been discounted by Western medicine and science, with several identifiable factors accounting for this. First, medical diagnoses in TCM and other ethnomedical systems are often portrayed in ways that are not readily understood by Western clinicians. Second, TCM and other ethnomedicines are often viewed as fundamentally lacking in the mechanistic, scientific bases that usually underpin claims of Western medical efficacy. Third, there has been a lack of rigorous, well-controlled clinical trials demonstrating clinical efficacy (and mechanisms) of TCM and other ethnomedicines. Fourth, existing scientific and clinical studies of TCM have often utilized plants that have been quality compromised, may be contaminated with pesticides or heavy metals, may have been botanically misidentified, or are lacking a consistent and reproducible resupply chain. As such, and as noted earlier, prior studies have frequently been compromised by quality control and botanical authentication issues, as well as lot-to-lot variability and lack of knowledge of precise growing locations and conditions, factors that have too often limited reproducibility. Finally, resupply of herbs for confirmation and follow-up studies is frequently problematic. The limiting factors mentioned earlier fall into two main categories: variables related to starting materials, and variables related to execution or interpretation of scientific and clinical studies. While the two are interdependent, without addressing the former, there is little value in pursuing the latter.
From the vantage point of ethnobotany, researchers have highlighted the difficulties in replicating the biological activity of a given plant when attempts are made to repeat an experiment after subsequent recollection [25]. As such, the challenge of reproducibility remains a formidable one.
There are also challenges in terms of sourcing plant species to be studied. These include: collecting them according to traditional techniques, documenting the precise collection sites using GPS technology, authenticating them visually, chemically and, through DNA sequencing, processing and extracting them according to established, traditional and reproducible techniques, storing them properly and so on.
Lastly, there is the inherent conundrum which speaks to the heart of the conceptual difference between traditional (i.e. ethnobotanical) herbal practice and modern (western) drug discovery and therapy. This conundrum can be conveyed through the articulation of two testable hypotheses, specifically; do herbal medicines work, when they work, predominantly because of single chemical compounds, albeit in small quantities? By extension, are herbal medicines and herbal medicine libraries merely repositories for sophisticated “bio-prospecting” in search of novel, and patentable, composition of matter discoveries (aka “new chemical entities”) or derivatives of already known chemical compounds which can be shown to be of new therapeutic application and benefit? One must also consider the fact that herbal therapy as practiced in traditional settings rarely involves the prescription of a single herb at a time. Instead, traditional conceptual frameworks, which preceded contemporary understanding of chemical compounds and their specific effects on precise, biological targets, almost always involve multiple herbs and a presumed additivity and/or synergy of effects on the host subject. The shared view among herbalists, in Asia and elsewhere, has been that a mono-therapy approach will be inferior to a multi-therapy, complex herbal mixture approach [26]. By way of example, a typical TCM prescription routinely includes 8–12 herbs, in varying ratios, with one of the herbs serving as the “king”, a second serving in the capacity of “minister”, a third as “adjutant” and a fourth as the “messenger” [27].
Given this disparity of conceptual models, the fundamental (second) testable hypothesis is that herbal therapies work, when they work, due to complex yet predictable effects of multiple compounds within complex mixtures of plant compounds. As evidenced by the near miraculous success of the three drug “AIDS Cocktail,” we now have unequivocal evidence that multi-drug therapy can sometimes be the best – or only –successful therapeutic option [28]. The work of Borisy et al. demonstrated the existence of synergistic effects involving multiple (FDA approved) compounds [29] and the work of Wagner has summarized the existence of synergistic effects involving multiple compounds found in a variety of natural products [30]. Might clinical successes attributed to herbal therapies involve additive and/or synergistic biological effects which can now be more meticulously described and, ideally, optimized for maximal therapeutic benefit?
Lastly, there is the non-technical challenge that Mao attempted to address a half century ago [1]; specifically, can the two schools of medical thought, Eastern and Western (modern biomedicine) develop shared research strategies which address the above mentioned known challenges without compromising the respective beliefs, practices and fundamental tenants of each tradition? Can ethnobotanists and modern drug discovery experts established an effective “united front,” and will this exercise result in novel therapeutic discoveries or not?
To reframe this question, can an artisan collection of authenticated TCM plants be established for the purpose of successful systematic biological evaluation and will such a library, when screened strategically, lead to the identification of novel compounds, new uses for known single compounds and/or their derivatives; and/or novel biological mechanisms? With regard to the second hypothesis mentioned earlier, might there also be a new opportunity to employ contemporary high-throughput screening facilities to search for anticipated as well as unanticipated combinations of single compounds which can be shown to be therapeutically effective due to biological additivity and/or synergy of multiple plant derived compounds? Will combinations with evidence of additivity and/or synergy confirm ethnobotanical knowledge about specific plant combinations (i.e. formulas) or might entirely unanticipated active combinations also be identified?
4.3. Considerations in the development of a prototype authenticated TCM plant library
Between 2006 and 2010, investigators from Harvard Medical School (HMS), in collaboration with colleagues from the Beijing University of Chinese Medicine (BUCM) and Hong Kong Baptist University (HKBU), embarked on efforts to build a prototype library of 202 botanically authenticated, quality controlled, collection site-documented Chinese medicinal plants. Each plant was to be tested for pesticide and heavy metal contamination and qualified using standards defined by the Chinese Pharmacopeia [31], an official publication of the State Food and Drug Administration (SFDA) of the People’s Republic of China. Such an artisan library could serve as a necessary prerequisite to reproducible pre-clinical studies of TCM herbs. These are mandatory prerequisites to the subsequent design and implementation of the next generation of animal and human studies of herbal remedies. Moreover, the ethnobotanical knowledge of TCM experts is essential to establish this library, and to efficiently guide drug development experts in their search for novel single compounds, novel uses of previously identified compounds and hypothetically, novel demonstrations of additivity and/or synergy involving multiple plant compounds within plants and within complex mixtures of medicinal plants.
In planning for, constructing and evaluating this authenticated TCM herbal library, many factors had to be considered including: the selection criteria of medicinal plants to be included in the research library; quality assurance including harvest sites and GPS documentation; botanical species authentication; collection protocols; plant processing details; creation of voucher specimens; testing for pesticides and heavy metal contamination; chemical and quality assessment according to existing standards of the Chinese Pharmacopeia; Chinese governmental cooperation, authorization and partnership; storage and shipping logistics of all specimens in China and the US; precise and reproducible extraction and fractionation procedures; selection of appropriate biological screening strategies; establishment of a suitable database and database management system; and, continuous efforts to maintain open communications and opportunities for expanded research collaboration among all participating co-investigators.
5. Methods
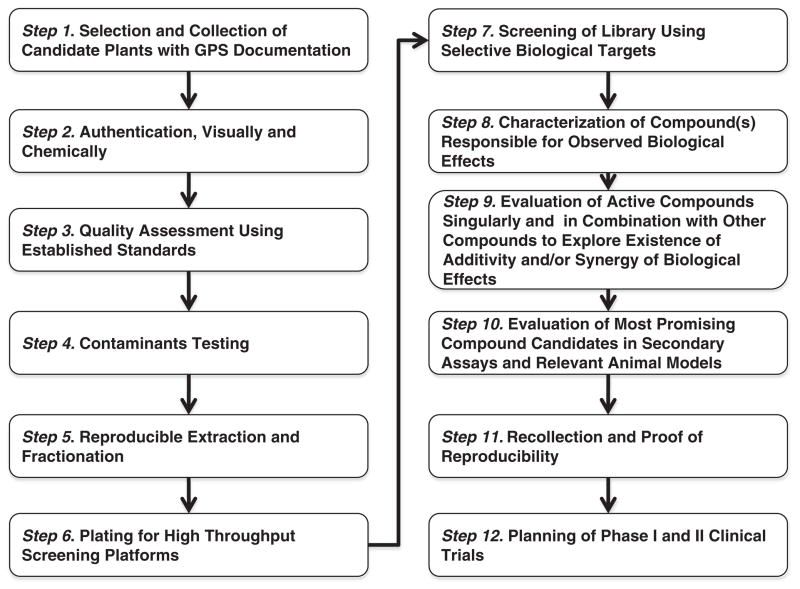
(See Fig. 1 for an overview of the methods employed in the creation of this prototype TCM library).
