Abstract
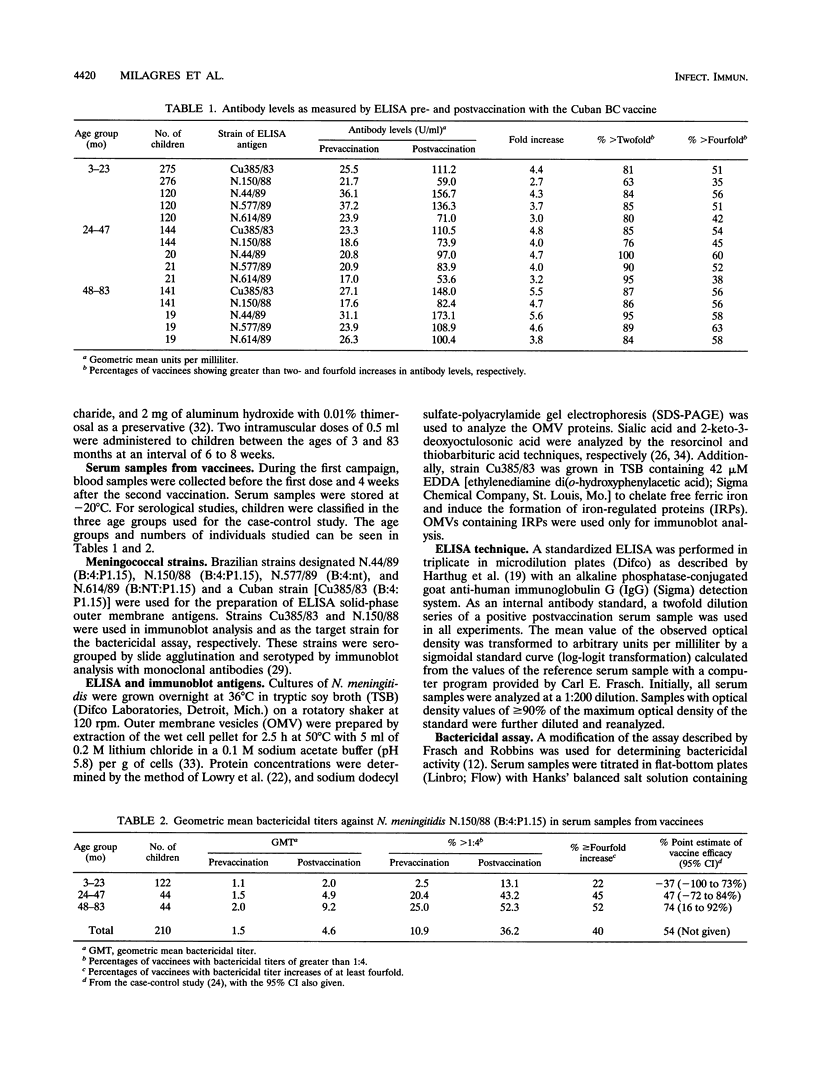
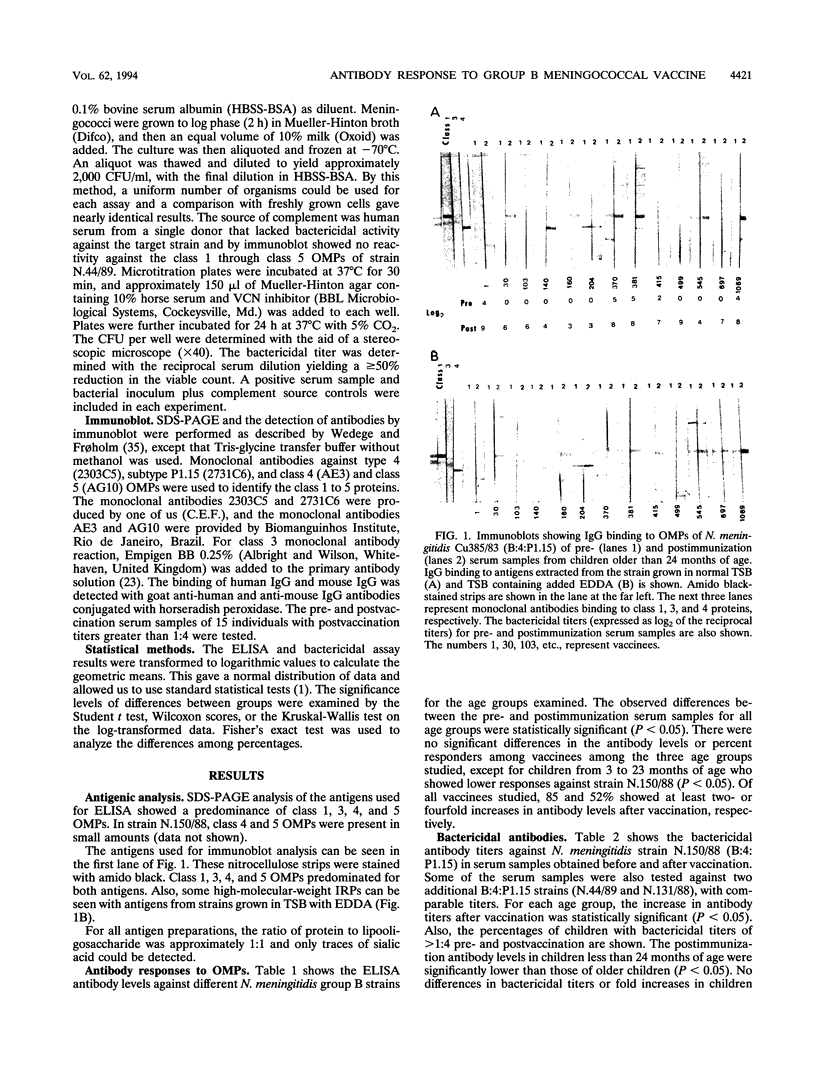
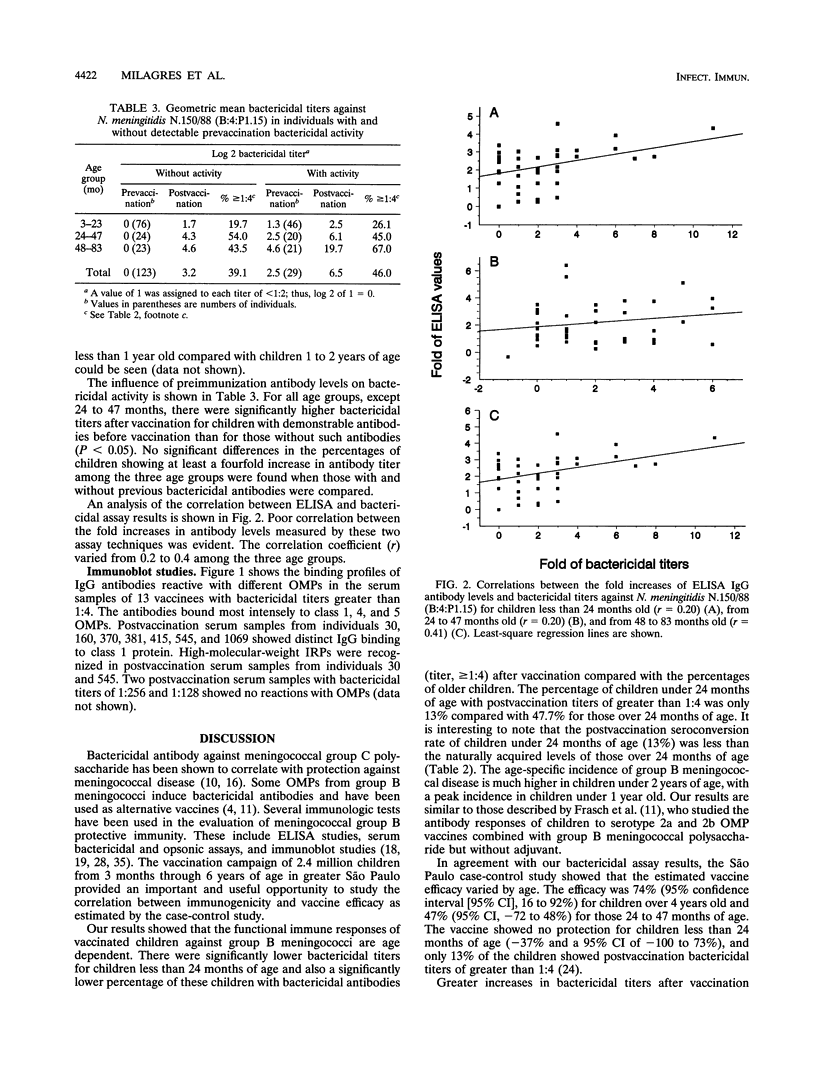
Since 1986, serogroup B Neisseria meningitidis has caused approximately 80% of the meningococcal disease in Brazil. In 1988, an epidemic caused by N. meningitidis B:4:P1.15 was recognized in the greater São Paulo area of Brazil. The São Paulo state government decided to vaccinate children from 3 to 83 months of age with a vaccine consisting of serotype 4 outer membrane protein and group C meningococcal polysaccharide that was produced in Cuba. About 2.7 million children were vaccinated during two immunization campaigns conducted in 1989 and 1990. Because of this, a case-control study was designed to determine vaccine efficacy against group B meningococcal disease. The purpose of our study was to compare the antibody response with the protection from disease estimated from the case-control study. We measured the immune responses of vaccinees by enzyme-linked immunosorbent assay (ELISA), immunoblot, and bactericidal assay. The development of bactericidal antibodies was age dependent and in good agreement with the results of the case-control study. Only 40% of vaccinees showed fourfold or greater increases in bactericidal antibody titers after vaccination. A poor correlation between antibody levels detected by ELISA and those by bactericidal assay was found. Immunoblot analysis showed that about 50% of the serum samples with bactericidal titers higher than 1:4 were reactive with class 1 outer membrane protein. We conclude that the bactericidal assay is a good, laboratory-based, functional assay for the study of vaccine immunogenicity and that an effective solution to group B meningococcal disease remains to be demonstrated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Høiby E. A., Grønnesby J. K., Arnesen O., Fredriksen J. H., Halstensen A., Holten E., Lindbak A. K., Nøkleby H., Rosenqvist E. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991 Nov 2;338(8775):1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- Black J. R., Dyer D. W., Thompson M. K., Sparling P. F. Human immune response to iron-repressible outer membrane proteins of Neisseria meningitidis. Infect Immun. 