Fig. 1.
Flow chart describing methods to develop a prototype authenticated Chinese medicinal plant library for systematic biological evaluation.
5.1. Criteria for inclusion
The selection of plants for the authenticated TCM herbal library was based on two main criteria: (1) the plants were listed in the official Pharmacopoeia of the People’s Republic of China (CP) [31] and, (2) the species were not endangered. This latter criterion is of concern since the over-harvesting of plants from wild sources for use in TCM is a growing problem [32]. For example, some plants used in Chinese medicine are on the Convention on International Trade in Endangered Species of Wild Flora and Fauna (CITES) list, including famous medicines such as Mu Xiang (Saussurea costus (Falc.) Lipsch.) and Rou Cong Rong (Cistanche deserticola Y. C. Ma) [33]. The official CP includes synthetic pharmaceutical medicines, but also many traditional medicines derived from natural sources. At the time this study was launched, the most recent edition was the 2005 Chinese Pharmacopeia.
The first volume of the 2005 CP includes monographs on Traditional Materia Medica and is comprised of substances derived from animals, minerals, plants, and fungi. A total of 471 monographs based on plant products are included in this volume, comprising 550 different species. The prototype library included a total of 200 species of plants and 2 species of fungi, representing more than one-third of all plant species listed in the CP. The 202 selected plants (see Appendix A) are some of the most commonly used in the CP and hence are estimated to represent approximately 75% of all plants used in routine TCM practice.
5.1.1. Monograph summarizing relevant information of each plant
Monographs were written for each species included in the project. The goals of the monographs included providing a comprehensive literature review of the traditional uses, as well as a summary of recent information about experimental studies of each plant. In particular, it was envisioned that the monographs would be a useful source of information for screeners of the library. Each monograph included the following sections: names and synonyms of the plant, collection and processing methods, therapeutic indications in TCM and western medicine, types of extracts, major chemical constituents, description of medicinal part of the plant, contraindications, common preparations and inclusion in common TCM formulae, and selected references in the TCM and western literature. The monographs are included in the project database (see section 5.7).
5.2. Collection protocol
Whenever possible, collection locations were selected from the region that is traditionally known for production of the plant species being collected. Additionally, each plant species was collected from three distinct locations in China separated by hundreds or thousands of miles and usually in different provinces, to ensure that at least one of these plants could meet the appropriate requirements of species identity, quality, and purity, including the absence of contamination by pesticides and/or excessive heavy metals. Plant collection consisted of three separate activities: environmental investigation, bulk harvest, and voucher collection. The environmental investigation included a survey to ensure that the collection area was suitable and capable of yielding enough plant material for the project. The bulk harvest consisted of the harvest of the medicinal part of the plant at the time that the herb is traditionally collected. During the bulk harvest, each sample was collected to give a total of 10 kg dry weight. Following the bulk harvest, all herbs were processed according to the traditional method as specified by the CP. This typically involved the removal of impurities such as other plant species or soil, followed by drying in the sun for a period of days or weeks. Lastly, vouchers of each sample, consisting of flowering or fruiting material of the plant, were collected according to standard protocols [34]. Vouchers have been stored along with a voucher of the bulk harvest medicinal part for future reference. The plant acquisition team, organized and overseen by BUCM co-investigators and consultants from HKBU, consisted of at least one Chinese herbal medicine resource expert from each of the 30 provinces and autonomous zones where plant acquisition took place. In addition, a 34 person Beijing-based acquisition team consisting of faculty and graduate students of BUCM as well as 8 additional TCM botanical experts oversaw the quality control and processing of all plant samples collected. Authentication of plant species was overseen by a separate group of faculty with TCM ethnobotanical expertise at HKBU. All steps of plant collection were documented using a combination of standardized collection forms, GPS data collection, photographs, and video. (See Appendices B–E for examples of photos imported into study’s database). After harvest and processing, plants were authenticated and tested for quality (see sections 5.3 and 5.4).
5.3. Authentication and quality assessment
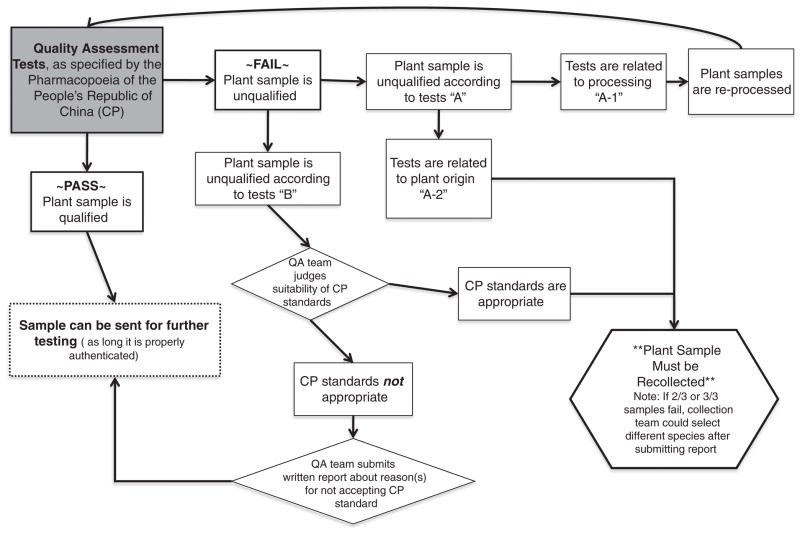
The taxonomic identification of each plant was confirmed by multiple experts in China. Plants were authenticated according to morphological and anatomical characteristics [35] by a team at HKBU and examined according to criteria listed in the CP for quality assessment at BUCM. The quality assessment tests were conducted according to the guidelines and standards provided in the CP 2005 edition [31]. The CP specifies the method for conducting relevant tests and provides standard reference values for results. In general, the quality assessment tests include methods for determining the identity and purity of the plant. For the purposes of the quality assessment tests, the identity of the plant is determined using methods such as qualitative thin-layer chromatography (TLC) and reactivity tests. There are also quantitative tests to determine the concentration of certain chemical ingredients. For example, the CP stipulates that licorice root (Glycyrrhiza glabra L.) must have at least 2.0% glycyrrhizic acid by dry weight [31]. Lastly, some of the quality assessment tests relate to determination of impurities in the plants. For example, many species require a test of water and ash content. The test of water content is to determine how well the sample may be preserved, and the test of ash content provides information about the amount of solid impurities, such as soil, that are included with the plant sample. Samples which did not meet quality assessment tests were considered to be unsuitable for subsequent study as they did not meet established and reproducible standards of quality assurance. The list of quality assessment tests stipulated by the CP is provided in Table 1. The methods used for deciding on the results of quality assessment tests and whether the results would necessitate plant recollection are summarized in Fig. 2.
Table 1.
Quality assessment tests based on the Chinese Pharmacopoeia.
| Test | Type of test* |
|---|---|
| Character | Test related to plant origin (A-2) |
| TLC identification | Test related to plant origin (A-2) |
| Foam reaction identification | Test related to plant origin (A-2) |
| Color reaction identification | Test related to plant origin (A-2) |
| Sublimation reaction identification | Test related to plant origin (A-2) |
| Impurity examination | Test related to plant processing (A-1) |
| Leaf examination | Test related to plant processing (A-1) |
| Water content examination | Test related to plant processing (A-1) |
| Total ash content examination | Test related to plant processing (A-1) |
| Acid-insoluble ash content examination | Test related to plant processing (A-1) |
| Peroxide value examination | Test related to plant origin (A-2) |
| Loss on drying examination | Test related to plant processing (A-1) |
| Volatiles | Test related to chemical content (B) |
| Extract | Test related to chemical content (B) |
| Content determination | Test related to chemical content (B) |
For relevance to decision tree with regard to accepting or rejecting plant samples see Fig. 2.
Fig. 2.
Decision Tree For Acceptance or Rejection of Plant Samples Based On Chinese Pharmacopoeia Quality Assessment (QA) Tests. See also Table 1 for details of quality assessment tests in the Chinese Pharmacopoeia.
5.4. Testing for heavy metals and pesticides
Following standards established by NSF International and American Standards Institute (ANSI) Standard 173 for acceptable metal levels in dietary supplements [36], all plants were tested for heavy metal and pesticide content, including five heavy metals (As, Cd, Cr, Pb and Hg) and pesticide residues.
There is currently considerable variation between different countries for testing pesticides in dietary supplements [37], and typically only a few pesticides are routinely examined. A comprehensive approach to pesticides testing was used in this project, and a broad screen including 162 pesticide residues was employed. More details pertaining to these research methods are reported separately [38].
5.5. Shipping, import, export and maintenance of plant materials
The logistics involved in the dried plant material supply chain, from when the material was transferred from BUCM to HMS possession, included abiding by the export/import regulations for all countries involved, freight forwarders, and insurance to protect the resources. Prior to shipment, it was necessary to apply for and obtain the appropriate import permits from the United States Department of Agriculture Animal and Plant Inspection Services (USDA APIS). With the USDA APIS permit in hand, service agreements were executed. At certain steps of the project, it was necessary that the dried plant material bulk shipments be maintained in a pest-free, climate-controlled storage facility. For example, the samples were held in China prior to shipment to the United States. After the samples arrived in the United States they were placed in storage. In both instances, bulk samples were maintained in a special storage facility with controlled temperature and humidity. The samples currently maintained in the United States are kept at 20 °C and 50% relative humidity in a state-of-the-art facility.
5.6. Selecting one of three samples for initial extraction and fractionation
Each species in the library was collected from three separate locations, but only one sample per species was to be initially extracted and fractionated for high throughput screening. Samples were selected for extraction and fractionation using two main criteria: (1) absence or lowest values of heavy metal and pesticide contamination among samples collected for each species; and (2) ease of collection. Heavy metal and pesticide amounts were determined as described elsewhere [38]. Ease of collection was judged by collection experts at BUCM. The explicit decision tree used to make determinations is summarized in Fig. 3.
Fig. 3.
Decision Tree for Choosing One of Several Plant Samples per Species for Extraction and Fractionation. Asterisk (*) indicates instances where clinical toxicological judgment may be required. For example, if all samples are contaminated, the preference is for the least toxic sample whether that is the lowest concentration of contaminant or contaminants with lowest clinical toxicity.
5.6.1. Extraction and fractionation procedures
Between 1 and 4 kg of the herb samples selected for extraction and fractionation were subjected to industrial-scale grinding, followed by extraction in 95% (v/v) ethanol for 30 min with sonication, using a solvent-to-herb volume ratio of 7:1. Using this method, compounds with a broad range of polarity can be extracted. Tannins were removed from the resulting ethanol extracts via polyamide column chromatography (polyamide: extract=10:1), followed by desalting and defatting on HP-20 resin (HP-20: extract=50:1). The HP-20 column was washed with water to remove salts, then with ethanol, and finally with 50% ethanol/dichloromethane to remove fatty acids. The resulting ethanol eluate was subjected to large-scale reverse-phase C-18 HPLC chromatography, with step-wise elution using 2% ethanol increments from 0 to 100% in 70 min and then 100% ethanol for 26 min, resulting in approximately 48 fractions per extract. Target quantities were 15 mg eluted material per fraction: 15 mg or less of each fraction was then dried in 4 ml glass vials for future formatting for screening. For each fraction for which more than 15 mg of material was obtained, the extra material was dried in appropriately sized glass vials. All dried fractions were stored at −20 °C until prepared for screening or used for further purification steps.
5.6.2. Preparing extract fractions for screening
To re-suspend fractions, 100% DMSO was added to each dried fraction in 4 ml vials for a final concentration of 15 mg/ml. DMSO was added using the Biomek FX (Beckman Coulter, Inc.) automated pipetting work station, allowing 352 fractions to be processed in each plating session. Small (10 mm) Teflon-coat stir bars (V&P Scientific) were added to each 4 ml glass vial and vials were stirred overnight at room temperature. Fractions that did not go into solution by stirring were hand-pipetted briefly the next morning to fully re-suspend them. Fractions were aliquoted by hand into 96-deep well plates (VWR 4002-011). To make plates for screening, extracts were re-formatted from the 96-well master plates into 384-well plates (ABgene AB-1056) using a Velocity-11 V-Prep (Agilent) automated pipetting work station. All plated extracts were stored at −20 °C.
5.7. Construction of electronic database
In order to meet the data requirements of this initiative, a novel database was designed to organize and store data related to all aspects of plant collections, authentication, shipping, processing and biological screening. The database, called the “Traditional Medicine Collection Tracking System (TM-CTS),” was designed to support the daily needs of the project in the context of the international collaboration between investigators in the United States and China, and to include information related to all aspects of the project from plant collection through extraction and fractionation. In addition, the database was constructed to work in concert with the screening database used in the project. The TM-CTS is described in a separate paper [39].
5.8. Initial screening strategies
Strategies used for screening the initial collection of several thousand pre-fractionated extracts were based on five major themes. First, assays needed to represent therapeutic areas of high unmet medical need. Second, assays were to represent the most “cutting-edge” biological advances in their respective therapeutic areas, in recognition of the fact that the goal was to identify and develop high-impact new drugs with therapeutic efficacies or scopes going beyond existing medicines. Third, assays needed to be carried out on small enough scales to support high throughput screening (HTS) in 96- or 384-well plate formats, and to not require unreasonably large amounts of material. More specifically, targeted assay volumes ranged from 0.1 to 1.0 μL per assay point, with occasional exceptions made for 8–10 μL per assay point for certain cell-based assays considered to be of uniquely high value. Fourth, sufficient down-stream assay capabilities, particularly the existence of appropriate in vivo animal models for the particular disease being targeted, needed to exist, in order to ensure that any lead compounds identified in initial screening campaigns could be further developed pharmacologically to a point where drug development value could be firmly established. Fifth, assays needed to offer the possibility of eventual patent-protected licensable opportunities (compound, target, or both), since it was recognized that eventual development of lead compounds would depend on interest by the pharmaceutical industry, which would likely require appropriate intellectual property protections allowing for a return on investment.
Guided by the above five principles, the first several thousand fractions were screened across a range of assays representing a variety of therapeutic areas. Although beyond the scope of this manuscript, active screens included targets in the following areas: oncology (angiogenesis, tumor-specific differential cytotoxicity, and cell survival pathways), anti-infectives (bacterial, anthrax), anti-virals (HIV-1), diabetes and neurodegeneration (Alzheimer’s disease, Parkinson’s disease). Other therapeutic areas under consideration included assays for novel targets in arthritis, cancer (and non-cancer) stem cells, cardiovascular disease, anti-infectives (Methicillin-resistant Staphylococcus aureus, tuberculosis, influenza A/H1N1), hepatitis C, immuno-oncology approaches, inflammation, multiple sclerosis, and pain.
5.9. Description of selected (initial) screens involving cell based and whole organism (e.g. Zebra fish) assays
Human fibroblasts and cancer cell lines [LNCaP, PC3 (Prostate), MCF7, MDA-231 (Breast), A549 (Lung), BJ Fibroblasts] were propagated using standard conditions as suggested by ATCC. Cell proliferation and/or survival were determined by using Cell Titer Glo (Promega) in a 384-well format assay as previously described [40].
For zebra fish screening, fractions were delivered robotically to 96 well plates. Four embryos were added per well by pipette at roughly 6 h post fertilization to allow early development to occur before treatment. More than 1000 extract fractions were initially screened at nominal concentrations (based on the assumption of a pure compound of MW 500) on embryos in 10% Hank’s Saline with 0.003% phenyl thiourea in water, observing from 6 h post fertilization to 72 h post fertilization. All wells were examined by light microscope to look for signs of altered development and impaired angiogenesis. Fractions that showed grossly defective development were not further analyzed.