1986 Dec;54(3):710–713. doi: 10.1128/iai.54.3.710-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danve B., Lissolo L., Mignon M., Dumas P., Colombani S., Schryvers A. B., Quentin-Millet M. J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993 Sep;11(12):1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Sacchi C. T., Brandiolone M. C., Vieiera V. S., Leite L. C. Development of a second generation group B meningococcal vaccine. NIPH Ann. 1991 Dec;14(2):225–231. [PubMed] [Google Scholar]
- Frasch C. E., Zahradnik J. M., Wang L. Y., Mocca L. F., Tsai C. M. Antibody response of adults to an aluminum hydroxide-adsorbed Neisseria meningitidis serotype 2b protein-group B polysaccharide vaccine. J Infect Dis. 1988 Oct;158(4):710–718. doi: 10.1093/infdis/158.4.710. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Altieri P. L., Pier G. B., Berman S. L. Safety and immunogenicity of group Y and group W135 meningococcal capsular polysaccharide vaccines in adults. Infect Immun. 1981 Dec;34(3):725–732. doi: 10.1128/iai.34.3.725-732.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen A., Lehmann A. K., Guttormsen H. K., Vollset S. E., Bjune G., Naess A. Serum opsonins to serogroup B meningococci after disease and vaccination. NIPH Ann. 1991 Dec;14(2):157–167. [PubMed] [Google Scholar]
- Harthug S., Rosenqvist E., Høiby E. A., Gedde-Dahl T. W., Frøholm L. O. Antibody response in group B meningococcal disease determined by enzyme-linked immunosorbent assay with serotype 15 outer membrane antigen. J Clin Microbiol. 1986 Dec;24(6):947–953. doi: 10.1128/jcm.24.6.947-953.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby E. A., Rosenqvist E., Frøholm L. O., Bjune G., Feiring B., Nøkleby H., Rønnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 1991 Dec;14(2):147–156. [PubMed] [Google Scholar]
- Irwin G. R., Allen R. G., Segal H. G., Allen A. M., Putnak J. R., Cannon H. G., Top F. H., Jr Serodiagnosis of hepatitis B virus infection by antibody to core antigen. J Infect Dis. 1977 Jul;136(1):31–36. doi: 10.1093/infdis/136.1.31. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lifely M. R., Moreno C., Lindon J. C. An integrated molecular and immunological approach towards a meningococcal group B vaccine. Vaccine. 