5.9.1. LC/MS and NMR techniques for compound characterization
LC/MS was performed on an Agilent 6130 Single Quadrupole system. NMR spectra of active compounds were obtained on a Varian 600 MHz spectrometer. Compounds were identified either using authentic samples or comparing their chemical shifts with those reported in the literature.
5.9.2. International collaboration agreements
Agreements covering the scope of work, ownership of materials and intellectual property, sharing of potential financial revenues and co-authorship of academic publications were negotiated by representatives of the participating US and Chinese Academic Institutions. These were also reviewed by representatives of the Chinese Ministry of Health, Chinese Ministry of Education, State Administration of TCM, Chinese FDA and the Chinese Ministry of Science and Technology.
6. Results
6.1. Current status of the authenticated TCM library
The initial plans of the participating co-investigators involved the collection of 202 individual TCM species in triplicate and the completion of all aspects of the methods described earlier as applied to all collected specimens. As envisioned, this would have resulted in an estimated 10,000 fractions. A total of 3709 fractions from 80 TCM authenticated species have been created and screened to date. Consequently, the results reported here are those that have been obtained based on work completed thus far.
At least one bulk sample has been collected from each of 202 species of TCM plants and fungi. Of these, 186 species have been authenticated according to macroscopic and microscopic characteristics, 158 species have been tested for quality according to standards in the CP [31] and 129 species have been tested for heavy metals and pesticides. The bulk samples of 136 species and voucher samples of 79 species have been delivered to HMS, with the remaining samples in China. All of the data and images related to collections, authentication, extraction, and fractionation, are currently stored in the project’s TM-CTS database for future reference [39].
The mean number of fractions per sample is 45 (median 47; range 19–52). In addition to the fractions that were eluted from the HPLC column, the total number of fractions per plant always included four additional fractions that represent the crude extract, the desalting and defatting washes of the crude extract, and the cleaned extract prior to fractionation. For example, the maximum number of fractions for one plant, 52, is equal to these four fractions plus a maximum of 48 possible fractions that are eluted from the HPLC fractionation column. These four additional fractions were included in the screening library. See Table 2 for a summary of the status of the library to date.
Table 2.
Status of prototype library of authenticated traditional Chinese medicinal plants to date. The number of samples collected, authenticated, processed, and quality tested to date is shown. In addition to total number of samples that were processed at each stage, comments regarding authentication, quality assessment, and contaminants are provided.
| Number of samples | Comments regarding quality assurance testing | |
|---|---|---|
| Total number TCM species planned for collection | 202 | |
| Total number samples (two or three) planned for collection | 582 | |
| Actual number that were collected | 562 | |
| Total samples authenticated | 451 | 7 (1.6%) failed authentication |
| Total samples which completed Chinese Pharmacopeia quality assessment | 382 | 54 (14.1%) failed quality assessment |
| Total number samples tested for heavy metals | 334* | |
| Total number samples tested for pesticides | 294* | |
| Total number of species extracted and fractionated | 80 | |
| Total number of fractions currently available for biological screening | 3709 |
Details about heavy metal and pesticide levels as well as their clinical implications are reported in a separate paper [38].
6.2. Summary of plant species included
Almost all (~99%) of the plants included in the prototype TCM library are flowering plants. The library includes 2 species of gymnosperm and 2 species of fungi. Of the flowering plants, the most commonly represented families are: Asteraceae, (10.7%), Rosaceae, (5.9%), Lamiaceae, (5.4%), Fabaceae, (4.9%), and Liliaceae, (4.9%). Of the bulk samples in the library, the majority come from underground parts of plants (42.4%), including bulbs, rhizomes, roots, and tubers. Other medicinal plant parts in the collection, consistent with TCM practice, include fruit and/or seed (29.8%), leaf and/or stem (17.1%), flower (5.4%), and whole plant (4.4%). The names of all species included in the library appear in Appendix A.
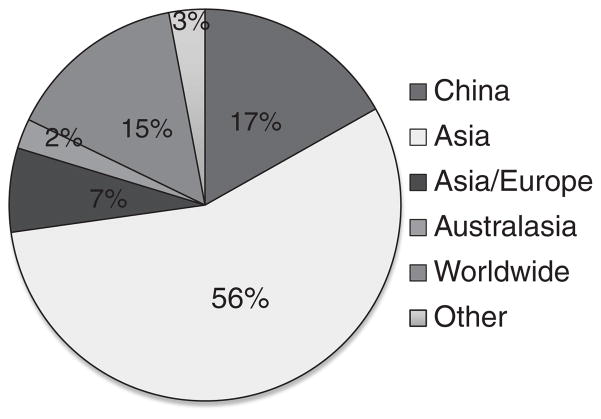
In terms of the natural geographic range of species in the collection, 17% of the species in the collection are endemic to China, as compared with estimates of 30–50% species endemic to China as a whole [41]. The most common distribution pattern of species in the collection is one that covers multiple countries in Asia (56%). For example, Schisandra chinensis (Turcz.) Baill. occurs in China, Japan, Korea, and Russia [41]. In addition, roughly 5–10% of the plants in the collection are originally exotic introductions from countries outside of China. For example, fennel (Foeniculum vulgare Mill.) and pomegranate (Punica granatum L.) are plants in the CP that are originally from the Mediterranean region of Europe. A summary of native range distributions of plants in the prototype TCM library is shown in Fig. 4.
Fig. 4.
Geographic Range of Species in the Authenticated Chinese Medicinal Plant Library. Data for 202 species are shown, based on the total range size of each plant as listed in the Flora of China [41]. Most plants in the collection (56%) have a distribution range that includes China plus other countries in Asia. For example, Wu Wei Zi, Schisandra chinensis (Turcz.) Bail., occurs in China, Japan, Korea, and Russia. About 1 in 5 plants (17%) in the collection are endemic to China exclusively. For example, Chuan Mu Xiang, Dolomiaea souliei (Franch.) C. Shih, only occurs in areas of high elevation in Southwest China. Additionally, about 15% of the plants have a worldwide distribution. For example, Xia Ku Cao, Prunella vulgaris L., occurs in Africa, Asia, Europe, and North America.
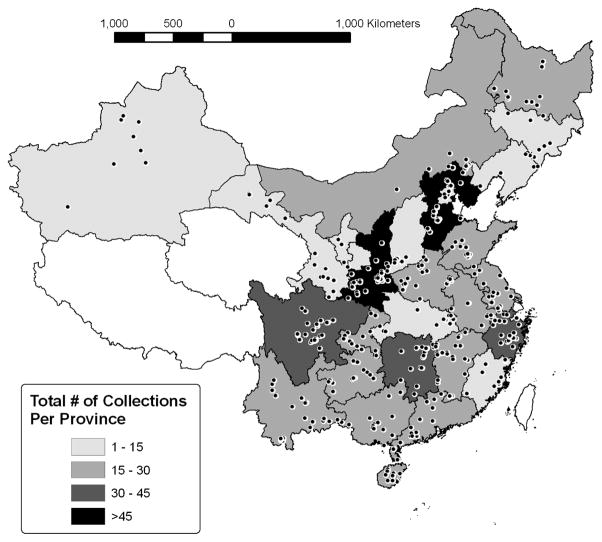
The plants in the library have been collected from a wide geographical range of sites throughout China, including sites in 21 of the 23 provinces and 4 out of 5 autonomous regions. The provinces of Shaanxi and Hebei had the most collection sites (see Fig. 5 for summary of site collections).
Fig. 5.
Map of Collection Locations in the People’s Republic of China. A total of 558 sample locations, representing 202 species, are shown. No collections were made in the provinces of Qinghai, Xizang (Tibet), or Taiwan. The highest number of collections were in Shaanxi (N=61) and Hebei provinces (N=47).
6.3. Heavy metal and pesticides screening
A total of 334 samples representing 126 species were tested for five heavy metals. Of those, 294 samples representing 112 species were also tested for 162 pesticide residues.
A full discussion of the patterns of heavy metal and pesticide content and the different methods for interpreting the clinical significance of these observations is beyond the scope of this manuscript and will be reported separately [38].
6.4. Quality assurance results
A total of 382 samples representing 142 species were tested for quality according to standards in the CP [31]. Of those, a total of 54 samples, or 14%, failed one or more of the quality assessment tests (see Tables 1 and 2). The most common reason for samples failing was due to the quantitative tests of chemical content. A total of 29 samples, representing 8% of the total, did not meet CP standards for chemical content. In those cases, a new sample was collected or the standard in the CP was re-assessed. Under certain circumstances, if our TCM experts reached a consensus that the existing CP standard was unrealistic and in need of revision, the sample was provisionally accepted. For example, in some cases, it was known that the CP was being changed for the 2010 edition. In these instances, a written justification was provided and assessed by experts at multiple universities of TCM in China. The next most common reason that samples failed was a result of tests of water or ash content, with roughly 5% of samples failing each. Samples that failed water or ash content were re-processed by drying the sample or re-cleaning it and tested again until they met the CP standard.
6.5. Shipment experience
In general, all shipments for the project went smoothly, possibly due to the fact that endangered or otherwise questionable species were not included in the project. For example, costus root (Saussurea costus (Falc.) Lipsch.) is listed in the CP but was excluded from the project because it is also a species with trade restrictions. However, shipments were regularly held in customs upon their entry in the United States and some were inspected. In particular, species of Chinese medicine that are related to either important agricultural species or potential weeds were inspected. For example, plants in the Citrus family (Rutaceae) like Chen Pi (Citrus reticulata Blanco) and Zhi Qiao (Citrus aurantium L.) were examined before they entered the United States presumably to protect the U.S. citrus industry.
6.6. Reproducibility of extraction process
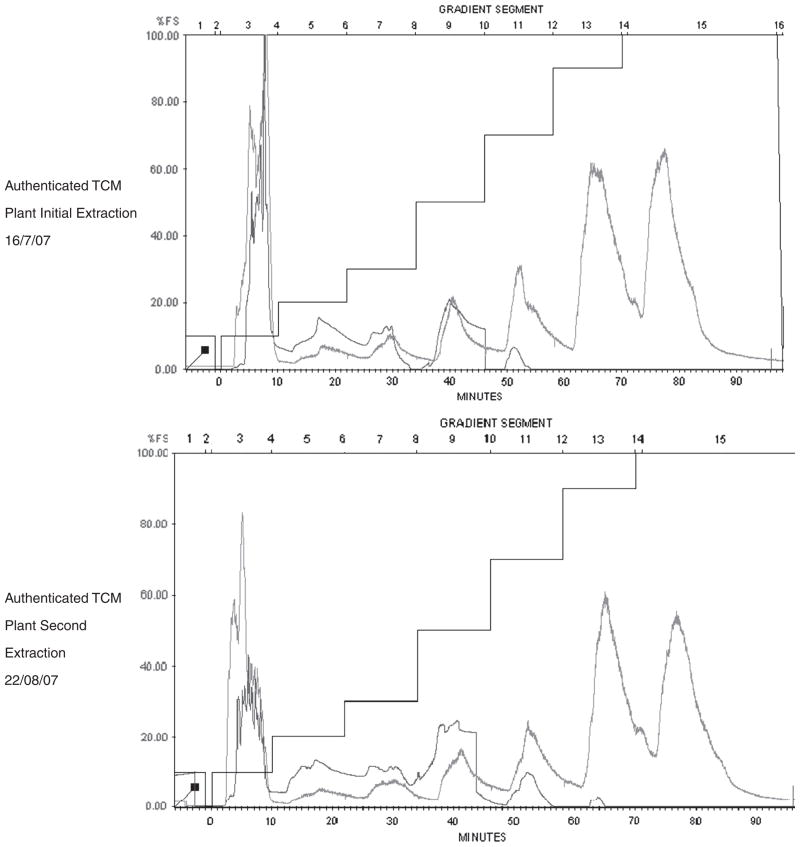
See Fig. 6 for evidence of reproducibility of the extraction protocol as applied to a single 2 kg shipment of authenticated TCM plant materials. Extracts made on two separate dates are characterized by comparable HPLC tracings.
Fig. 6.
Evidence of Reproducibility of Extraction Methods (BioXPlore HPLC) Involving A Single Species Dried Material Extracted on Different Dates.
6.7. Initial high-throughput screening targets
See Appendix F for a listing of initial screening targets applied to preliminary evaluation of the 3709 TCM fractions from 82 authenticated TCM species.
6.8. Examples of selected initial screens as “proof of principle:” cytotoxicity screens and zebra fish assays
Six human cell lines were initially screened against 1686 library fractions to identify those fractions that induced cell death and/or inhibited cell proliferation. We identified 6 fractions (out of 1686) that preferentially affected the human fibroblast cell line BJ and a number of fractions that showed specificity for each of the other cell lines. Of note, we found several fractions that affected both prostate cancer and breast cancer cell lines. All fractions were retested, and identification of the active compounds is on-going.
Fractions were also screened for their efficacy in blocking angiogenesis using a whole organism, zebra fish embryo assay. Of fractions screened thus far only a small number showed impaired angiogenesis with few other defects. Of these, one specific fraction from the plant Stellaria dichotoma var. lanceolata Bge. showed the most striking anti-angiogenic effects (see Fig. 7).
Fig. 7.
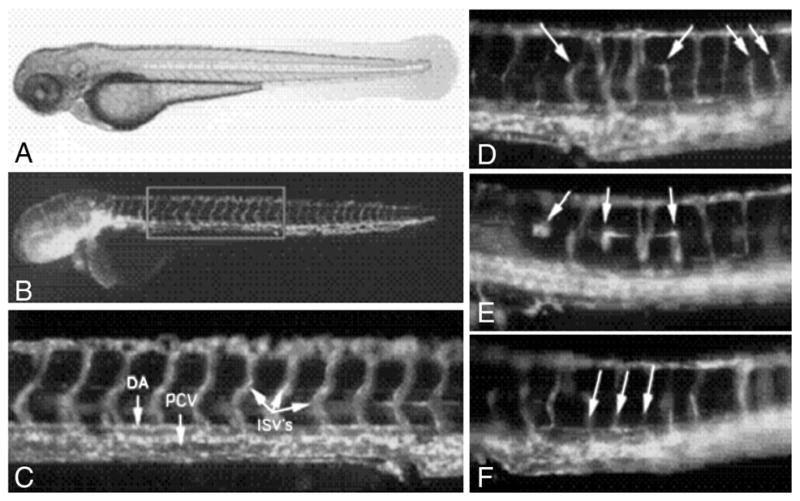
Zebra Fish Aniti-Angiogenesis Assay - Example of Proof of Principle. (A) Zebrafish embryo at 27 hrs; (B) Normal blood vessels; (C) Higher magnification of normal vessels; (D) (E) (F) Developmental abnormalities in intersegmetal vessels due to a style plant extract fraction. DA = dorsal artery, PCV = posterior cardinal vein, ISV’s = intersegmental vessels.
A more detailed evaluation of the qualitative changes that this fraction had on blood vessel development in the zebra fish involved a transgenic line which expresses green fluorescent protein specifically in the endothelial cells of blood vessels. We observed that the dorsal artery and posterior cardinal vein develop normally when exposed to the extract, indicating that vasculogenesis was not adversely affected. However, the angiogenic process whereby intersegmental vessels grow dorsally from these two major vessels was inhibited. As shown in Fig. 7, at 72 h post-fertilization, many intersegmental vessels were either absent, stunted in growth, thin or occasionally misguided compared to untreated controls. The only other obvious gross phenotypic abnormality which was observed following treatment with this fraction was pericardial edema, which is visible by 48 h post fertilization. Pericardial edema is commonly seen in a wide range of cardiovascular developmental abnormalities, including angiogenesis defects.