1987 Mar;5(1):11–26. doi: 10.1016/0264-410x(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Maeland J. A., Wedege E. Serum antibodies to cross-reactive Neisseria outer membrane antigens in healthy persons and patients with meningococcal disease. APMIS. 1989 Sep;97(9):774–780. doi: 10.1111/j.1699-0463.1989.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Munkley A., Tinsley C. R., Virji M., Heckels J. E. Blocking of bactericidal killing of Neisseria meningitidis by antibodies directed against class 4 outer membrane protein. Microb Pathog. 1991 Dec;11(6):447–452. doi: 10.1016/0882-4010(91)90041-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Poolman J. T. Polysaccharides and membrane vaccines. Adv Biotechnol Processes. 1990;13:57–86. [PubMed] [Google Scholar]
- Sacchi C. T., Pessoa L. L., Ramos S. R., Milagres L. G., Camargo M. C., Hidalgo N. T., Melles C. E., Caugant D. A., Frasch C. E. Ongoing group B Neisseria meningitidis epidemic in São Paulo, Brazil, due to increased prevalence of a single clone of the ET-5 complex. J Clin Microbiol. 1992 Jul;30(7):1734–1738. doi: 10.1128/jcm.30.7.1734-1738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Leinonen M., Abdillahi H., Poolman J. T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989 Aug;7(4):325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger Y., Granoff D. M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992 Mar 18;267(11):1489–1494. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J Bacteriol. 1980 Jan;141(1):169–176. doi: 10.1128/jb.141.1.169-176.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wedege E., Frøholm L. O. Human antibody response to a group B serotype 2a meningococcal vaccine determined by immunoblotting. Infect Immun. 1986 Feb;51(2):571–578. doi: 10.1128/iai.51.2.571-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes J. C., Perkins B. A., Camargo M. C., Hidalgo N. T., Barbosa H. A., Sacchi C. T., Landgraf I. M., Gattas V. L., Vasconcelos H. de G., Gral I. M. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992 Oct 31;340(8827):1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]