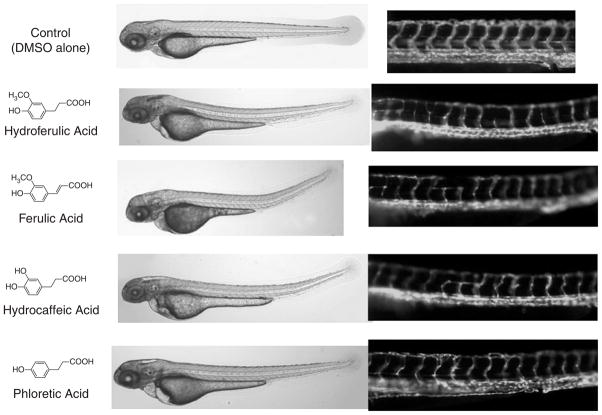
LC/MS and NMR analyses showed that the fraction contained a relatively pure major chemical entity, which was identified as Dihydroferulic acid. Reference material (Sigma) when tested in the zebra fish assay showed the previously observed vascular phenotype at concentrations above 50 μM. A series of chemically related compounds was tested in an attempt to obtain a structure activity relationship (see Fig. 8). Of all structurally related compounds tested, the most active was cinnamaldehyde, which impaired angiogenesis at concentrations above 0.1 μM. Notably, the most active compounds were known to be agonists of TRP channels. To explore the relationship further, Dihydroferulic acid was tested in a TRP channel assay and found to be active at concentrations above 50 μM. We also tested other known agonists of TRP channels and found them to be active in the zebra fish assay. Unfortunately, further experiments to confirm the identity of the target of the zebra fish fraction have not given definitive results to date.
Fig. 8.
Testing related compounds for relative anti-angiogenesis potency.
Interestingly the TCM literature suggests that the plant containing this fraction, S. dichotoma, is effective in treating disorders of the blood. Western scientific literature further suggests that the active component from S. dichotoma, Dihydroferulic acid, is effective in blocking endothelial cell growth while cinnamaldehyde has been shown to be active in blocking angiogenesis.
7. Discussion
This manuscript summarizes our rationale for the development of a library of authenticated medicinal plants, methods used, the status of this library and preliminary research conducted to date. The discussion will be limited to lessons learned based on the work completed thus far, followed by a preliminary list of researchable hypotheses and proposed next steps.
Importantly, through the present collaborative study, US and Chinese co-investigators have jointly proposed and implemented a potentially reproducible strategy to identify, collect, authenticate, quality control, extract and pre-fractionate medicinal (TCM) plant species for the purpose of systematic biological, i.e. pre-clinical, evaluation using state of the science drug discovery technologies. While expensive and complex, this methodological approach has the potential to address many of the criticisms aimed at previous investigations involving herbal (natural) products and sets the stage for future pre-clinical research investigations involving medicinal plants.
US and Chinese co-investigators with expertise in (TCM), botany, chemistry and drug discovery, have jointly established a prototype library consisting of 202 authenticated medicinal herbal and fungal species that collectively represent the therapeutic content of the majority of all commonly prescribed TCM herbal prescriptions. Currently housed at HMS, the library consists of duplicate or triplicate kilogram quantities of each authenticated and processed species, as well as “detanninized” extracts and sub-fractions of each mother extract. Importantly, each species has been collected at two or three unique sites, each separated geographically by hundreds of miles, with precise GPS documentation of each collection location as well as information about the seed origins of plant. Each of the plant samples has been authenticated visually and chemically prior to testing for contamination by heavy metals and/or pesticides. An explicit decision process has been developed whereby samples of each species with the least contamination were selected to undergo ethanol extraction and reproducible HPLC sub-fractionation procedures in preparation for high throughput screening across a broad array of biological targets including, but not limited to, targets relating to cancer biology. (See Appendix G for a graphic summary of methods used to develop this prototype library).
In the process of developing this prototype TCM library, we tested all collected specimens for their heavy metal and/or pesticide content. A detailed summary of these findings and their clinical significance is beyond the scope of this article and is being reported in a separate manuscript [38].
Preliminary biological screening of this library has led to the following observations. We have been able to document evidence of biological activity in a variety of screens ranging from standard cell based growth assays to screens involving whole animals (i.e. zebra fish). As anticipated, the fractionation process has resulted in many fractions that are dominated by single compounds and this has greatly facilitated the process of identifying active components in a given screen. The finding that TRP channel agonists may alter angiogenesis is provocative but has yet to be reduced to a molecular mechanism. Indeed, the cellular targets are still unknown as we might have obtained the observed effects by stimulating channels on the endothelial cells or some other cell type that plays a role in angiogenesis.
Given that the work presented here only includes preliminary screens and compound characterization, a number of testable hypotheses remain unanswered. First, is the question of how this library, developed according to a systematic protocol differs from comparable libraries consisting of TCM plant species, extracts and fractions elsewhere. As examples, at least five such collections of research TCM extracts are known to exist throughout East Asia and these include the: (1) TCM natural product library at the Dalian Institute of Chemical, Physics, Chinese Academy of Sciences; (2) Kunming Institute of Botany, the Chinese Academy of Sciences; (3) Beijing Tong Ren Tang Chinese Medicine Co. Ltd.; (4) Shanghai Institute of Materia Medica; and (5) Sun Ten Pharmaceuticals Co. Ltd. and Sun Ten Phytotech in Taiwan. While several of these collections include a minority of samples which were individually grown and/or collected by the named entity mentioned earlier, the majority of plant samples in these extract collections were purchased on the open market and almost certainly came from multiple collection sites (i.e. numerous farms and/or wild sites) with uncertain (and heterogeneous) collection and processing procedures. As such, these other established TCM library collections are less stringent with regard to quality assurance and the potential for reproducibility as compared with the present library.
As noted in the Introduction, any study of botanical medicines must adhere to strict and repeatable methods for ensuring the quality of the botanical product. It has been well documented that the bioactive ingredients of plant-based medicines vary in ways that have pharmacological consequences [42–44]. This variation can exist within a single population of one named species [45]. Furthermore, plant chemistry is known to vary according to many factors, including ontogeny [46], geography [47,48], and season [49,50], even within the same genetic individual. Consequently, one plant-based medicine may consist of multiple genotypes, even within one species. Each genotype may consist of multiple chemotypes, depending on the place where they live and the time of collection. Efforts to rigorously test the biological activity of plant-based medicines and reliably produce standardized plant extracts for clinical use must account for the combined possibility of genetic and chemical variability within a single botanical medicine [51]. Therefore, it is of the utmost importance to ensure that the botanical provenance and details of the collection and processing are recorded in detail and to the maximal extent possible. Every step in the creation of this prototype library, from collection to extraction and fractionation has been stored in a database created specifically for this project (i.e. the TM-CTS) [39]. While these steps maximize the prospect of demonstrating reproducibility of biological effects from compounds derived from specific plant collections, explicit proof of reproducibility remains another critically important next step.
The larger question, of course, is whether the effort and expense required to create such a library of authenticated medicinal plants will result in scientific discoveries that justify the investment. Some would argue that this investment, while risky, is justifiable and essential to the comprehensive and authoritative assessment of commonly used medicinal plants worldwide. In their important review of this subject, Schmidt and colleagues [52] remind us that most medical systems developed prior to the 20th Century relied almost exclusively on multicomponent medicines obtained from natural sources. Today, however, the modern pharmaceutical industry relies almost exclusively on single-ingredient drugs, typically referred to as new chemical entities (NCE’s). However, the rate of NCE discovery has slowed in recent decades [53,54]. In addition more diseases are being treated with combinations of single-component drugs. These combination therapies are designed to lower the incidence of resistance or target several pathological processes simultaneously. They are particularly important in treating infectious diseases such as HIV [28], tuberculosis [55], malaria [56] and complex chronic diseases like cancer [57] and metabolic syndrome [58].
By contrast, traditional herbal medicinal systems, including those of China, India, Africa, the Asian Pacific and Hawaii classically relied on complex mixtures of plants to treat common symptoms and diseases. Schmidt et al. contend that traditional medicinal use of multicomponent botanicals may be better suited to prevent or control complex multi-factorial diseases as compared with the efficacy of single active ingredient pharmaceuticals [52]. This conceptual model inextricably leads to a testable hypothesis, namely, that multicomponent botanical therapies (MCBTs), if standardized using unequivocal botanical identification techniques and quality assurance methods, can be shown to contain individual chemical compounds which, when combined in specific ratios, result in predictable additive and/or synergistic biological effects capable of altering the natural course of established disease. How then might we get from our current state of knowledge to a confirmation or rejection of this testable hypothesis?
The specific methods detailed in this manuscript, which can be used to expand the entire library of TCM medicinal plants or, alternatively, to build new parallel libraries of medicinal plants from other cultures and continents (e.g., those of India, Africa, the Mideast, Hawaii, etc.) begin to address issues pertaining to standardization and quality control of MCBTs. The next logical challenge is to use such potentially reproducible libraries to produce evidence of: (1) novel single compounds (i.e. NCE’s) with demonstrable provocative biological effects; or, (2) single compounds which are already known but which can be shown through HTS screening to possess here-to-fore unknown biological properties; or (3) evidence of entirely novel biological mechanisms; and (4) evidence that single compounds of interest, from this library (aka, “singletons”), when paired with one or more additional compounds from the same plant, a different plant in a MCBT (as is the case in most traditionally used herbal formulas and “patent medicines”) or hypothetically, in combination with a small molecule which has already been recognized as an FDA approved drug, will result in additive or synergistic effects. Such combination studies could readily be performed under automated HTS conditions using well-established techniques for combination-based screening based on the combination index methodology originally established by Chou and Talalay [59].
The present library sets the stage for these subsequent investigations. Initial screening of the 3709 fractions from 80 authenticated TCM plant species has resulted in a spectrum of leads, the majority of which remain in early stages of investigation for their reproducibility, relative potency, pharmacokinetics, safety and therapeutic potential as prospective single compound drug candidates. While we remain intrigued by the potential of combinatorial studies, due to funding challenges, we have yet to initiate combinatorial studies to explore the existence of additivity or synergy of biological effects involving lead candidate compounds within the TCM library and/or with FDA approved small drug molecules.
Several implications of this ongoing work are noteworthy. First, the successful academic partnerships between US and Chinese institutions and co-investigators have resulted in a series of agreements which are consistent with the Convention on Biological Diversity [60] and demonstrate the willingness of multiple international stakeholder groups to collaborate across professional disciplines and international boundaries. These agreements and working relationships properly acknowledge the indigenous and traditional medical knowledge of our TCM co-investigators and their home country, China. Indeed, the ethnobotanical expertise of our Chinese colleagues has been a prerequisite to the implementation of this prototype library, and will be essential for the continued, targeted scientific evaluation of this library over the coming years. Future investigations of medicinal plants in other countries may choose to refine this collaborative strategy for use in their respective international settings.
Second, in spite of the fact that there has been a relative decline in natural product-based drug discovery by most major pharmaceutical companies, the history of modern drug discovery and the relatively high proportion of FDA approved prescription drugs derived from plants. “....begs the question of whether plant (based compounds), secondary metabolites and related synthetic compounds perform better as drugs than randomly synthesized compounds [52].” For this reason, we plan to continue investigations of this prototype library in search of compounds and/or combinations of compounds with therapeutic potential.
Third, while the therapeutic value of MCBT’s has not, as of yet, been firmly established, an important hypothetical adjunct favoring them is that they have the potential “…to provide combination therapies which can simultaneously target various elements of human diseases, providing efficacy and safety unmatched by NCE’s.” This multi-targeted “birdshot” approach may provide a viable alternative to the “silver bullet” NCE approach better designed to work with one symptom or pathogen at a time [52].
There is also the undeniable realization that generations of traditional herbal practitioners who serve as repositories of millennia of empirical ethnobotanical knowledge are thinning in numbers. Unless reproducible research strategies are soon established to promote thoughtful, culturally sensitive and scientifically authoritative evaluation of herbal medicine systems, and evidence of their value, we may witness a decline in the number of well-educated trainees interested in maintaining these ethnomedical traditions worldwide. As a result, we may diminish opportunities for scientific discovery and the perpetuation of ethnobotanical study as a direct result of our failed collective stewardship of this shared global heritage.
The challenge to “form a firmly united front” [1] by engaging experts in both traditional and modern medicine is still timely today. The difference is that we now have far more powerful technologies to illuminate our shared path towards this goal.
Only time will tell if the methods proposed here to establish a testable library of authenticated, commonly used medicinal plants will result in the discovery of new knowledge and the advent of novel therapeutic options. We will continue screening the existing library as resources allow. It is encouraging that the NIH and NCCAM have listed “Research on Complementary and Alternative Medicine pharmacologic interventions,” which presumably includes TCM and other herbal therapies, as a prioritized strategic objective for the next five years of NIH funding [61]. It is hoped that this prototype TCM library as well as other natural product repositories with similar features, will enhance ongoing efforts to systematically evaluate commonly used herbal therapies worldwide.
An anonymous Chinese proverb says: “The methods used by one may be faulty. The methods used by many will be better.”
Supplementary materials related to this article can be found online at doi:10.1016/j.fitote.2010.11.017.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health National Cancer Institute (U19 CA128534), the National Center for Complementary and Alternative Medicine (AT03002 PIRC), the Bernard Osher Foundation, and Hope Funds for Cancer Research.
INBio (Instituto Nacional de Biodiversidad) performed the chemical extraction and fractionation of all of the samples for the project.
NSF International Center for Applied Research, Ann Arbor, Michigan performed the heavy metal and pesticide analyses.
The sponsors were not involved in the collection, management, analysis or interpretation of the data.
Footnotes
Dedicated to Dr. Norman R. Farnsworth of the University of Illinois at Chicago for his pioneering work on botanical natural products, his superb inspiration and leadership as world authority in the field of pharmacognosy.
References
- 1.Zedong M. Mao Zedong’s Manuscripts since the Funding of People’s Republic of China. Beijing, China: The Central Literature Publishing House; 1991. [Google Scholar]
- 2.Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Boyd MR, Cardellina JH, Newman DJ, Snader KM, McCloud TG, et al. Ethnobotany and drug discovery: the experience of the US National Cancer Institute. Ciba Found Symp. 1994;185:178–90. doi: 10.1002/9780470514634.ch13. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–52. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PM, Adams PF, Schiller JS. Summary health statistics for the U.S. population: National Health Interview Survey, 2001. Vital Health Stat. 2003;10:1–82. [PubMed] [Google Scholar]
- 9.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States. Natl Health Stat Report. 2007;2008:1–23. [PubMed] [Google Scholar]
- 10.Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States. Natl Health Stat Report. 2007;2009:1–14. [PubMed] [Google Scholar]
- 11.Li B, Wu S, Lui C. China pharmaceuticals: investing in Traditional Chinese Medicine (TCM) Hong Kong: 2009. [Google Scholar]
- 12.National Center for Complementary and Alternative Medicine (NCCAM) Centers for Research on Complementary and Alternative Medicine (CRC) Program. [Accessed September 19, Accessed 2010];CY 2003 Research Priorities. A priority to elucidate mechanisms of action and conduct small, well-developed phase I and II trials. 2002 December 2; at http://web.archive.org/web/20021225053952/http://nccam.nih.gov/research/priorities/indexhtm#2.
- 13.Oh WK, Kantoff PW, Weinberg V, et al. Prospective, multicenter, randomized phase II trial of the herbal supplement, PC-SPES, and diethylstilbestrol in patients with androgen-independent prostate cancer. J Clin Oncol. 2004;22:3705–12. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 14.Wolsko PM, Solondz DK, Phillips RS, Schachter SC, Eisenberg DM. Lack of herbal supplement characterization in published randomized controlled trials. Am J Med. 2005;118:1087–93. doi: 10.1016/j.amjmed.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 15.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal M, Farnsworth NR. Echinacea angustifolia in rhinovirus infections. N Engl J Med. 2005;353:1971–2. doi: 10.1056/NEJM200511033531818. author reply -2. [DOI] [PubMed] [Google Scholar]
- 17.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 18.DiPaola RS, Morton RA. Proven and unproven therapy for benign prostatic hyperplasia. N Engl J Med. 2006;354:632–4. doi: 10.1056/NEJMe058301. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed 10/6/10];NCCAM Policy: Natural Product Integrity. at http://nccam.nih.gov/research/policies/naturalproduct.htm.
- 20.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 21.Holzbeieriein J. Hsp90: a drug target? Curr Oncol Rep. 2010;12:95–101. doi: 10.1007/s11912-010-0086-3. [DOI] [PubMed] [Google Scholar]
- 22.Richardson P. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose–escalation study. Br J Haematol. 2010;150:438–45. doi: 10.1111/j.1365-2141.2010.08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suffness M, Douros J. Current status of the NCI plant and animal product program. J Nat Prod. 1982;45:1–14. doi: 10.1021/np50019a001. [DOI] [PubMed] [Google Scholar]
- 24.Verpoorte R. Pharmacognosy in the new millennium: leadfinding and biotechnology. J Pharm Pharmacol. 2000;52:253–62. doi: 10.1211/0022357001773931. [DOI] [PubMed] [Google Scholar]
- 25.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bensky D, Clavey S, Stoger E, Gamble A. Chinese Herbal Medicine: Materia Medica. Seattle: Eastland Press; 2004. [Google Scholar]
- 27.Scheid V, Bensky D, Ellis A, Barolet R. Chinese herbal medicine: Formulas and strategies. 2. Seattle: Eastland Press; 2009. [Google Scholar]
- 28.Agrawal L, Lu X, Jin Q, Alkhatib G. Anti-HIV therapy: current and future directions. Curr Pharm Des. 2006;12:2031–55. doi: 10.2174/138161206777442100. [DOI] [PubMed] [Google Scholar]
- 29.Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, Stockwell BR, Keith CT. Systematic discovery of multicomponent therapeutics. PNAS. 2003;100:7977–82. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: People’s Medical Publishing House; 2005. [Google Scholar]
- 32.Larsen HO, Olsen CS. Unsustainable Collection and Unfair Trade? Uncovering and Assessing Assumptions Regarding Central Himalayan Medicinal Plant Conservation. Biodiversity and Conservation. 2007;16(6):1679–1697. [Google Scholar]
- 33. [Accessed September 19, 2010];Convention on International Trade in Endangered Species of Wild Flora and Fauna and Flora (CITES) Appendices I, II and III. at http://www.cites.org/eng/app/appendices.shtml.
- 34.Hildreth J, Hrabeta-Robinson E, Applequist W, Betz J, Miller J. Standard operating procedure for the collection and preparation of voucher plant specimens for use in the nutraceutical industry. Anal Bioanal Chem. 2007;389:13–7. doi: 10.1007/s00216-007-1405-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZZ, Xiao PG, Xiao Y, Yuen JPS. Quality assurance of Chinese Herbal Medicines (CHMs) J Food Drug Anal. 2007;15:337–46. [Google Scholar]
- 36.NSF International Standard/American National Standard. #173 for Dietary Supplements. Ann Arbor: NSF International; 2008. [Google Scholar]
- 37.World Health Organization. Operational Guidance: Information needed to support clinical trials of herbal products UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) 2005. [Google Scholar]
- 38.Harris ESJ, Woolf AD, Eisenberg DM, et al. Heavy Metal and Pesticide Content in Commonly Prescribed Individual Raw Chinese Herbal Medicines. In Preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris ESJ, Erickson SD, Tolopko AN, Cao SG, Craycroft JA, Scholten R, Fu YL, Wang WQ, Liu Y, Zhao ZZ, Clardy J, Shamu C, Eisenberg DM. Traditional Medicine Collection Tracking System (TM-CTS): A Database for Ethnobotanically-Driven Drug-Discovery Programs. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, et al. CDK8 is a colorectal cancer oncogene that regulates B-catenin activity. Nature. 2008;455:547–51. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu ZY, Raven PH. Flora of China, Multiple Volumes. St. Louis: Missouri Botanical Garden Press; 1994. [Google Scholar]
- 42.Delabays N, Simonnet X, Gaudin M. The genetics of artemisinin content in Artemisia annua L and the breeding of high yielding cultivars. Curr Med Chem. 2001;8:1795–801. doi: 10.2174/0929867013371635. [DOI] [PubMed] [Google Scholar]
- 43.Goodger JQD, Whincup AL, Field AR, Holtum JAM, Woodrow IE. Variation in huperzine A and B in Australasian Huperzia species. Biochem Syst Ecol. 2008;36:612–8. [Google Scholar]
- 44.Viljoen AM, Subramoney S, van Vuuren SF, Baser KH, Demirci B. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J Ethnopharmacol. 2005;96:271–7. doi: 10.1016/j.jep.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Hong DY, Lau AJ, Yeo CL, et al. Genetic diversity and variation of saponin contents in Panax notoginseng roots from a single farm. J Agric Food Chem. 2005;53:8460–7. doi: 10.1021/jf051248g. [DOI] [PubMed] [Google Scholar]
- 46.Rehill BJ, Whitham TG, Martinsen GD, Schweitzer JA, Baily JK, Lindroth RL. Developmental trajectories in cottonwood phytochemistry. J Chem Ecol. 2006;32:2269–85. doi: 10.1007/s10886-006-9141-9. [DOI] [PubMed] [Google Scholar]
- 47.Han T, Hu Y, Zhou SY, Li HL, Zhang QY, Zhang H, Huang BK, Rahman K, Zheng HC, Qin LP. Correlation between the genetic diversity and variation of total phenolic acids contents in Fructus Xanthii from different populations in China. Biomed Chromatogr. 2008;22:478–86. doi: 10.1002/bmc.956. [DOI] [PubMed] [Google Scholar]
- 48.Su S, He CM, Li LC, Chen JK, Zhou TS. Genetic characterization and phytochemical analysis of wild and cultivated populations of Scutellaria baicalensis. Chem Biodivers. 2008;5:1353–63. doi: 10.1002/cbdv.200890123. [DOI] [PubMed] [Google Scholar]
- 49.Dong TT, Cui XM, Song ZH, et al. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–23. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 50.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–6. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 51.Ribnicky DM, Poulev A, Schmidt B, Cefalu WT, Raskin I. Evaluation of botanicals for improving human health. Am J Clin Nutr. 2008;87:472S–5S. doi: 10.1093/ajcn/87.2.472S. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat Chem Biol. 2007;3:360–6. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- 53.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–53. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 54.Sams-Dodd F. Drug discovery: selecting the optimal approach. Drug Discov Today. 2006;11:465–72. doi: 10.1016/j.drudis.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Blomberg B, Fourie B. Fixed-dose combination drugs for tuberculosis: application in standardised treatment regimens. Drugs. 2003;63:535–53. doi: 10.2165/00003495-200363060-00002. [DOI] [PubMed] [Google Scholar]
- 56.Schellenberg D, Abdulla S, Roper C. Current issues for anti-malarial drugs to control P. falciparum malaria. Curr Mol Med. 2006;6:253–60. doi: 10.2174/156652406776055168. [DOI] [PubMed] [Google Scholar]
- 57.Waterhouse DN, Gelmon KA, Klasa R, et al. Development and assessment of conventional and targeted drug combinations for use in the treatment of aggressive breast cancers. Curr Cancer Drug Targets. 2006;6:455–89. doi: 10.2174/156800906778194586. [DOI] [PubMed] [Google Scholar]
- 58.Deedwania PC, Volkova N. Current treatment options for the metabolic syndrome. Curr Treat Options Cardiovasc Med. 2005;7:61–74. doi: 10.1007/s11936-005-0007-1. [DOI] [PubMed] [Google Scholar]
- 59.Chou T, Talalay P. Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 60.UNEP. Convention on Biological Diversity. [Accessed September 19, 2010];United Nations Environment Programme. at http://www.cbd.int.
- 61.National Center for Complementary and Alternative Medicine. Accessed at http://nccam.nih.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.